The Marine Natural Product Furospinulosin 1 Induces Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cell Spheroids, But Not in Cells Grown Traditionally with Longer Treatment
Abstract
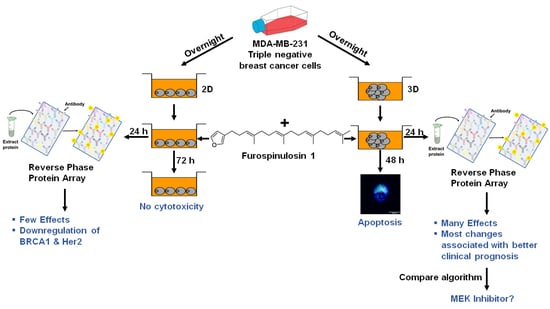
:1. Introduction
2. Results
2.1. Screening Assay Results
2.2. Identification of the Compound Responsible for the Activity
2.3. Determination of Concentration Required to Obtain 50% Activity
2.4. Differential Protein Expression Analysis
2.5. Bioinformatic Analysis of Proteomic Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. 3D Spheroid Multiparametric Assay
4.4. 2D Cell Viability Assay (MTT)
4.5. IC50 Determination
4.6. Reverse Phase Protein Array (RPPA)
4.7. Purification and Identification of Furospinulosin 1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Facts and Figures 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 7 April 2021).
- Narod, S.A.; Dent, R.A.; Foulkes, W.D. CCR Anniversary Commentary: Triple-Negative Breast Cancer in 2015-Still in the Ballpark. Clin. Cancer Res. 2015, 21, 3813–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triple-Negative Breast Cancer; Breastcancer.org 2020. Available online: https://www.breastcancer.org/ (accessed on 16 March 2020).
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. BCR 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugurama, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyahi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- Mayer, A.M.S. 16. Marine Pharmacology and the Late 2011 Marine Pharmaceuticals Pipeline. Toxicon 2012, 60, 104. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Ivascu, A.; Kubbies, M. Diversity of cell-mediated adhesions in breast cancer spheroids. Int. J. Oncol. 2007, 31, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Wiedner, S.D.; Burnum, K.E.; Pederson, L.M.; Anderson, L.N.; Fortuin, S.; Chauvigne-Hines, L.M.; Shukla, A.K.; Ansong, C.; Panisko, E.A.; Smith, R.D.; et al. Multiplexed activity-based protein profiling of the human pathogen Aspergillus fumigatus reveals large functional changes upon exposure to human serum. J. Biol. Chem. 2012, 287, 33447–33459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Li, X.M.; Meinkoth, J.; Pittman, R.N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Chan, J.J.; Tan, T.J.Y.; Dent, R.A. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther. Adv. Med Oncol. 2019, 11, 1758835919880429. [Google Scholar] [CrossRef]
- Lin, F.M.; Yost, S.E.; Wen, W.; Frankel, P.H.; Schmolze, D.; Chu, P.G.; Yuan, Y.C.; Liu, Z.; Yim, J.; Chen, Z.; et al. Differential gene expression and AKT targeting in triple negative breast cancer. Oncotarget 2019, 10, 4356–4368. [Google Scholar] [CrossRef]
- Yonashiro, R.; Eguchi, K.; Wake, M.; Takeda, N.; Nakayama, K. Pyruvate Dehydrogenase PDH-E1beta Controls Tumor Progression by Altering the Metabolic Status of Cancer Cells. Cancer Res. 2018, 78, 1592–1603. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. CR 2019, 38, 195. [Google Scholar] [CrossRef]
- Obayashi, S.; Horiguchi, J.; Higuchi, T.; Katayama, A.; Handa, T.; Altan, B.; Bai, T.; Bao, P.; Bao, H.; Yokobori, T.; et al. Stathmin1 expression is associated with aggressive phenotypes and cancer stem cell marker expression in breast cancer patients. Int. J. Oncol. 2017, 51, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Cassimeris, L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef]
- Lovric, J.; Dammeier, S.; Kieser, A.; Mischak, H.; Kolch, W. Activated Raf induces the hyperphosphorylation of stathmin and the reorganization of the microtubule network. J. Biol. Chem. 1998, 273, 22848–22855. [Google Scholar] [CrossRef]
- Braganza, A.; Quesnelle, K.; Bickta, J.; Reyes, C.; Wang, Y.; Jessup, M.; St Croix, C.; Arlotti, J.; Singh, S.V.; Shiva, S. Myoglobin induces mitochondrial fusion, thereby inhibiting breast cancer cell proliferation. J. Biol. Chem. 2019, 294, 7269–7282. [Google Scholar] [CrossRef]
- De Roos, M.A.; van der Vegt, B.; Peterse, J.L.; Patriarca, C.; de Vries, J.; de Bock, G.H.; Wesseling, J. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome in ‘pure’ ductal carcinoma in situ of the breast. Histopathology 2007, 51, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Van der Vegt, B.; de Roos, M.A.; Peterse, J.L.; Patriarca, C.; Hilkens, J.; de Bock, G.H.; Wesseling, J. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome of invasive ductal breast carcinoma. Histopathology 2007, 51, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Sullivan, R.J.; Lauffenburger, D.A. Molecular Pathways: Receptor Ectodomain Shedding in Treatment, Resistance, and Monitoring of Cancer. Clin. Cancer Res. 2017, 23, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidger, A.M.; Keyse, S.M. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin. Cell Dev. Biol. 2016, 50, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, H.; Deng, S.; Kobayashi, J.; Ohizumi, Y.; Hirata, Y. Dictyoceratin-A and B; antimicrobial terpenoids from the okinawan sponge, Hippospongia sp. Tetrahedron 1986, 42, 4197–4201. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Minale, L. Polyprenyl derivatives from the sponge Ircinia Spinosula 2-Polyprenylbenzoquinones, 2 polyprenylbenzoquinols, prenylated furans and a C-31 difuranoterpene. Tetrahedron 1972, 28, 1315–1324. [Google Scholar] [CrossRef]
- Erdogan, I.; Sener, B. Two metabolites from the marine sponge Spongia officinalis L. Acta Pharm. Turc. 2001, 43, 17–19. [Google Scholar]
- McPail, K.; Davies-Coleman, M.T.; Coetzee, P. A New Furanosesterterpene from the South African Nudibranch Hypselodoris capensis and a Dictyoceratida Sponge. J. Nat. Prod. 1998, 61, 961–964. [Google Scholar] [CrossRef]
- Walker, R.P.; Thompson, J.E.; Faulkner, D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980, 45, 4976–4979. [Google Scholar] [CrossRef]
- Tasdemir, D.; Bugni, T.S.; Mangalindan, G.C.; Concepcion, G.P.; Harper, M.K.; Ireland, C.M. Cytotoxic bromoindole derivatives and terpenes from the Philippine marine sponge Smenospongia sp. Z. Nat. C J. Biosci. 2002, 57, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Sener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs 2010, 8, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues Felix, C.; Gupta, R.; Geden, S.; Roberts, J.; Winder, P.; Pomponi, S.A.; Diaz, M.C.; Reed, J.K.; Wright, A.E.; Rohde, K.H. Selective Killing of Dormant Mycobacterium tuberculosis by Marine Natural Products. Antimicrob. Agents Chemother. 2017, 61, 8. [Google Scholar] [CrossRef] [Green Version]
- Sirenko, O.; Mitlo, T.; Hesley, J.; Luke, S.; Owens, W.; Cromwell, E.F. High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. ASSAY Drug Dev. Technol. 2015, 13, 402–414. [Google Scholar] [CrossRef]
- Guzmán, E.A. Regulated Cell Death Signaling Pathways and Marine Natural Products That Target Them. Mar. Drugs 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, E.A.; Maers, K.; Roberts, J.; Kemami-Wangun, H.V.; Harmody, D.; Wright, A.E. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs 2015, 33, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.K. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2018, 39, 38–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadevaia, S.; Lu, Y.; Morales, F.C.; Mills, G.B.; Ram, P.T. Identification of optimal drug combinations targeting cellular networks: Integrating phospho-proteomics and computational network analysis. Cancer Res. 2010, 70, 6704–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibes, R.; Qiu, Y.; Lu, Y.; Hennessy, B.; Andreeff, M.; Mills, G.B.; Kornblau, S.M. Reverse phase protein array: Validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol. Cancer Ther. 2006, 5, 2512–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubenfels, M.W. New sponges from the Puerto Rican deep. Smithson. Misc. Collect. 1934, 91, 1–28. [Google Scholar]
- Wiedenmayer, F. Shallow-Water Sponges of the Western Bahamas; Birkhäuser: Basel, Switzerland, 1977; Volume 28, p. 331. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.A.; Pitts, T.P.; Winder, P.L.; Wright, A.E. The Marine Natural Product Furospinulosin 1 Induces Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cell Spheroids, But Not in Cells Grown Traditionally with Longer Treatment. Mar. Drugs 2021, 19, 249. https://doi.org/10.3390/md19050249
Guzmán EA, Pitts TP, Winder PL, Wright AE. The Marine Natural Product Furospinulosin 1 Induces Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cell Spheroids, But Not in Cells Grown Traditionally with Longer Treatment. Marine Drugs. 2021; 19(5):249. https://doi.org/10.3390/md19050249
Chicago/Turabian StyleGuzmán, Esther A., Tara P. Pitts, Priscilla L. Winder, and Amy E. Wright. 2021. "The Marine Natural Product Furospinulosin 1 Induces Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cell Spheroids, But Not in Cells Grown Traditionally with Longer Treatment" Marine Drugs 19, no. 5: 249. https://doi.org/10.3390/md19050249
APA StyleGuzmán, E. A., Pitts, T. P., Winder, P. L., & Wright, A. E. (2021). The Marine Natural Product Furospinulosin 1 Induces Apoptosis in MDA-MB-231 Triple Negative Breast Cancer Cell Spheroids, But Not in Cells Grown Traditionally with Longer Treatment. Marine Drugs, 19(5), 249. https://doi.org/10.3390/md19050249