Sulfated and Sulfur-Containing Steroids and Their Pharmacological Profile
Abstract
:1. Introduction
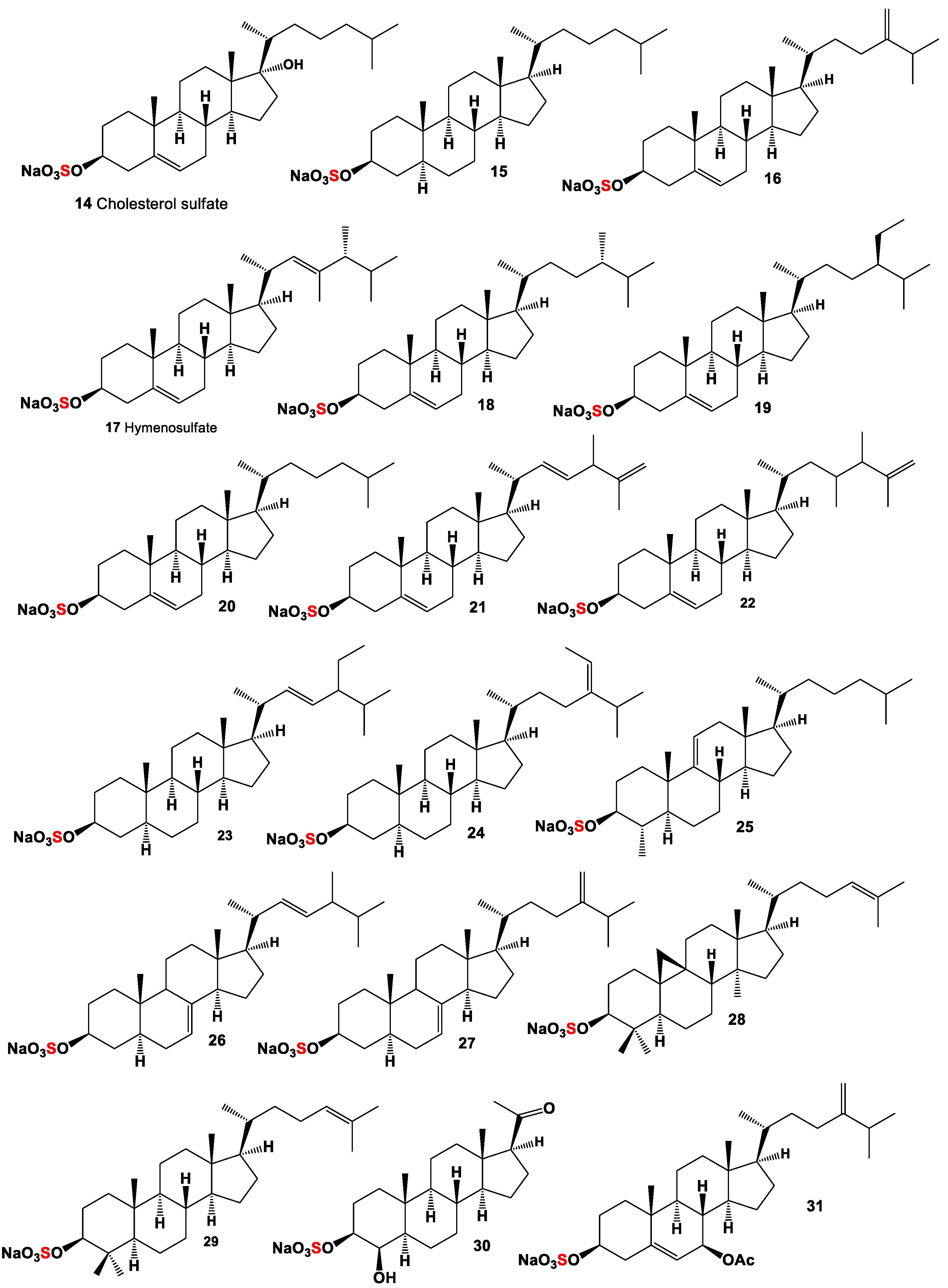
2. Mono Sulfated Steroids Derived from Marine Sources
3. Di- and Poly-Sulfated Steroids Derived from Marine Sources
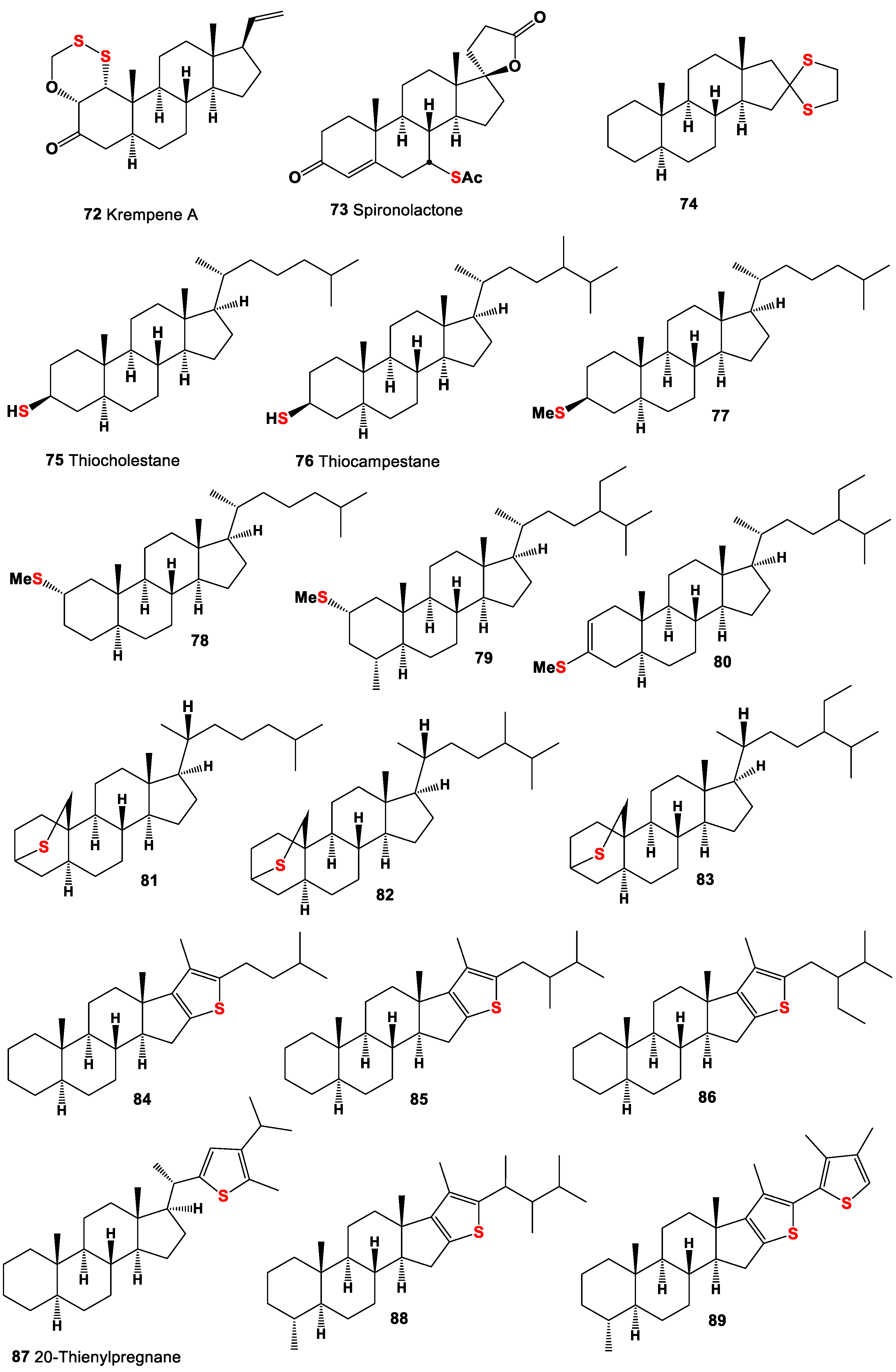
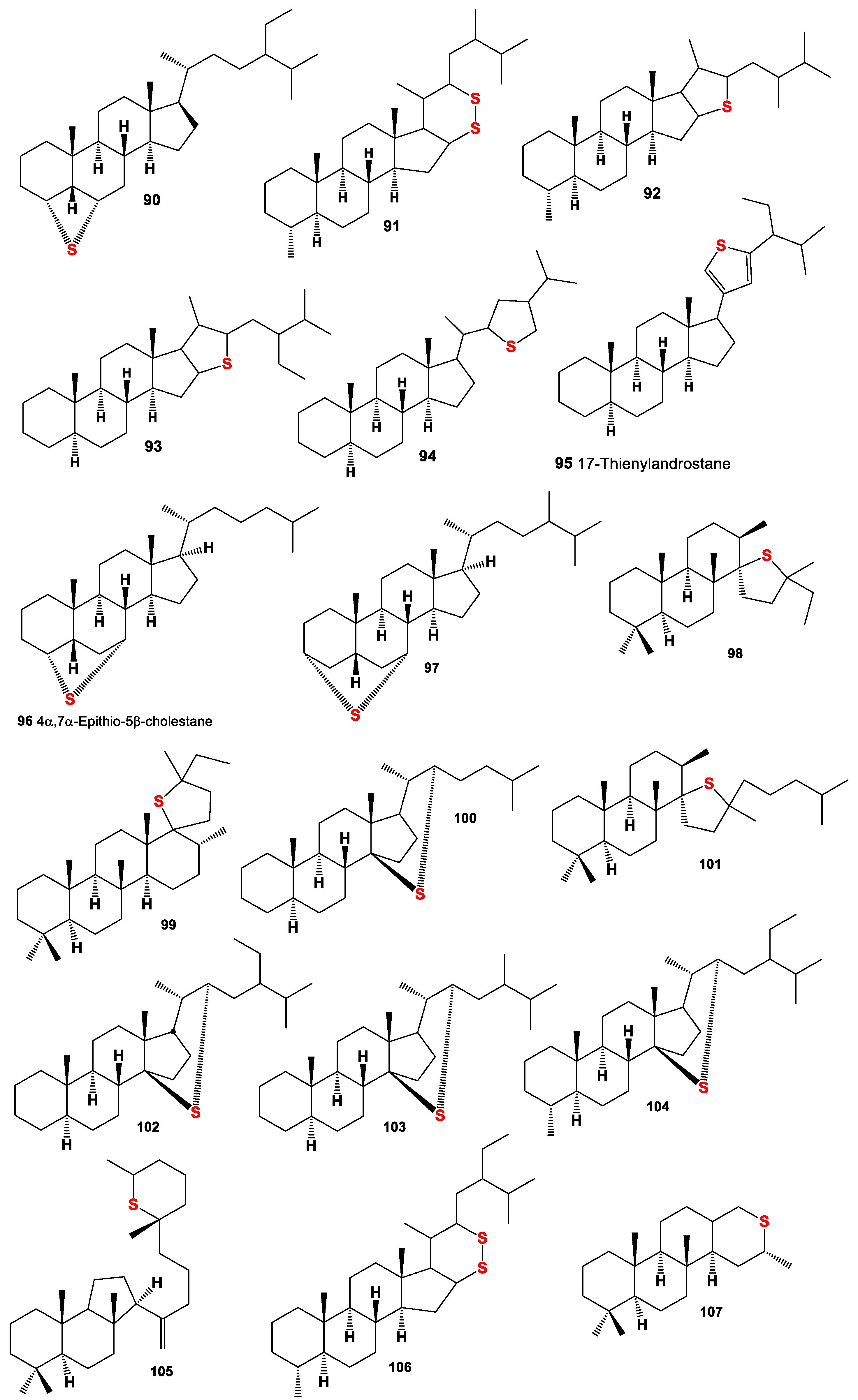
4. Sulfur-Containing Steroids Derived from Marine and Terrestrial Sources
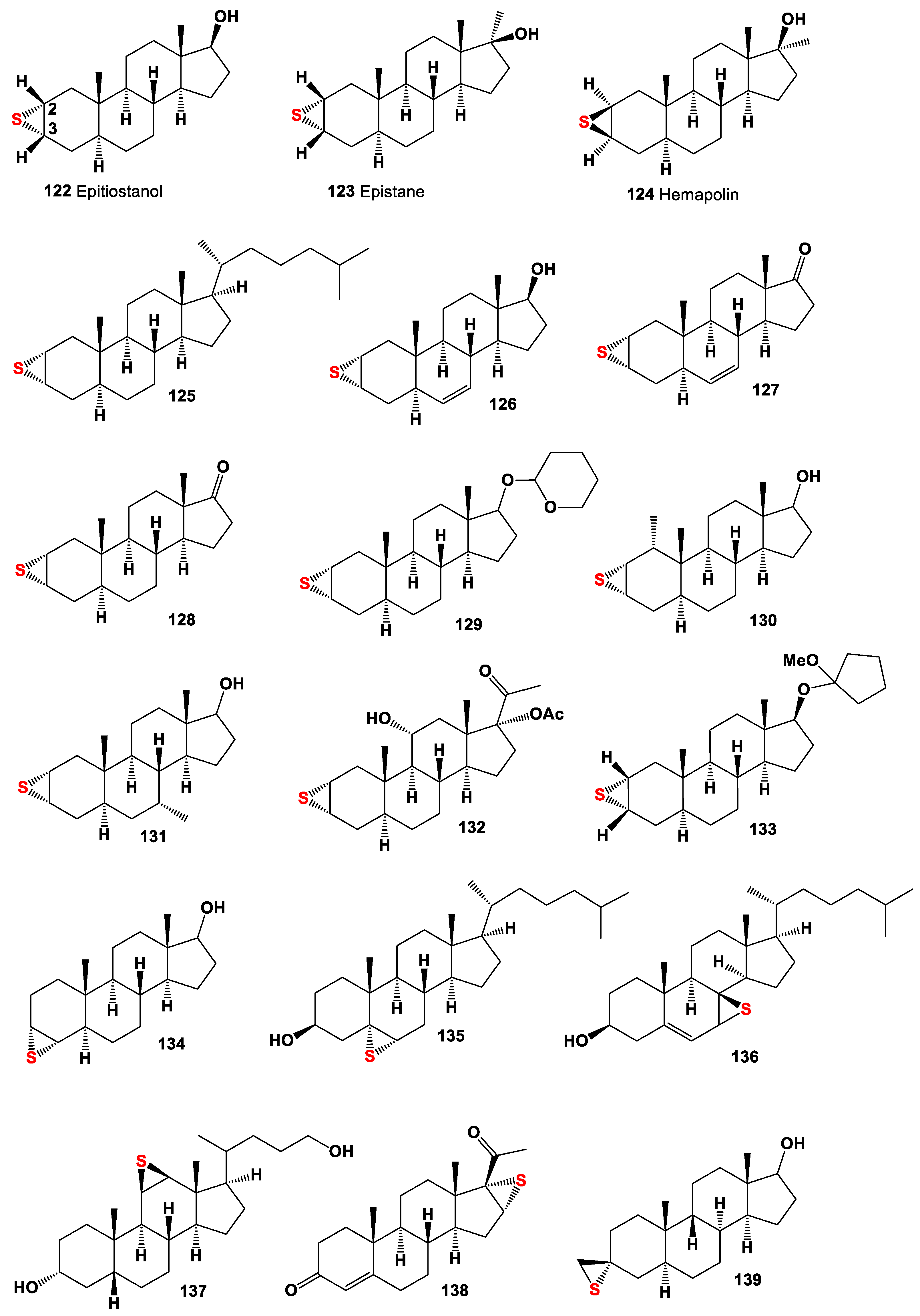
5. Epithio Steroids
6. Comparison of Biological Activities of Sulfated and Sulfur-Containing Steroids
6.1. Antitumor Activity of Natural Mono-, Di-, and Poly-Sulfated Steroids
6.2. Biological Activity of Sulfur-Containing and Epithio Steroids
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries. A review. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Kornprobst, J.M.; Sallenave, C.; Barnathan, G. Sulfated compounds from marine organisms. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1998, 119B, 1–51. [Google Scholar]
- Mougous, J.D.; Green, R.E.; Williams, S.J. Sulfotransferases and sulfatases in mycobacteria. Chem. Biol. 2002, 9, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Sousa, E.; Pinto, M.M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Chemistry and biodiversity of the biologically active natural glycosides. Chem. Biodivers. 2004, 1, 673–781. [Google Scholar] [CrossRef]
- Almeida, J.R.; Da-Silva, M.C.; Sousa, E. Antifouling potential of nature-inspired sulfated compounds. Sci. Rep. 2017, 7, 42424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teles, Y.C.F.; Sallett, M.; Souza, R. Sulphated flavonoids: Biosynthesis, structures, and biological activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, taxonomic distribution, biogenesis and biological activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- Mensah-Nyagan, A.G.; Beaujean, D.; Do-Rego, J.L. In vivo evidence for the production of sulfated steroids in the frog brain. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2000, 126, 213–219. [Google Scholar] [CrossRef]
- Pauli, G.F.; Friesen, J.B.; Gödecke, T. Occurrence of progesterone and related animal steroids in two higher plants. J. Nat. Prod. 2010, 73, 338–345. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J. The regulation of steroid action by sulfation and desulfation. Endocrin. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef] [PubMed]
- Suchański, J.; Ugorski, M. The biological role of sulfatides. Postepy Hig. Med. Dosw. (Online) 2016, 70, 489–504. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Minale, L.; Riccio, R. Polyoxygenated steroids of marine origin. Chem. Rev. 1993, 93, 1839–1895. [Google Scholar] [CrossRef]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, M.E. Sources and biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, M.V.; Paloma, L.G.; Minale, L. Isolation and structure characterization of two novel bioactive sulphated polyhydroxysteroids from the Antarctic Ophiuroid, Ophioderma longicaudum. Nat. Prod. Lett. 1993, 3, 197–208. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Stonik, V.A.; Ivanchina, N.V.; Kicha, A.A. New polar steroids from starfish. Nat. Prod. Commun. 2008, 3, 158–172. [Google Scholar] [CrossRef] [Green Version]
- Savidov, N.; Gloriozova, T.A.; Dembitsky, V.M. Pharmacological activities of sulphated steroids derived from marine sources. Life Sci. Press. 2018, 2, 48–58. [Google Scholar]
- Kellner Filho, L.C.; Picão, B.W.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Dias, G.M.; Copp, B.R.; Bertanha, C.S.; Januario, A.H. Bioactive aliphatic sulfates from marine invertebrates. Mar. Drugs 2019, 17, 527. [Google Scholar] [CrossRef] [Green Version]
- Burdige, D.J. Geochemistry of Marine Sediments; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Volkman, J.K. Lipids of Geochemical Interest in Microalgae. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Handbook of Hydrocarbon and Lipid Microbiology; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Harris, P.T. The fate of microplastic in marine sedimentary environments: A review and synthesis. Mar. Pollut. Bull. 2020, 158, 111398. [Google Scholar] [CrossRef]
- La Rowe, D.E.; Arndt, S.; Bradley, J.A.; Estes, E.R.; Hoarfrostf, A.; Langg, S.Q.; Lloydh, K.G. The fate of organic carbon in marine sediments-New insights from recent data and analysis. Earth Sci. Rev. 2020, 204, 103146. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Shilling, A.; Verde, C.; Baker, B.J.; Giordano, D. Marine terpenoids from polar latitudes and their potential applications in biotechnology. Mar. Drugs 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Volkman, J.K.; Holdsworth, D.G.; Neill, G.P.; Bavor, H.J., Jr. Identification of natural, anthropogenic and petroleum hydrocarbons in aquatic sediments. Sci. Total Environ. 1992, 112, 203–219. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Ether lipids of the organic world: Formation and biotransfor-mation. In Fats for Future; Cambie, R.C., Ed.; Ellis Horwood Ltd. Publ.: Oxford, UK, 1989; Chapter 12; pp. 173–188. [Google Scholar]
- Volkman, J. Sterols in microorganisms. Appl. Microbiol. Biotech. 2003, 60, 495–506. [Google Scholar] [CrossRef]
- Cranwell, P.A. Lipids of aquatic sediments and sedimenting particulates. Prog. Lipid Res. 1982, 21, 271–308. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally occurring boron containing compounds: Structures and biological activities. J. Nat. Prod. Resour. (India) 2017, 3, 147–152. [Google Scholar]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I.; Mansour, M.P.; Sikes, E.L.; Gelin, F. Microalgal biomarkers: A review of recent research developments. Org. Geochem. 1998, 29, 1163–1179. [Google Scholar] [CrossRef]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Nováková, L.; Solich, P. Determination of steroid hormones in biological and environmental samples using green microextraction techniques: An overview. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Ali, H.A.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Quntar, A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 209–237. [Google Scholar] [CrossRef]
- Wolkenstein, K.; Gross, J.H.; Falk, H. Boron-containing organic pigments from a Jurassic red alga. Proc. Natl. Acad. Sci. USA 2010, 107, 19374–19378. [Google Scholar] [CrossRef] [Green Version]
- Wolkenstein, K.; Sun, H.; Falk, H.; Griesinger, C. Structure and absolute configuration of Jurassic polyketide-derived spiroborate pigments obtained from microgram quantities. J. Am. Chem. Soc. 2015, 137, 13460–13463. [Google Scholar] [CrossRef] [PubMed]
- Summons, R.E.; Jahnke, L.L.; Simoneit, B.R. Lipid biomarkers for bacterial ecosystems: Studies of cultured organisms, hydrothermal environments and ancient sediments. Ciba Found. Symp. 1996, 202, 174–193. [Google Scholar]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; van Diggelen, O.; Op den Camp, H.J.M.; Visser, E.J.W.; Lucassen, E.C.; Vile, A.; Jetten, M.S.M.; Smolders, A.J.P.; Roelofs, J.G.M. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A Review. Front. Microbiol. 2012, 3, 156–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, H.W.; Stewart, J.W.B.; Cole, C.V. Concepts of sulfur, carbon, and nitrogen transformations in soil: Evaluation by simulation modelling. Biogeochemistry 1986, 2, 163–177. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021. [Google Scholar] [CrossRef]
- Bruisson, S.; Berg, G.; Garbeva, P.; Weisskopf, L. Volatile interplay between microbes: Friends and Foes. In Bacterial Volatile Compounds as Mediators of Airborne Interactions; Ryu, C.M., Weisskopf, L., Piechulla, B., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Janjua, H.T. Microbial secondary metabolites and defense of plant stress. Microb. Serv. Restorat. Ecol. 2020, 11, 37–46. [Google Scholar]
- Lee, Y.J.; Cho, Y.; Tran, H.N.K. Secondary metabolites from the marine sponges of the genus Petrosia: A literature review of 43 years of research. Mar. Drugs 2021, 19, 122. [Google Scholar] [CrossRef]
- Roy, S.R.; Minei, A.; Ahmadi, P.; Hermawan, I.; Kurnianda, V.; Dick, M.H.; Tanaka, J. Two new steroid sulfates from a cheilostome bryozoan, Calyptotheca sp. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Kicha, A.A.; Malyarenko, T.V.; Kalinovsky, A.I.; Popov, R.S.; Malyarenko, O.S.; Ermakova, S.P.; Ivanchina, N.V. Polar steroid compounds from the Arctic starfish Asterias microdiscus and their cytotoxic properties against normal and tumor cells in vitro. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Huong, T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Agafonova, I.G.; Long, P.Q.; Stonik, V.A. Highly hydroxylated steroids of the starfish Archaster typicus from the Vietnamese waters. Steroids 2010, 75, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhskiy, L.A.; Shkel, T.V.; Ivanchina, N.I.; Kicha, A.A.; Grabovec, I.P.; Gilep, A.A. Structural analogues of lanosterol from marine organisms of the class Asteroidea as potential inhibitors of human and Candida albicans lanosterol 14α-demethylases. Nat. Prod. Commun. 2017, 12, 1843–1846. [Google Scholar] [CrossRef] [Green Version]
- Shubina, L.K.; Makarieva, T.N.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; Dmitrenok, P.S.; von Amsberg, G.; Stonik, V.A. Gracilosulfates A–G, monosulfated polyoxygenated steroids from the marine sponge Haliclona gracilis. Mar. Drugs 2020, 18, 454. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Ermolaeva, S.D.; Yurchenko, E.A.; Pislyagin, E.A.; van Minh, C.; Dmitrenok, P.S. Granulatosides D, E and other polar steroid compounds from the starfish Choriaster granulatus. Their immunomodulatory activity and cytotoxicity. Nat. Prod. Res. 2019, 33, 2623–2630. [Google Scholar] [CrossRef]
- Palagiano, E.; Zollo, F.; Minale, L.; Gomez Paloma, L.; Iorizzi, M.; Bryan, P.; McClintock, J.; Hopkins, T.; Riou, D.; Roussakis, C. Downeyosides A and B, two new sulfated steroid glucuronides from the starfish Henricia downeyae. Tetrahedron 1995, 51, 12293–12300. [Google Scholar] [CrossRef]
- Palagiano, E.; Zollo, F.; Minale, L.; Iorizzi, M.; Bryan, P.; McClintock, J.; Hopkins, T. Isolation of 20 glycosides from the starfish Henricia downeyae, collected in the Gulf of Mexico. J. Nat. Prod. 1996, 59, 348–354. [Google Scholar] [CrossRef]
- Björkman, L.R.; Karlsson, K.A.; Pascher, I.; Samuelsson, B.E. The identification of large amounts of cerebroside and cholesterol sulfate in the sea star. Asterias rubens. Biochim. Biophys. Acta 1972, 270, 260–265. [Google Scholar] [CrossRef]
- Björkman, L.R.; Karlsson, K.A.; Nilsson, K. On the existence of cerebroside and cholesterol sulfate in tissues of the sea star. Asterias rubens. Comp. Biochem. Physiol. 1972, 43, 409–410. [Google Scholar]
- Makarieva, T.N.; Stonik, V.A.; Kapustina, I.I. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids 1993, 58, 508–517. [Google Scholar] [CrossRef]
- Kates, M.; Volcani, B.E. Lipids of diatoms. Biochem. Biophys. Acta 1966, 116, 264–272. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishibashi, M.; Nakamura, H. Hymenosulphate, a novel sterol sulphate with Ca-releasing activity from the cultured marine haptophyte Hymenomonas sp. J. Chem. Soc. Perkin Trans. 1 1989, 2, 101–103. [Google Scholar] [CrossRef]
- Gallo, C.; d’Ippolito, G.; Nuzzo, G.; Sardo, A.; Fontana, A. Autoinhibitory sterol sulfates mediate programmed cell death in a bloom-forming marine diatom. Nat. Commun. 2017, 8, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.; Kim, E.J. Steroidal constituents from the edible sea urchin Diadema savignyi Michelin induce apoptosis in human cancer cells. J. Med. Food 2015, 18, 45–53. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Venugopal, M.J.R.V.; Minale, L.; Iorizzi, M.; Pelagiano, E. Chemical examination of a sea cucumber of Holothuria genus of the Indian Ocean. Indian J. Chem. 1998, 37B, 262–268. [Google Scholar]
- Prinsep, M.R.; Blunt, J.W.; Munro, M.H.G. A new sterol sulfate from the marine sponge Stylopus australis. J. Nat. Prod. 1989, 52, 657–659. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Stonik, V.A.; D’yachuk, O.G.; Dmitrenok, A.S. Annasterol sulfate, a novel marine sulfated steroid, inhibitor of glucanase activity from the deep water sponge Poecillastra laminaris. Tetrahedron Lett. 1995, 36, 129–134. [Google Scholar] [CrossRef]
- Nakatsu, T.; Walker, R.P.; Thompson, J.E.; Faulkner, D.J. Biologically active sterol sulfates from the marine sponge Toxadocia zumi. Experientia 1983, 39, 759–761. [Google Scholar] [CrossRef]
- Li, H.Y.; Matsunaga, S.; Fusetani, N.; Fujiki, H. Echinoclasterol sulfate phenethyl-ammonium salt, a unique steroid sulfate from the marine sponge. Echinoclathria subhispida. Tetrahedron Lett. 1993, 34, 5733–5736. [Google Scholar] [CrossRef]
- Sperry, S.; Crews, P. Haliclostanone sulfate and halistanol sulfate from an Indo-Pacific Haliclona sponge. J. Nat. Prod. 1997, 60, 29–32. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids 1995, 60, 666–673. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I. Hemolytic polar steroidal constituents of the starfish Aphelasterias japonica. J. Nat. Prod. 2000, 63, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Matsunaga, S.; Fusetani, N.; van Soest, R.W.M. Acanthosterol sulfates A−J: ten new antifungal steroidal sulfates from a marine songe Acanthodendrilla sp. J. Nat. Prod. 1998, 61, 1374–1378. [Google Scholar] [CrossRef]
- Iorizzi, M.; Bryan, P.; McClintock, J. Chemical and biological investigation of the polar constituents of the starfish Luidia clathrata, collected in the Gulf of Mexico. J. Nat. Prod. 1995, 58, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Andriyaschenko, P.V.; Levina, E.V.; Kalinovsky, A.I. Chemistry of natural compounds and bioorganic chemistry: Steroid compounds from the Pacific starfishes Luidia quinaria and Distolasterias elegans. Izv. Akad. Nauk Ser. Khim. 1996, 3, 473–481. [Google Scholar]
- Lee, Y.J.; Han, S.; Kim, S.H. Three new cytotoxic steroidal glycosides isolated from Conus pulicarius collected in Kosrae, Micronesia. Mar. Drugs 2017, 15, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malyarenko, T.V.; Malyarenko-Vishchuk, O.S.; Ivanchina, N.V. Four new sulfated polar steroids from the Far Eastern starfish Leptasterias ochotensis: Structures and activities. Mar. Drugs 2015, 13, 4418–4435. [Google Scholar] [CrossRef] [Green Version]
- Imperatore, C.; D’Aniello, F.; Aiello, A. Phallusiasterols A and B: Two new sulfated sterols from the Mediterranean tunicate Phallusia fumigata and their effects as modulators of the PXR receptor. Mar. Drugs 2014, 12, 2066–2078. [Google Scholar] [CrossRef] [Green Version]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I. Asterosaponin P2 857 from the Far-Eastern starfish Patiria (Asterina) pectinifera. Russ. Chem. Bull. 2000, 49, 1794–1795. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, J.; Huang, R.; Wang, Y.; Xu, T. Polyhydroxy steroids and saponins from China sea starfish Asterina pectinifera and their biological activities. Chem. Pharm. Bull. 2010, 58, 856–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Riccardis, F.; Minale, L.; Riccio, R. A novel group of polyhydroxycholanic acid derivatives from the deep-water starfish Styracaster caroli. Tetrahedron Lett. 1993, 34, 4381–4384. [Google Scholar] [CrossRef]
- Kong, F.; Andersen, R.J. Polymastiamides B−F, novel steroid/amino acid conjugates isolated from the Norwegian marine sponge Polymastia boletiformis. J. Nat. Prod. 1996, 59, 379–385. [Google Scholar] [CrossRef]
- Kong, F.; Andersen, R.J. Polymastiamide A, a novel steroid/amino acid conjugate isolated from the Norwegian marine sponge Polymastia boletiformis (Lamarck, 1815). J. Org. Chem. 1993, 58, 6924–6928. [Google Scholar] [CrossRef]
- Chludil, H.D.; Maier, M.S. Minutosides A and B, antifungal sulfated steroid xylosides from the patagonian starfish Anasterias minuta. J. Nat. Prod. 2005, 68, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Gunasekera, M.; Cross, S.S. New antiviral sterol disulfate ortho esters from the marine sponge Petrosia weinbergi. J. Org. Chem. 1991, 56, 1322–1325. [Google Scholar] [CrossRef]
- Sun, H.H.; Gross, S.S.; Gunaseker, M.; Koehn, F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge petrosia weinbergi. Tetrahedron 1991, 47, 1185–1190. [Google Scholar] [CrossRef]
- McKee, T.C.; Cardellina II, J.H.; Riccio, R.; D’Auria, M.V.; Iorizzi, M.; Minale, L.; Moran, R.A.; Gulakowski, R.J.; McMahon, J.B. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrates. J. Med. Chem. 1994, 37, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Finamore, E.; Zollo, F.; Minale, L.; Yasumoto, T. Starfish saponins, part 47. Steroidal glycoside sulfates and polyhydroxysteroids from Aphelasterias japonica. J. Nat. Prod. 1992, 55, 767–771. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Lipids of marine origin. A study of Ophiura sarsi phospholipids. Bioorg Chem. (USSR) 1980, 6, 426–429. [Google Scholar]
- Sato, D.; Ando, Y.; Tsujimoto, R. Identification of novel nonmethylene-interrupted fatty acids, 7E,13E-20∶2, 7E,13E,17Z-20∶3,9E,15E,19Z-22∶3, and 4Z,9E,15E,19Z-22∶4, in Ophiuroidea (Brittle star) lipids. Lipids 2001, 36, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Plasmalogens in phospholipids of marine invertebrates. Biol. Morya (Vladivostok) 1979, 5, 86–90. [Google Scholar]
- Dembitsky, V.M. Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 1996, 35, 1–51. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Vaskovsky, V.E. A quantitative distribution of plasmalogens in different phospholipid classes of marine invertebrates. Biol. Morya (Vladivostok) 1976, 5, 68–72. [Google Scholar]
- Shubina, L.K.; Fedorov, S.N.; Levina, E.V. Comparative study on polyhydroxylated steroids from echinoderms. Comp. Biochem. Physiol. 1998, 119B, 505–516. [Google Scholar] [CrossRef]
- Voogt, P.A. Biosynthesis and composition of 3-sterols in the ophiuroids Ophiura albida and Ophioderma longicauda. Comp. Biochem. Physiol. 1973, 45B, 593–601. [Google Scholar] [CrossRef]
- Comin, M.J.; Maier, M.S.; Roccatagliata, A.J. Evaluation of the antiviral activity of natural sulfated polyhydroxysteroids and their synthetic derivatives and analogs. Steroids 1999, 64, 335–340. [Google Scholar] [CrossRef]
- Levina, E.V.; Andriyaschenko, P.V.; Kalinovsky, A.I. New Ophiuroid-type steroids from the starfish Pteraster tesselatus. J. Nat. Prod. 1998, 61, 1423–1429. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S.; Konosu, S. Bioactive marine metabolites II. Halistanol sulfate, an antimicrobial novel steroid sulfate from the marine sponge Halichondria cf. moorei bergquist. Tetrahedron Lett. 1981, 22, 1985–1991. [Google Scholar] [CrossRef]
- Nakamura, F.; Kudo, N.; Tomachi, Y. Halistanol sulfates I and J, new SIRT1–3 inhibitory steroid sulfates from a marine sponge of the genus Halichondria. J. Antibiot. 2018, 71, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, X.D.; Zhao, Q.; Wang, C.Y. Topsensterols A–C, cytotoxic polyhydroxylated sterol derivatives from a marine sponge Topsentia sp. Mar. Drugs 2016, 14, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitson, E.L.; Bugni, T.S.; Chockalingam, P.S. Spheciosterol sulfates, PKCzeta inhibitors from a philippine sponge Spheciospongia sp. J. Nat. Prod. 2008, 71, 1213–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdyshev, E.V. Fatty aldehydes of the phosphatidylethanolamines of marine invertebrates. Chem. Nat. Compd. 1989, 25, 525–529. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Quantification of plasmalogen, alkylacyl and diacyl glycerophospholipids by micro-thin-layer chromatography. J. Chromatogr. 1988, 436A, 467–473. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gorina, I.A.; Fedorova, I.P.; Solovieva, M.V. Comparative investigation of plasmalogens, alkylacyl and diacyl glycerophospholipids of the marine sponges (type Porifera, class Demospongiae). Comp. Biochem. Physiol. 1989, 92, 733–736. [Google Scholar] [CrossRef]
- Berdyshev, E.V. Mass spectrometry of fatty aldehydes. Biochim. Biophys. Acta 2011, 1811, 680–693. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Y.M.; Kaisin, M.; Djerassi, C. Steroids from starfish. Steroids 1973, 22, 835–850. [Google Scholar] [CrossRef]
- Whitson, E.L.; Bugni, T.S.; Prdiya, S.; Chockalingam, P.S. Fibrosterol sulfates from the Philippine sponge Lissodendoryx (Acanthodoryx) fibrosa: Sterol dimers that inhibit PKC ζ. J. Org. Chem. 2009, 74, 5902–5908. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Shubina, L.K.; Kalinovsky, A.I. Steroids in porifera. II. Steroid derivatives from two sponges of the family. Sokotrasterol sulfate, a marine steroid with a new pattern of side chain alkylation. Steroids 1983, 42, 267–271. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Sennett, S.H.; Kelly-Borges, M. Ophirapstanol trisulfate, a new biologically active steroid sulfate from the deep-water marine sponge Topsentia ophiraphidites. J. Nat. Prod. 1994, 57, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.N.; Girich, E.V.; Yurchenko, E.A. Metabolites of marine sediment-derived fungi: Actual trends of biological activity studies. Mar. Drugs 2021, 19, 88. [Google Scholar] [CrossRef]
- Chiang, Y.R.; Ismail, W. Anaerobic biodegradation of steroids. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids, Handbook of Hydrocarbon and Lipid Microbiology; Boll, M., Ed.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 165–195. [Google Scholar]
- Mazurek, M.A.; Simoneit, B.R.T. Higher molecular weight terpenoids as indicators of organic emissions from terrestrial vegetation. ACS Symp. Ser. 1997, 671, 92–108. [Google Scholar]
- Pancost, R.D.; Pressley, S.; Coleman, J.M.; Benning, L.G.; Mountain, B.W. Lipid biomolecules in silica sinters: Indicators of microbial biodiversity. Environ. Microbiol. 2005, 7, 66–77. [Google Scholar] [CrossRef]
- Sinninghe Damsté, J.S.; De Leeuw, J.W.; Kock-Van Dalen, A.C.; De Zeeuw, M.A. The occurrence and identification of series of organic sulphur compounds in oils and sediment extracts. I. A study of Rozel Point Oil (U.S.A.). Geochim. Cosmochim. Acta 1987, 51, 2369–2391. [Google Scholar] [CrossRef]
- De Leeuw, J.W.; Sinninghe Damsté, J.S. Organic sulfur compounds and other biomarkers as indicators of paleosalinity. ACS Symp. Ser. 1990, 429, 417–443. [Google Scholar]
- Zamanpour, M.K.; Kaliappan, R.S.; Rockne, K.J. Gas ebullition from petroleum hydrocarbons in aquatic sediments: A review. J. Environ. Manag. 2020, 271, 110997. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Riolo, J.; Hussler, G.; Albrecht, P.; Connan, J. Distribution of aromatic steroids in geological samples: Their evaluation as geochemical parameters. Org. Geochem. 1986, 10, 981–990. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Gloriozova, T.A. Natural sulphur-containing steroids: Origin and biological activities. Vietnam J. Chem. 2018, 56, 533–541. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, L.; Huang, T.; Chu, Z.; Xie, Z. Transformation of sulfur species in lake sediments at Ardley Island and Fildes Peninsula, King George Island, Antarctic Peninsula. Sci. Total Environ. 2020, 703, 135591. [Google Scholar] [CrossRef] [PubMed]
- Tang, K. Chemical diversity and biochemical transformation of biogenic organic sulfur in the ocean. Front. Mar. Sci. 2020, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, A.; Algeo, T.J.; Krystynd, L.; Rowe, H.; Brookfield, M.; Williams, J.; Nye, S.W., Jr.; Hannigan, R. Marine sulfur cycle evidence for upwelling and eutrophic stresses during Early Triassic cooling events. Earth Sci. Rev. 2019, 195, 68–82. [Google Scholar] [CrossRef]
- Amend, J.P.; Edwards, K.J.; Lyons, T.W. Sulfur biogeochemistry: Past and present. Geol. Soc. Amer. 2004, 379. [Google Scholar] [CrossRef]
- Darnet, S.; Schaller, H. Metabolism and biological activities of 4-methyl-sterols. Molecules 2019, 24, 451. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Bhattacharya, S.; Roy, C.; Rameez, M.J.; Sarkar, J.; Mapder, T.; Fernandes, S.; Peketi, A.; Mazumdar, A.; Ghosh, W. Cryptic roles of tetrathionate in the sulfur cycle of marine sediments: Microbial drivers and indicators. Biogeosciences 2020, 17, 4611–4631. [Google Scholar] [CrossRef]
- Shen, Y.; Thiel, V.; Suarez-Gonzalez, P.; Rampen, S.W.; Reitner, J. Sterol preservation in hypersaline microbial mats. Biogeosciences 2020, 17, 649–666. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Deng, Z.; Zhu, X.; van Ofwegen, L.; Proksch, P.; Lin, W. Krempenes A–D: A series of unprecedented pregnane-type steroids from the marine soft coral Cladiella krempfi. Helv. Chim. Acta 2006, 89, 2020–2026. [Google Scholar] [CrossRef]
- Karim, A.; Brown, E.A. Isolation and identification of novel sulfur-containing metabolites of spironolactone (Aldactone®). Steroids 1972, 20, 41–62. [Google Scholar] [CrossRef]
- de Marvao, A.; Alexander, D.; Bucciarelli-Ducci, C. Heart disease in women: A narrative review. Anaesthesia 2021, 76, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Segar, J.L. Neonatal diuretic therapy: Furosemide, thiazides, and spironolactone. Clin. Perinatol. 2012, 39, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.L.; Catena, C.; Sechi, L.A. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J. Hyperten. 2013, 31, 3–15. [Google Scholar] [CrossRef]
- Carone, L.; Oxberry, S.G.; Twycross, R. Spironolactone. J. Pain Symptom Manag. 2017, 53, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Ruba, A.A.; Mohan, V.R. GC-MS analysis of active compounds in the whole plant of Andrographis echioides (L) nees (Acanthaceae). Eur. J. Biomed. Pharm. Sci. 2014, 1, 443–452. [Google Scholar]
- El Fels, L. Suivi Physico-Chimique, Microbiologique et Écotoxicologique du Compostage de Boues de. STEP Mélangées à des Déchets de Palmier: Validation de Nouveaux Indices de Maturité. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2014. [Google Scholar]
- El Fels, L.; El Ouaqoudi, F.-Z.; Lemee, L.; Geffroy, C.; Ambles, A.; Hafidi, M. Occurrence of plant and fecal steroid and their evolution during co-composting of sewage sludge and lignocellulosic waste. Biochem. Eng. J. 2016, 105, 497–504. [Google Scholar] [CrossRef]
- Kohnen, M.E.L.; Sinninghe Damsté, J.S.; Haven, H.T.L.; Ckock-Van Dalen, A.; Schouten, S.; De Leeuw, J.W. Identification and geochemical significance of cyclic di-and trisulphides with linear and acyclic isoprenoid carbon skeletons in immature sediments. Geochim. Cosmochim. Acta 1991, 55, 3685–3695. [Google Scholar] [CrossRef] [Green Version]
- Sinninghe Damsté, J.S.; Kohnen, M.E.L.; Baas, M.; Kock-van Dalen, A.C.; de Leeuw, J.W. Sulphur-bound steroid and phytane carbon skeletons in geomacromolecules: Implications for the mechanism of incorporation of sulphur into organic matter. Geochim. Cosmochim. Acta 1993, 57, 2515–2528. [Google Scholar]
- Lu, H.; Peng, P.; Hsu, C.S. Geochemical explication of sulfur organics characterized by Fourier transform ion cyclotron resonance mass spectrometry on sulfur-rich heavy oils in Jinxian Sag, Bohai Bay basin, Northern China. Energy Fuels 2013, 27, 5861–5866. [Google Scholar] [CrossRef] [Green Version]
- Adam, P.; Schneckenburger, P.; Schaeffer, P.; Albrecht, P. Clues to early diagenetic sulfurization processes from mild chemical cleavage of labile sulfur-rich geomacromolecules. Geochim. Cosmochim. Acta 2000, 64, 3485–3503. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.T.; Jiang, L.X. Identification and formation of sulfur-containing steroids in sulfur-rich heavy oils in the Jinxian Sag, Bohai Bay Basin, North China. Sci. China Earth Sci. 2013. [Google Scholar] [CrossRef] [Green Version]
- Sinninghe Damsté, J.S.; Rijpstra, W.I.C.; Kock-van Dalen, A.C.; de Leeuw, J.W.; Schenck, P.A. The occurrence and identification of series of organic sulphur compounds in oils and sediment extracts II. Their presence in samples from hypersaline and non-hypersaline depositional environments and possible application as source, palaeo environmental and maturity indicators. Geochim. Cosmochim. Acta 1989, 53, 1323–1341. [Google Scholar]
- Sinninghe Damsté, J.S.; Schouten, S.; de Leeuw, J.W.; Tvan Duin, A.C.; Geenevasen, J.A.J. Identification of novel sulfur-containing steroids in sediments and petroleum: Probable incorporation of sulfur into δ5,7-sterols during early diagenesis. Geochim. Cosmochim. Acta 1999, 63, 31–38. [Google Scholar] [CrossRef]
- Schouten, S.; Sephton, S.; Baas, M.; Sinninghe Damsté, J.S. Steroid carbon skeletons with unusually branched C-3 alkyl side chains in sulphur-rich sediments. Geochim. Cosmochim. Acta 1998, 62, 1127–1132. [Google Scholar] [CrossRef]
- Van Kaam-Peters, H.M.E.; Rijpstra, W.I.C.; De Leeuw, J.W.; Sinninghe Damsté, J.S. A high-resolution biomarker study of different lithofacies of organic sulfur-rich carbonate rocks of a Kimmeridgian lagoon (French southern Jura). Org. Geochem. 1998, 28, 151–177. [Google Scholar] [CrossRef]
- Werne, J.P.; Hollander, D.J.; Behrens, A.; Schaeffer, P.; Albrecht, P.; Sinninghe Damsté, J.S. Timing of early diagenetic sulfurization of organic matter: A precursor-product relationship in Holocene sediments of the anoxic Cariaco Basin, Venezuela. Geochim. Cosmochim. Acta 2000, 64, 1741–1751. [Google Scholar] [CrossRef]
- Poinsot, J.; Schneckenburger, P.; Adam, P.; Schaeffer, P.; Trendel, J.M.; Riva, A.; Albrecht, P. Novel polycyclic sulfides derived from regular polyprenoids in sediments: Characterization, distribution, and geochemical significance. Geochim. Cosmochim. Acta 1998, 62, 805–814. [Google Scholar] [CrossRef]
- Strausz, O.P.; Lown, E.M.; Payzant, J.D. Nature and geochemistry of sulfur-containing compounds in Alberta petroleums. In Geochemistry of Sulfur in Fossil Fuels; American Chemical Society: Washington, DC, USA, 1990; Chapter 22; pp. 366–396. [Google Scholar]
- Petrov, P.D.; Fernández-Murga, L.; Conde, I. Epistane, an anabolic steroid used for recreational purposes, causes cholestasis with elevated levels of cholic acid conjugates, by upregulating bile acid synthesis (CYP8B1) and cross-talking with nuclear receptors in human hepatocytes. Arch. Toxicol. 2020, 94, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Weththasinghe, S.A. Synthesis and in vitro Metabolism Studies of Selected Steroids for Anti-Doping Analysis. Ph.D. Thesis, Australian National University, Canberra, Australia, 2020. [Google Scholar]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Invest. 2016, 126, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological activities of epithio steroids. J. Pharm. Res. Intern. 2017, 18, 1–19. [Google Scholar] [CrossRef]
- Díaz, F.C.; Sáez-González, E.; Benlloch, S.; Álvarez-Sotomayor, D. Albumin dialysis with MARS for the treatment of anabolic steroid-induced cholestasis. Ann. Hepatol. 2016, 15, 939–943. [Google Scholar]
- Okano, M.; Sato, M.; Ikekita, A. Analysis of non-ketoic steroids 17α-methyl-epithiostanol and desoxy-methyl-testosterone in dietary supplements. Drug Test. Anal. 2009, 1, 518–525. [Google Scholar]
- Ichihashi, T.; Kinoshita, H.; Yamada, H. Absorption and disposition of epithiosteroids in rats: Avoidance of first-pass metabolism of mepitiostane by lymphatic absorption. Xenobiotica 1991, 21, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Abbate, V.; Kicman, A.T.; Evans-Brown, M.; McVeigh, J. Anabolic steroids detected in bodybuilding dietary supplements-a significant risk to public health. Drug Test. Anal. 2014. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.C.; Weththasinghe, S.A.; McClure, L.; Cawley, A.T.; Suann, C.; Suann, E.; Sutherland, E.; Cooper, E.; Heather, A.; McLeod, M.D. In vivo metabolism of the designer anabolic steroid hemapolin in the thoroughbred horse. Drug Test. Anal. 2020, 12, 752–762. [Google Scholar] [CrossRef]
- Izuo, M.; Yoshida, M.; Tominaga, T.; Abe, O.; Enomoto, K. A phase III trial of oral high-dose methoxy-progesterone acetate versus mepitiostane in advanced postmenopausal breast cancer. Cancer 1985, 56, 2576–2579. [Google Scholar] [CrossRef]
- Komeno, T. Steromal 2,3-Diol Cyclic Trithiocarbonate. U.S. Patent 3,139,128, 19 December 1961. [Google Scholar]
- Korneno, T.; Kawanami, E. 11,12-Epithio Steroids of Pregnane Series. U.S. Patent 3,160,627, 8 December 1964. [Google Scholar]
- Komeno, T. 2,3-Epithio-Steroids and Production Thereof. U.S. Patent 3,230,215, 18 January 1966. [Google Scholar]
- Komeno, T. 2,3-Epithio-5-Androst-6-ene Compounds. U.S. Patent 379778, 25 December 1967. [Google Scholar]
- Kellis, J.T., Jr.; Childers, W.E.; Robinson, C.; Vickery, L.E. Inhibition of aromatase cytochrome P-450 by 10-oxirane and 10-thiirane substituted androgens. J. Biol. Chem. 1987, 262, 4421–4426. [Google Scholar] [CrossRef]
- Takeda, K.; Komeno, T.; Kawanami, J.; Ishihara, S. Bile acids and steroids. XXVII: Thiosteroids (12)1 steroidal 2,3- and 3,4-episulphides and related compounds. Tetrahedron 1965, 21, 329–351. [Google Scholar] [CrossRef]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. Roy. Soc. Edinburg 1868, 25, 224–242. [Google Scholar]
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochemistry (Moscow) 2020, 14B, 216–227. [Google Scholar] [CrossRef]
- Vázquez, J.; López, M.; Gibert, E.; Herrero, E.; Luque, F.J. Merging ligand-based and structure-based methods in drug discovery: An overview of combined virtual screening approaches. Molecules 2020, 25, 4723. [Google Scholar] [CrossRef] [PubMed]
- Stumpfe, D.; Hu, H.; Bajorath, J. Evolving concept of activity cliffs. ACS Omega 2019, 4, 14360–14368. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Aldous, D.; Raboisson, R.; Rognan, D. (Eds.) The Practice of Medicinal Chemistry, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2015; p. 902. [Google Scholar]
- Mervin, L.H.; Afzal, A.M.; Drakakis, G.; Lewis, R.; Engkvist, O.; Bender, A. Target prediction utilizing negative bioactivity data covering large chemical space. J. Cheminform. 2015, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Center Biol. Act. Comp. 1990, 1, 4–25. [Google Scholar]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Dmitriev, A.V.; Pogodin, P.V.; Poroikov, V.V. Computer-aided prediction of biological activity spectra for chemical compounds: Opportunities and limitations. Biomed. Chem. Res. Meth. 2018, 1, e00004. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.way2drug.com/passonline/ (accessed on 2 March 2021).
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef]
- Horishny, V.; Kartsev, V.; Matiychuk, V.; Geronikaki, A.; Anthi, P.; Pogodin, P.; Poroikov, V.; Ivanov, M.; Kostic, M.; Sokovic, M.D. 3-Amino-5-(indol-3-yl) methylene-4-oxo-2-thioxothiazolidine derivatives as antimicrobial agents: Synthesis, computational and biological evaluation. Pharmaceuticals 2020, 13, 229. [Google Scholar] [CrossRef]
- Amiranashvili, L.; Nadaraia, N.; Merlani, M.; Kamoutsis, C.; Petrou, A.; Geronikaki, A.; Pogodin, P.; Druzhilovskiy, D.; Poroikov, V.; Ciric, A. Antimicrobial activity of nitrogencontaining 5-alpha-androstane derivatives: In silico and experimental studies. Antibiotics 2020, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Proroikov, V.V. Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: Origin, structures and biological activity. Molecules 2021, 26, 686. [Google Scholar] [CrossRef] [PubMed]
- Vizer, S.A.; Sycheva, E.S.; Al Quntar, A.A.A.; Kurmankulov, N.B.; Yerzhanov, K.B.; Dembitsky, V.M. Propargylic sulfides: Synthesis, properties, and application. Chem. Rev. 2015, 115, 1475–1502. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Anabolic cyanosteroids and their biological activities-A brief review. World J. Pharm. Pharm. Sci. 2017, 6, 127–151. [Google Scholar]
- Poroikov, V.V.; Gloriozova, T.A.; Dembitsky, V.M. Natural occurring thiirane containing compounds: Origin, chemistry, and their pharmacological activities. Pharm. Chem. J. 2017, 4, 107–120. [Google Scholar]
- Lazcano-Becerra, M.; Velarde-Ruiz, V.J.A.; Aldana-Ledesma, J.M.; Gómez-Castaños, P.C.; Díaz-Aceves, P.E.; García-Jiménez, E.S. Antisecretory drug evolution: Pharmacology and clinical uses. Rev. Médica MD 2019, 10, 175–184. [Google Scholar]
- Patel, D.; Bertz, R.; Ren, S. A systematic review of gastric acid-reducing agent-mediated drug–drug interactions with orally administered medications. Clin. Pharmacokinet. 2020, 59, 447–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, P.; Malik, J.K. A comprehensive review on botanical as anti-ulcer therapeutics: Prospective avenues of biocompatible drug discovery. Scholars Int. J. Tradition. Complem. Med. 2020, 3, 27–32. [Google Scholar] [CrossRef]









| No. | Antitumor & Related Activity, (Pa) * | Violation of Lipid Metabolism, (Pa )* | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 1 | Antineoplastic (0.887) Chemopreventive (0.815) | Anti-hypercholesterolemic (0.901) Cholesterol synthesis inhibitor (0.822) | Hemostatic (0.931) Biliary tract disorders treatment (0.814) |
| 2 | Antineoplastic (0.906) Chemopreventive (0.816) | Anti-hypercholesterolemic (0.895) Cholesterol synthesis inhibitor (0.816) | Hemostatic (0.927) Biliary tract disorders treatment (0.819) |
| 3 | Antineoplastic (0.828) Chemopreventive (0.735) Apoptosis agonist (0.703) | Atherosclerosis treatment (0.786) Cholesterol synthesis inhibitor (0.733) Anti-hypercholesterolemic (0.711) | Hepatoprotectant (0.832) Anti-inflammatory (0.807) Biliary tract disorders treatment (0.717) |
| 4 | Antineoplastic (0.778) Chemopreventive (0.802) | Cholesterol synthesis inhibitor (0.762) Atherosclerosis treatment (0.744) | Biliary tract disorders treatment (0.752) Anti-ischemic, cerebral (0.774) |
| 5 | Chemopreventive (0.843) Antineoplastic (0.804) | Atherosclerosis treatment (0.721) Cholesterol synthesis inhibitor (0.744) | Biliary tract disorders treatment (0.713) Wound-healing agent (0.831) |
| 6 | Antineoplastic (0.880) Apoptosis agonist (0.865) | Cholesterol synthesis inhibitor (0.774) Anti-hypercholesterolemic (0.821) | Wound-healing agent (0.866) Biliary tract disorders treatment (0.764) |
| 7 | Antineoplastic (0.877) Chemopreventive (0.722) Apoptosis agonist (0.712) | Cholesterol synthesis inhibitor (0.776) Cholesterol synthesis inhibitor (0.755) Anti-hypercholesterolemic (0.732) | Wound-healing agent (0.852) Hemostatic (0.757) Biliary tract disorders treatment (0.741) |
| 8 | Chemopreventive (0.857) Antineoplastic (0.765) | Anti-hypercholesterolemic (0.884) Cholesterol synthesis inhibitor (0.766) | Biliary tract disorders treatment (0.871) Hemostatic (0.736) |
| 9 | Antineoplastic (0.904) Chemopreventive (0.843) Apoptosis agonist (0.721) | Atherosclerosis treatment (0.889) Cholesterol synthesis inhibitor (0.821) Anti-hypercholesterolemic (0.820) | Biliary tract disorders treatment (0.911) Anti-inflammatory (0.821) Biliary tract disorders treatment (0.724) |
| 10 | Chemopreventive (0.879) Apoptosis agonist (0.850) Antineoplastic (0.824) | Cholesterol synthesis inhibitor (0.862) Atherosclerosis treatment (0.862) Anti-hypercholesterolemic (0.812) | Wound-healing agent (0.872) Biliary tract disorders treatment (0.753) Hemostatic (0.731) |
| 11 | Chemopreventive (0.882) Antineoplastic (0.773) Apoptosis agonist (0.748) | Cholesterol synthesis inhibitor (0.869) Atherosclerosis treatment (0.858) Anti-hypercholesterolemic (0.823) | Biliary tract disorders treatment (0.762) Hemostatic (0.739) |
| 12 | Chemopreventive (0.873) Antineoplastic (0.778) Apoptosis agonist (0.751) | Cholesterol synthesis inhibitor (0.861) Atherosclerosis treatment (0.844) Anti-hypercholesterolemic (0.832) | Biliary tract disorders treatment (0.812) Wound-healing agent (0.775) |
| 13 | Antineoplastic (0.902) Chemopreventive (0.878) | Cholesterol synthesis inhibitor (0.855) Anti-hypercholesterolemic (0.831) | Wound-healing agent (0.884) |
| No. | Antitumor & Related Activity, (Pa) * | Violation of Lipid Metabolism, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 14 | Chemopreventive (0.828) Antineoplastic (0.798) | Anti-hypercholesterolemic (0.919) Cholesterol synthesis inhibitor (0.803) | Wound-healing agent (0.975) Biliary tract disorders treatment (0.861) |
| 15 | Antineoplastic (0.780) Chemopreventive (0.766) | Anti-hypercholesterolemic (0.874) Cholesterol synthesis inhibitor (0.789) | Biliary tract disorders treatment (0.955) Wound-healing agent (0.945) |
| 16 | Chemopreventive (0.841) Antineoplastic (0.804) Prostate disorders treatment (0.675) | Anti-hypercholesterolemic (0.907) Cholesterol synthesis inhibitor (0.818) Atherosclerosis treatment (0.724) | Wound-healing agent (0.846) Biliary tract disorders treatment (0.823) Hepatoprotectant (0.816) |
| 17 | Antineoplastic (0.858) Chemopreventive (0.797) Apoptosis agonist (0.719) | Anti-hypercholesterolemic (0.886) Cholesterol synthesis inhibitor (0.753) Atherosclerosis treatment (0.699) | Biliary tract disorders treatment (0.801) Wound-healing agent (0.783) Hepatoprotectant (0.771) |
| 18 | Antineoplastic (0.791) Prostate disorders treatment (0.692) | Anti-hypercholesterolemic (0.909); Cholesterol synthesis inhibitor (0.806); | Wound-healing agent (0.938) Biliary tract disorders treatment (0.850) |
| 19 | Chemopreventive (0.887) Antineoplastic (0.803) | Anti-hypercholesterolemic (0.940) Cholesterol synthesis inhibitor (0.815); | Wound-healing agent (0.952) Hepatoprotectant (0.876) |
| 20 | Chemopreventive (0.828) Antineoplastic (0.798) | Anti-hypercholesterolemic (0.919); Cholesterol synthesis inhibitor (0.803) | Wound-healing agent (0.975) Biliary tract disorders treatment (0.861) |
| 21 | Chemopreventive (0.934) Antineoplastic (0.867) Apoptosis agonist (0.857) | Anti-hypercholesterolemic (0.912) Atherosclerosis treatment (0.776) Cholesterol synthesis inhibitor (0.750) | Hemostatic (0.928) Biliary tract disorders treatment (0.764) Wound-healing agent (0.727) |
| 22 | Chemopreventive (0.891) Antineoplastic (0.791) | Anti-hypercholesterolemic (0.789) Cholesterol synthesis inhibitor (0.788); | Wound-healing agent (0.816) Biliary tract disorders treatment (0.807) |
| 23 | Antineoplastic (0.822) Chemopreventive (0.806) | Anti-hypercholesterolemic (0.926) Atherosclerosis treatment (0.804) | Biliary tract disorders treatment (0.879); Hepatoprotectant (0.764) |
| 24 | Antineoplastic (0.781) Chemopreventive (0.780) | Anti-hypercholesterolemic (0.929); Cholesterol synthesis inhibitor (0.762) | Biliary tract disorders treatment (0.910); Hepatoprotectant (0.876) |
| 25 | Chemopreventive (0.837) Antineoplastic (0.777) | Acute neurologic disorders treatment (0.808) Cholesterol synthesis inhibitor (0.719); | Wound-healing agent (0.895) Hepatoprotectant (0.888); |
| 26 | Chemopreventive (0.902) Apoptosis agonist (0.894) Antineoplastic (0.866); | Anti-hypercholesterolemic (0.931) Atherosclerosis treatment (0.807) Cholesterol synthesis inhibitor (0.732) | Biliary tract disorders treatment (0.844) Hepatic disorders treatment (0.818) Wound-healing agent (0.556); |
| 27 | Chemopreventive (0.850) Antineoplastic (0.777) | Anti-hypercholesterolemic (0.897); Cholesterol synthesis inhibitor (0.819); | Hepatoprotectant (0.879) Biliary tract disorders treatment (0.866) |
| 28 | Chemopreventive (0.944) Apoptosis agonist (0.808) | Cholesterol synthesis inhibitor (0.714); Atherosclerosis treatment (0.708); | Hepatoprotectant (0.872) Antifungal (0.831) |
| 29 | Chemopreventive (0.913) Antineoplastic (0.796) Apoptosis agonist (0.790) | Cholesterol synthesis inhibitor (0.762) Anti-hypercholesterolemic (0.726) Atherosclerosis treatment (0.717) | Wound-healing agent (0.965) Hepatoprotectant (0.933) Biliary tract disorders treatment (0.791); |
| 30 | Antineoplastic (0.772) Chemopreventive (0.715); | Atherosclerosis treatment (0.611) Cholesterol synthesis inhibitor (0.550) | Hepatoprotectant (0.893) Erythropoiesis stimulant (0.789); |
| 31 | Chemopreventive (0.882) Antineoplastic (0.803) | Anti-hypercholesterolemic (0.906) Cholesterol synthesis inhibitor (0.823) | Biliary tract disorders treatment (0.847) Hepatoprotectant (0.812); |
| No. | Antitumor & Related Activity, (Pa) * | Violation of Lipid Metabolism, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 32 | Antineoplastic (0.845) Chemopreventive (0.834) Apoptosis agonist (0.826); | Anti-hypercholesterolemic (0.895); Cholesterol synthesis inhibitor (0.776); Atherosclerosis treatment (0.712) | Hepatoprotectant (0.933) Biliary tract disorders treatment (0.895) Wound-healing agent (0.885) |
| 33 | Chemopreventive (0.821) Antineoplastic (0.805); | Cholesterol synthesis inhibitor (0.667); Atherosclerosis treatment (0.561) | Hepatoprotectant (0.859) Antiinflammatory (0.832) |
| 34 | Antineoplastic (0.830) Apoptosis agonist (0.792) Chemopreventive (0.783) | Atherosclerosis treatment (0.691) Cholesterol synthesis inhibitor (0.682); | Hepatoprotectant (0.968) Anti-inflammatory (0.817) Biliary tract disorders treatment (0.704); |
| 35 | Chemopreventive (0.777) Antineoplastic (0.779) | Anti-hypercholesterolemic (0.748) Cholesterol synthesis inhibitor (0.717) | Anti-ischemic, cerebral (0.850) Biliary tract disorders treatment (0.728) |
| 36 | Chemopreventive (0.821) Antineoplastic (0.765) | Cholesterol synthesis inhibitor (0.727) Anti-hypercholesterolemic (0.671) | Biliary tract disorders treatment (0.833) Wound-healing agent (0.794) |
| 37 | Antineoplastic (0.829) | Cholesterol synthesis inhibitor (0.618) | Anti-ischemic, cerebral (0.785) |
| 38 | Antineoplastic (0.813) | Atherosclerosis treatment (0.629) | Biliary tract disorders treatment (0.639) |
| 39 | Antineoplastic (0.823) | Atherosclerosis treatment (0.715) | Biliary tract disorders treatment (0.931) |
| 40 | Chemopreventive (0.924) Antineoplastic (0.837) | Anti-hypercholesterolemic (0.926) Atherosclerosis treatment (0.671) | Wound-healing agent (0.953) Hepatoprotectant (0.925) |
| 41 | Antineoplastic (0.854) Apoptosis agonist (0.790) | Anti-hypercholesterolemic (0.902) Atherosclerosis treatment (0.675) | Wound-healing agent (0.963) Hepatoprotectant (0.915) |
| 42 | Chemopreventive (0.918) Antineoplastic (0.806) | Anti-hypercholesterolemic (0.942) Cholesterol synthesis inhibitor (0.712) | Wound-healing agent (0.975) Hepatoprotectant (0.915) |
| 43 | Chemopreventive (0.935) Antineoplastic (0.818) | Anti-hypercholesterolemic (0.901) Atherosclerosis treatment (0.685) | Wound-healing agent (0.980) Hepatoprotectant (0.972) Biliary tract disorders treatment (0.958) |
| 44 | Chemopreventive (0.922) Antineoplastic (0.841) | Anti-hypercholesterolemic (0.887) Atherosclerosis treatment (0.679) | Wound-healing agent (0.977) Hepatoprotectant (0.965) |
| 45 | Chemopreventive (0.931) Antineoplastic (0.861); | Anti-hypercholesterolemic (0.716) Atherosclerosis treatment (0.704) | Hepatoprotectant (0.893) Wound-healing agent (0.887) |
| 46 | Antineoplastic (0.831) | Anti-hypercholesterolemic (0.782) | Wound-healing agent (0.888) |
| 47 | Chemopreventive (0.860) | Anti-hypercholesterolemic (0.850) | Wound-healing agent (0.861) |
| No. | Antitumor & Related Activity, (Pa) * | Violation of Lipid Metabolism, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 48 | Chemopreventive (0.913) Antineoplastic (0.844) | Anti-hypercholesterolemic (0.825) Cholesterol synthesis inhibitor (0.678) | Hepatoprotectant (0.924) Biliary tract disorders treatment (0.727) |
| 49 | Antineoplastic (0.716) | Anti-hypercholesterolemic (0.865) | Biliary tract disorders treatment (0.794) |
| 50 | Antineoplastic (0.686) | Anti-hypercholesterolemic (0.679) | Hepatoprotectant (0.653) |
| 51 | Antineoplastic (0.626) | Cholesterol antagonist (0.820) | Anti-inflammatory (0.614) |
| 52 | Antineoplastic (0.691) | Cholesterol antagonist (0.934) | Antifungal (0.696) |
| 53 | Antineoplastic (0.874) | Anti-hypercholesterolemic (0.697) | Anti-inflammatory (0.718) |
| 54 | Antineoplastic (0.849) | Atherosclerosis treatment (0.748) | Anti-inflammatory (0.777) |
| 55 | Antineoplastic (0.869) | Autoimmune disorders treatment (0.762) | Angiogenesis inhibitor (0.926) |
| 56 | Antineoplastic (0.796) | Atherosclerosis treatment (0.646) | Anti-inflammatory (0.811) |
| 57 | Antineoplastic (0.836) Chemopreventive (0.712) Apoptosis agonist (0.649) | Atherosclerosis treatment (0.684) Cholesterol synthesis inhibitor (0.656) Anti-hypercholesterolemic (0.617) | Biliary tract disorders treatment (0.928) Hepatic disorders treatment (0.889) Wound-healing agent (0.888) |
| 58 | Antineoplastic (0.788) | Cholesterol synthesis inhibitor (0.755) | Biliary tract disorders treatment (0.843) |
| 59 | Antineoplastic (0.793) | Atherosclerosis treatment (0.636) | Biliary tract disorders treatment (0.713) |
| 60 | Antineoplastic (0.782) | Cholesterol synthesis inhibitor (0.671) | Wound-healing agent (0.933) |
| 61 | Antineoplastic (0.820) | Cholesterol synthesis inhibitor (0.734) | Wound-healing agent (0.942) |
| 62 | Antineoplastic (0.711) | Cholesterol synthesis inhibitor (0.696) | Biliary tract disorders treatment (0.963) |
| 63 | Antineoplastic (0.764) | Atherosclerosis treatment (0.684) | Biliary tract disorders treatment (0.957) |
| 64 | Chemopreventive (0.948) | Cholesterol synthesis inhibitor (0.562) | Hepatoprotectant (0.915) |
| 65 | Antineoplastic (0.729) | Cholesterol synthesis inhibitor (0.572) | Biliary tract disorders treatment (0.835) |
| 66 | Antineoplastic (0.734) | Cholesterol synthesis inhibitor (0.661) | Biliary tract disorders treatment (0.725) |
| 67 | Antineoplastic (0.790) | Cholesterol synthesis inhibitor (0.655) | Wound-healing agent (0.812) |
| 68 | Antineoplastic (0.868) | Atherosclerosis treatment (0.645) | Hepatoprotectant (0.918) |
| 69 | Antineoplastic (0.868) | Atherosclerosis treatment (0.645) | Hepatoprotectant (0.918) |
| 70 | Antineoplastic (0.748) Chemopreventive (0.692) | Cholesterol synthesis inhibitor (0.688) Atherosclerosis treatment (0.664) | Biliary tract disorders treatment (0.943) Hepatic disorders treatment (0.934) |
| 71 | Antineoplastic (0.767) Chemopreventive (0.704) | Cholesterol synthesis inhibitor (0.696) Atherosclerosis treatment (0.665) | Biliary tract disorders treatment (0.963) Hepatic disorders treatment (0.934); |
| No. | Antitumor & Related Activity, (Pa) * | Specific Activities, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 72 | Antineoplastic (0.842) | Erythropoiesis stimulant (0.722) Prostate disorders treatment (0.688) | Anti-eczematic (0.773) Anti-seborrheic (0.733) Anti-psoriatic (0.658) |
| 73 | Antineoplastic (0.868) | Diuretic (0.991) Anti-hyperaldosteronism (0.963) Antihypertensive (0.940) Renal disease treatment (0.934) | Cardiotonic (0.850) Anti-ischemic, cerebral (0.822) Antiarthritic (0.741) Antithrombotic (0.691) |
| 74 | Antineoplastic (0.824) | Anti-osteoporotic (0.917) | Anti-seborrheic (0.845) |
| 75 | Antineoplastic (0.723) | Prostate disorders treatment (0.735) | Anti-eczematic (0.787) |
| 76 | Antineoplastic (0.736) | Prostate disorders treatment (0.745) | Anti-eczematic (0.813) |
| 77 | Antineoplastic (0.774) | Anesthetic general (0.774) | Anti-eczematic (0.809) |
| 78 | Antineoplastic (0.774) | Anesthetic general (0.774) Respiratory analeptic (0.675) | Anti-eczematic (0.809) Anti-psoriatic (0.686) |
| 79 | Antineoplastic (0.773) Antimetastatic (0.682) | Anti-osteoporotic (0.683) | Anti-eczematic (0.776) Anti-psoriatic (0.643) |
| 80 | Antineoplastic (0.729) Apoptosis agonist (0.680) | Anti-osteoporotic (0.789) | Anti-eczematic (0.782) |
| 81 | Antineoplastic (0.681) | Anti-osteoporotic (0.730) | Anti-eczematic (0.798) |
| 82 | Antineoplastic (0.664) Apoptosis agonist (0.624) | Anti-osteoporotic (0.742) | Anti-eczematic (0.771) Anti-psoriatic (0.619) |
| 83 | Antineoplastic (0.700) | Anti-osteoporotic (0.744) | Anti-eczematic (0.766) |
| 84 | Anesthetic general (0.761) | Anti-seborrheic (0.823) | |
| 85 | Antineoplastic (0.618) | Prostate disorders treatment (0.667) | Anti-seborrheic (0.817) |
| 86 | Prostate disorders treatment (0.652) | Anti-seborrheic (0.810) | |
| 87 | Antineoplastic (0.756) | Antiallergic (0.785) | Anti-psoriatic (0.818) Anti-eczematic (0.756) |
| 88 | Antineoplastic (0.658) | Prostate disorders treatment (0.669) | Dermatologic (0.692) Anti-eczematic (0.678) |
| 89 | Antineoplastic (0.730) | Anti-osteoporotic (0.696) |
| No. | Antitumor & Related Activity, (Pa) * | Specific Activities, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 90 | Antineoplastic (0.740) | Anti-osteoporotic (0.750) | Anti-eczematic (0.824) |
| 91 | Antineoplastic (0.773) | Hepatic disorders treatment (0.676) | Anti-seborrheic (0.692) |
| 92 | Antineoplastic (0.658) | Antiprotozoal (Plasmodium) (0.627) | Anti-seborrheic (0.656) |
| 93 | Antihypertensive (0.705) | Anti-seborrheic (0.809) | |
| 94 | Hepatic disorders treatment (0.728) | Anti-eczematic (0.736) | |
| 95 | Anesthetic general (0.762) | Anti-seborrheic (0.787) | |
| 96 | Anti-osteoporotic (0.641) | Anti-eczematic (0.840) | |
| 97 | Atherosclerosis treatment (0.680) | Anti-eczematic (0.808) Anti-psoriatic (0.649) | |
| 98 | Apoptosis agonist (0.947) Antineoplastic (0.862) | Inflammatory bowel disease treatment (0.842) | Anti-eczematic (0.898) Anti-psoriatic (0.828) Septic shock treatment (0.619) |
| 99 | Apoptosis agonist (0.947) Antineoplastic (0.869) | Inflammatory bowel disease treatment (0.842) | Anti-eczematic (0.898) Anti-psoriatic (0.828) Septic shock treatment (0.619) |
| 100 | Apoptosis agonist (0.929) Antineoplastic (0.822) | Antipruritic (0.590) | Anti-eczematic (0.859) Anti-psoriatic (0.798) |
| 101 | Anti-obesity (0.750) | Anti-seborrheic (0.797) Anti-eczematic (0.696) | |
| 102 | Anti-obesity (0.616) | Anti-seborrheic (0.782) Anti-eczematic (0.691) | |
| 103 | Anti-obesity (0.768) | Anti-seborrheic (0.791) Anti-eczematic (0.687) | |
| 104 | Antihypertensive (0.650) | Anti-eczematic (0.719) Anti-seborrheic (0.640) | |
| 105 | Apoptosis agonist (0.933) Antineoplastic (0.872) | Dermatologic (0.781) | Anti-eczematic (0.847) Anti-psoriatic (0.822) |
| 106 | Antineoplastic (0.708) | Antihypertensive (0.655) | Anti-seborrheic (0.676) |
| 107 | Apoptosis agonist (0.649) | Anti-inflammatory (0.626) | Dermatologic (0.639) |
| No. | Antitumor & Related Activity, (Pa) * | Specific Activities, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 108 | Antineoplastic (0.764) Antimetastatic (0.657) | Hypolipemic (0.700) | Anti-eczematic (0.766) |
| 109 | Antineoplastic (0.756) | Hepatic disorders treatment (0.794) | Anti-eczematic (0.753) |
| 110 | Antineoplastic (0.764) Antimetastatic (0.657) | Hypolipemic (0.700) | Anti-eczematic (0.766) |
| 111 | Antineoplastic (0.747) | Hepatic disorders treatment (0.690) | Anti-eczematic (0.739) |
| 112 | Antineoplastic (0.671) | Anti-osteoporotic (0.710) | Anti-eczematic (0.808) Anti-psoriatic (0.669) |
| 113 | Antineoplastic (0.692) | Anti-osteoporotic (0.687) | Anti-eczematic (0.803) |
| 114 | Antineoplastic (0.702) | Anti-osteoporotic (0.727) | Anti-eczematic (0.816) |
| 115 | Antineoplastic (0.766) | Hepatic disorders treatment (0.731) | Anti-seborrheic (0.836) |
| 116 | Antineoplastic (0.768) | Hepatic disorders treatment (0.728) | Anti-seborrheic (0.844) |
| 117 | Antineoplastic (0.751) | Prostate disorders treatment (0.776) | Anti-eczematic (0.792) |
| 118 | Antineoplastic (0.749) | Prostate disorders treatment (0.782) | Anti-eczematic (0.770) |
| 119 | Antineoplastic (0.834) | Anti-osteoporotic (0.717) | Anti-eczematic (0.806) |
| 120 | Antineoplastic (0.828) | Anti-osteoporotic (0.722) | Anti-eczematic (0.811) |
| 121 | Antineoplastic (0.829) | Anti-osteoporotic (0.720) | Anti-eczematic (0.809) |
| No. | Antitumor & Related Activity, (Pa) * | Specific Activities, (Pa) * | Additional Predicted Activity, (Pa) * |
|---|---|---|---|
| 122 | Antineoplastic (0.964) Cytostatic (0.798) Antineoplastic (breast cancer) (0.598) | Anti-secretoric (0.948) Estrogen antagonist (0.860) | Erythropoiesis stimulant (0.760) Cardiotonic (0.729) |
| 123 | Antineoplastic (0.966) Cytostatic (0.681) Prostatic (benign) hyperplasia treatment (0.673) | Anti-secretoric (0.952) | Anti-inflammatory (0.754) Bone diseases treatment (0.663) Anabolic (0.648) |
| 124 | Antineoplastic (0.966) Cytostatic (0.681) Prostatic (benign) hyperplasia treatment (0.673) | Anti-secretoric (0.952) | Anti-inflammatory (0.754) Bone diseases treatment (0.663) Anabolic (0.648) |
| 125 | Antineoplastic (0.932) | Anti-secretoric (0.863) Anti-hypercholesterolemic (0.759) | Anti-eczematic (0.840) Dermatologic (0.747) Anti-psoriatic (0.659) |
| 126 | Antineoplastic (0.955) Cytostatic (0.676) | Anti-secretoric (0.938) Estrogen antagonist (0.807) | Anti-seborrheic (0.814) Dermatologic (0.639) |
| 127 | Antineoplastic (0.962) | Anti-secretoric (0.841) | |
| 128 | Antineoplastic (0.971) Antineoplastic (breast cancer) (0.671) | Anti-secretoric (0.861) | Anti-seborrheic (0.830) Cardiotonic (0.701) |
| 129 | Antineoplastic (0.970) | Estrogen antagonist (0.686) Anti-secretoric (0.677) | Cardiotonic (0.672) Dermatologic (0.649) |
| 130 | Antineoplastic (0.883) Cytostatic (0.661) | Anti-secretoric (0.906) Estrogen antagonist (0.750) | Anti-seborrheic (0.926) Dermatologic (0.743) |
| 131 | Antineoplastic (0.960) Cytostatic (0.724) | Anti-secretoric (0.965) Estrogen antagonist (0.915) | Anti-seborrheic (0.848) Anti-osteoporotic (0.729) |
| 132 | Antineoplastic (0.939) Cytostatic (0.787) | Anti-secretoric (0.967) Estrogen antagonist (0.946) | Anti-inflammatory (0.929) Anti-seborrheic (0.849) |
| 133 | Antineoplastic (0.974) Prostatic (benign) hyperplasia treatment (0.583) | Estrogen antagonist (0.870) Anti-secretoric (0.827) | Antiprotozoal (Plasmodium) (0.642) Anabolic (0.616) |
| 134 | Antineoplastic (0.868) | Cardiotonic (0.925) Anti-arrhythmic (0.858) | Anti-seborrheic (0.869) Anti-inflammatory (0.733) |
| 135 | Antineoplastic (0.780) | Anesthetic general (0.847) Anti-secretoric (0.804) | Anti-eczematic (0.811) Anti-inflammatory (0.739) |
| 136 | Antineoplastic (0.779) Apoptosis agonist (0.707) | Cholesterol antagonist (0.946) Anti-hypercholesterolemic (0.930) | Respiratory analeptic (0.963) Anesthetic general (0.913) |
| 137 | Antineoplastic (0.775) | Cholesterol antagonist (0.932) Anti-hypercholesterolemic (0.900) | Anesthetic general (0.923) Respiratory analeptic (0.919) |
| 138 | Antineoplastic (0.912) | Cardiotonic (0.936) | Respiratory analeptic (0.781) Anesthetic general (0.746) |
| 139 | Antineoplastic (0.768) | Cholesterol antagonist (0.745) | Anti-seborrheic (0.905) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and Sulfur-Containing Steroids and Their Pharmacological Profile. Mar. Drugs 2021, 19, 240. https://doi.org/10.3390/md19050240
Pounina TA, Gloriozova TA, Savidov N, Dembitsky VM. Sulfated and Sulfur-Containing Steroids and Their Pharmacological Profile. Marine Drugs. 2021; 19(5):240. https://doi.org/10.3390/md19050240
Chicago/Turabian StylePounina, Tatyana A., Tatyana A. Gloriozova, Nick Savidov, and Valery M. Dembitsky. 2021. "Sulfated and Sulfur-Containing Steroids and Their Pharmacological Profile" Marine Drugs 19, no. 5: 240. https://doi.org/10.3390/md19050240
APA StylePounina, T. A., Gloriozova, T. A., Savidov, N., & Dembitsky, V. M. (2021). Sulfated and Sulfur-Containing Steroids and Their Pharmacological Profile. Marine Drugs, 19(5), 240. https://doi.org/10.3390/md19050240






