Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri
Abstract
:1. Introduction
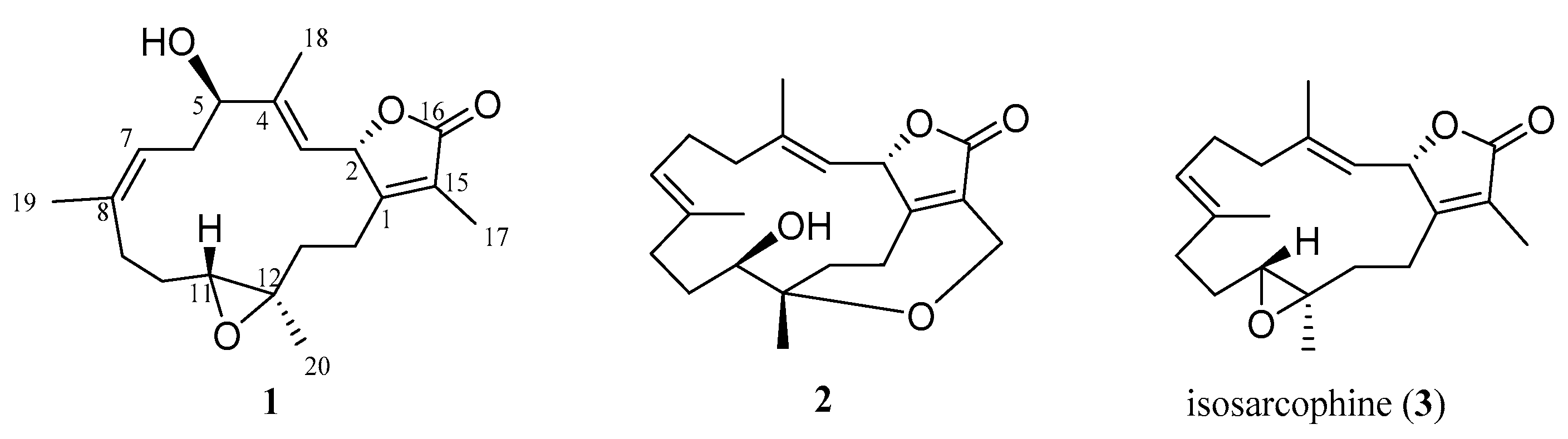
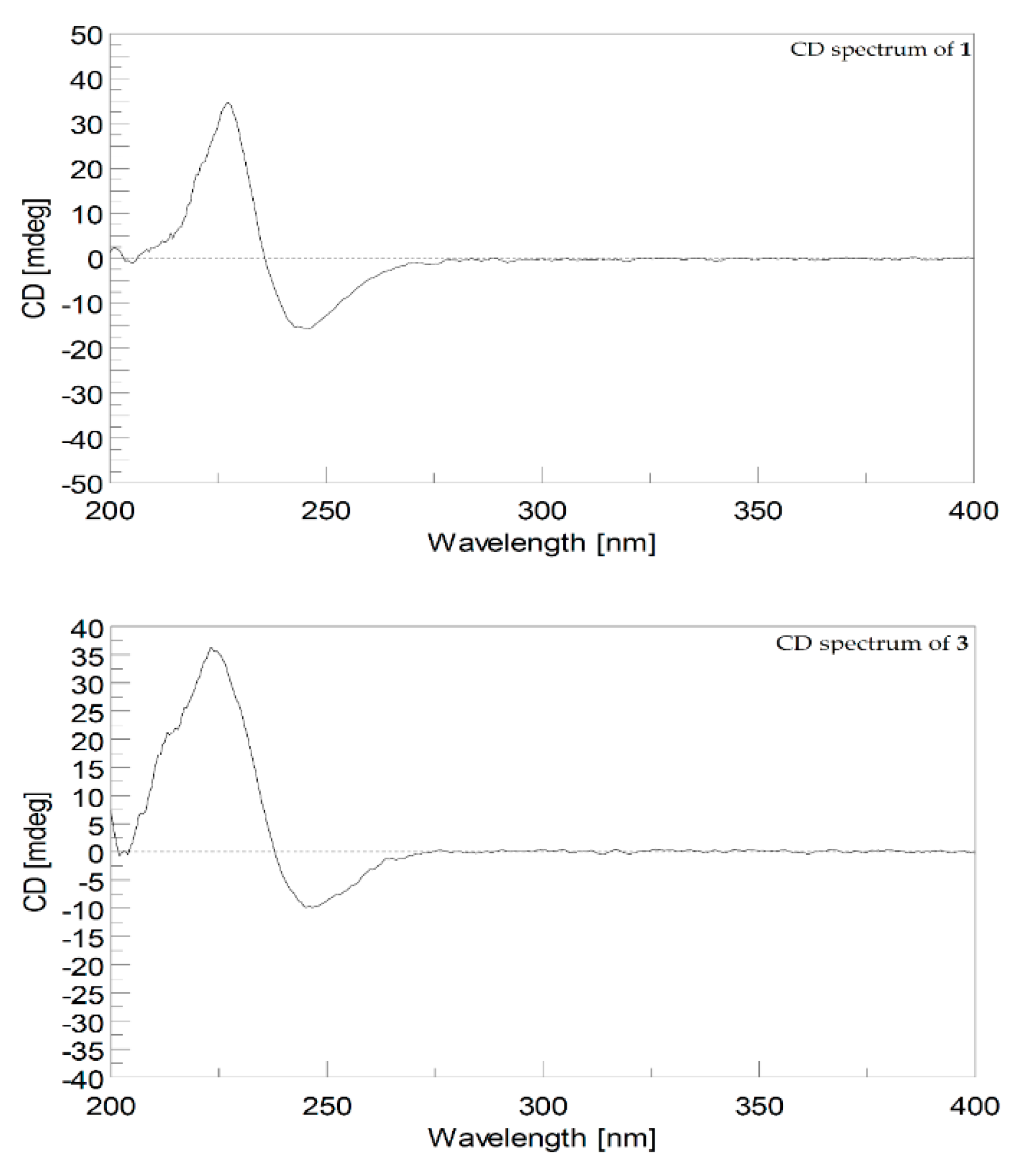
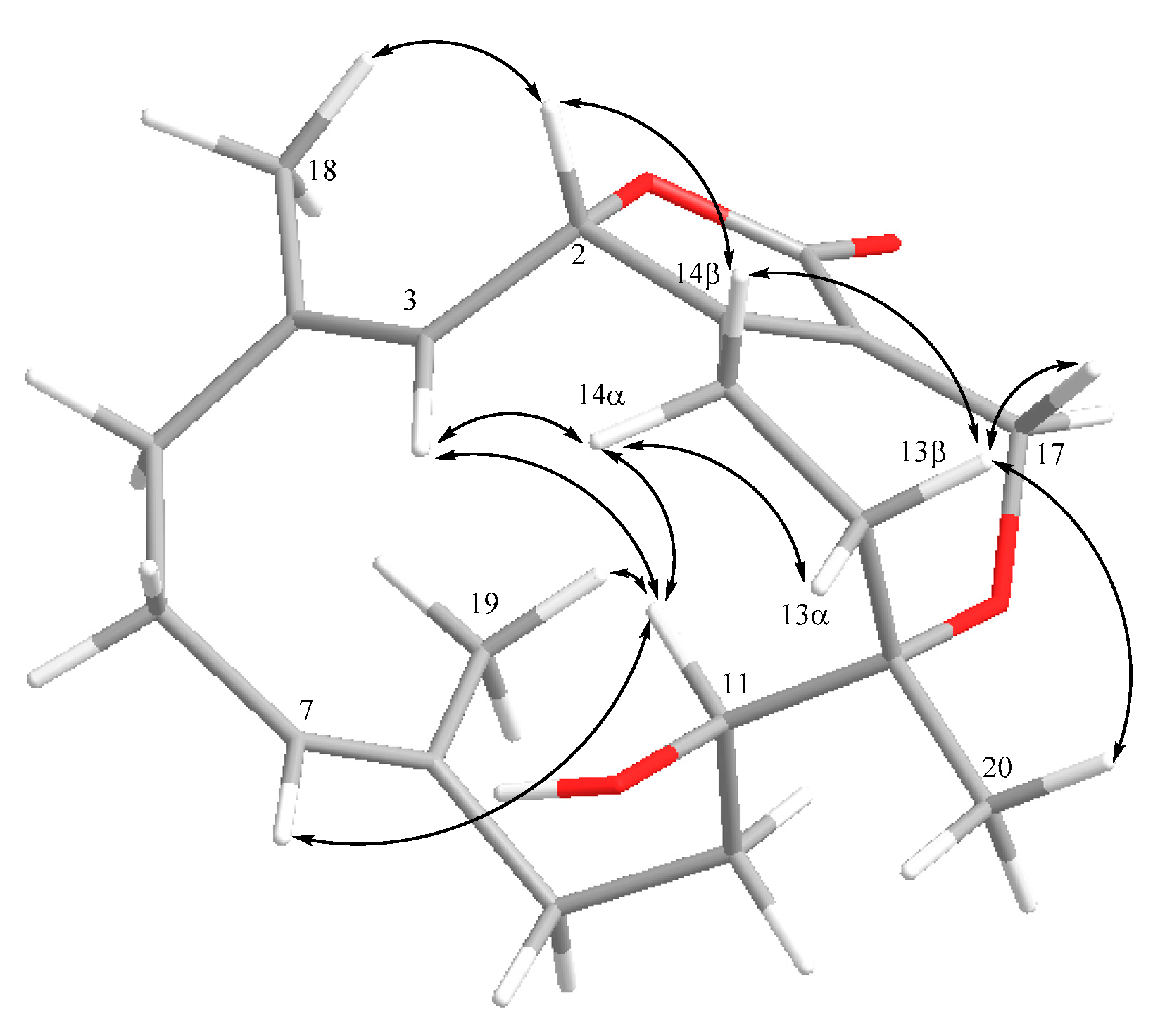
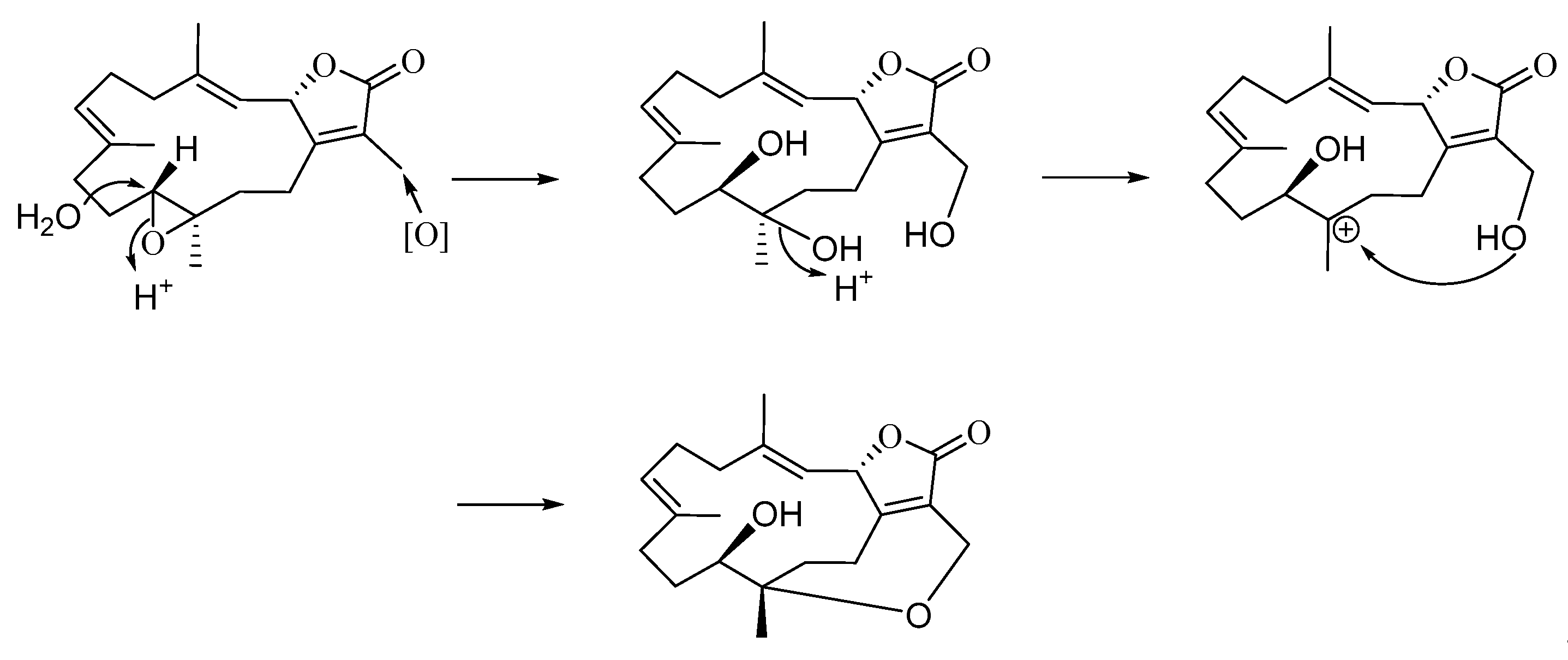
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
- Cherbonolide M (1): colorless amorphous oil, +29.0 (c 1.00, CHCl3), IR (KBr) νmax 3445, 2927, 2862, 1749, 1679, 1455, 1387, 1093, 1006, 755 cm−1; CD (1.2 × 10–4 M, MeOH) λmax Δε 245.5 (−15.6), and 227.0 (+34.8) nm; for 13C and 1H data see Table 1; electrospray ionization mass spectrometry (ESIMS) m/z 355; HRESIMS m/z 355.1882 [M + Na]+ (calculated for C20H28O4Na: 355.1880).
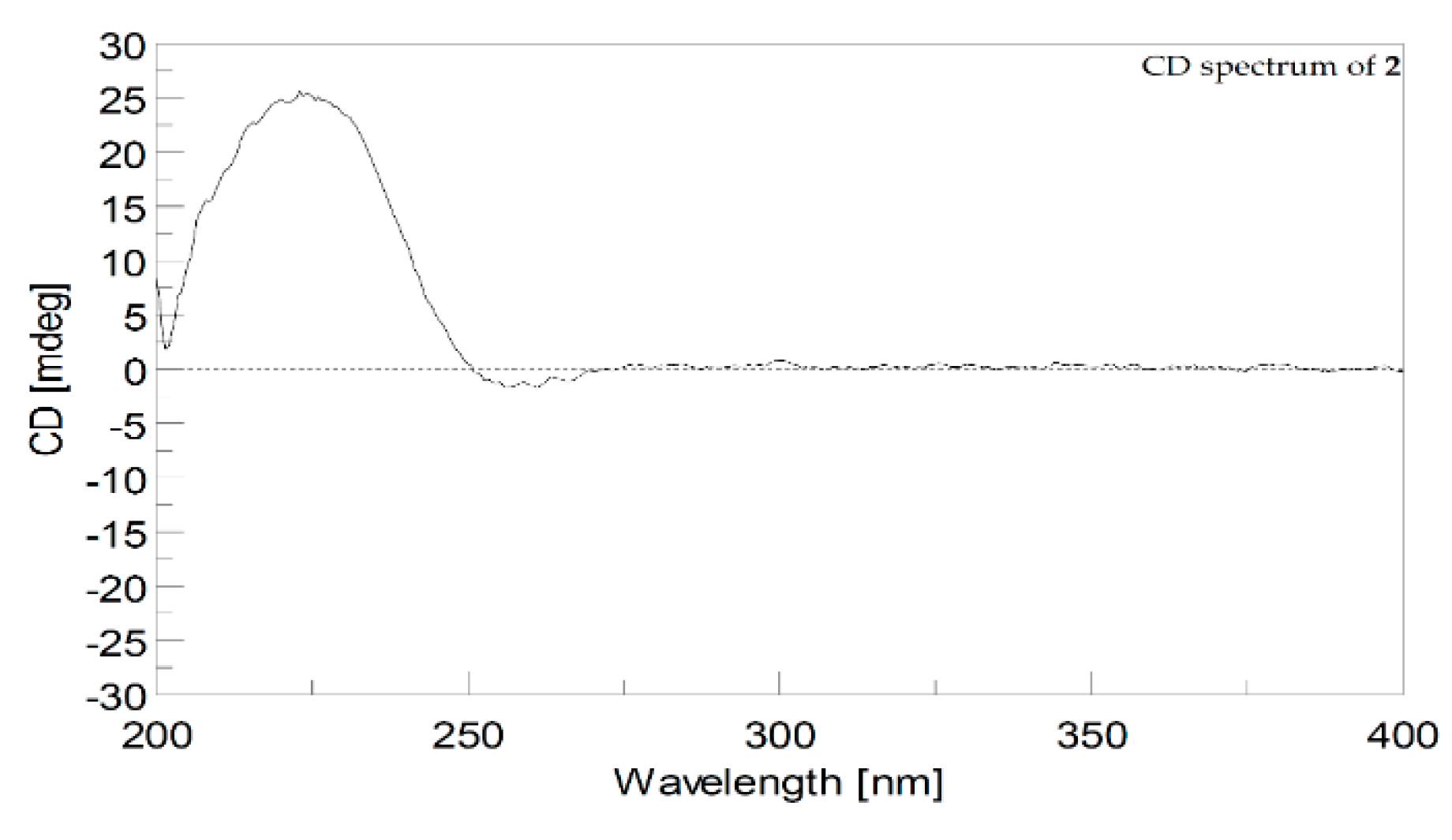
- Cherbonolide N (2): colorless amorphous oil, +15.0 (c 1.00, CHCl3), IR (KBr) νmax 3481, 2930, 1741, 1678, 1437, 1383, 1102, 1061, 986, 754 cm−1; CD (1.2 × 10−4 M, MeOH) λmax Δε 255.4 (−2.0), and 225.0 (+25.0) nm; for 13C and 1H data see Table 1; ESIMS m/z 355; HRESIMS m/z 355.1880 [M + H]+ (calculated for C20H28O4Na: 355.1879).
3.4. Cytotoxicity Testing
3.5. Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [Green Version]
- Aratake, S.; Tomura, T.; Saitoh, S.; Yokokura, R.; Kawanishi, Y.; Shinjo, R.; Reimer, J.D.; Tanaka, J.; Maekawa, H. Soft coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes. PLoS ONE 2012, 7, e30410. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Sung, P.J.; Duh, C.Y.; Chen, B.W.; Sheu, J.H.; Yang, N.S. Anti-inflammatory activities of nature products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.Y.; Huang, C.Y.; Chen, S.R.; Weng, J.R.; Tu, T.H.; Cheng, Y.B.; Wu, S.H.; Sheu, J.H. New hydroquinone monoterpenoid and ccembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum. Mar. Drugs 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Huang, C.Y.; Chao, C.H.; Lin, C.C.; Dai, C.F.; Su, J.H.; Sung, P.J.; Wu, S.H.; Sheu, J.H. New biscembranoids sardigitolides A-D and known cembranoid-related compounds from Sarcophyton digitatum: Isolation, structure elucidation, and bioactivities. Mar. Drugs 2020, 18, 452. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Mohamed, T.A.; Elshamy, A.I.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Umeyama, A.; Paré, P.W.; Efferth, T. Sarcoehrenbergilides D–F: Cytotoxic cembrene diterpenoids from the soft coral Sarcophyton ehrenbergi. RSC Adv. 2019, 9, 27183–27189. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.B.; Babu, D.C.; Bharadwaj, T.V.; Srikanth, D.; Vardhan, K.S.; Raju, T.V.; Bunce, R.A.; Venkateswarlu, Y. Isolation, structural assignment and synthesis of (SE)-2-methyloctyl 3-(4-methoxyphenyl) propenoate from the marine soft coral Sarcophyton ehrenbergi. Nat. Prod. Res. 2015, 29, 70–76. [Google Scholar]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Cytotoxic cembranoids from the Red Sea soft coral, Sarcophyton auritum. Tetrahedron Lett. 2014, 55, 3984–3988. [Google Scholar] [CrossRef]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Paré, P.W.; Hegazy, M.E.F. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Hsieh, M.K.; Duh, C.Y. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs 2013, 11, 4318–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.C.; Huang, C.Y.; Ahmed, A.F.; Hwang, T.L.; Sheu, J.H. Anti-inflammatory cembranoids from a Formosa soft coral Sarcophyton cherbonnieri. Mar. Drugs 2020, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Huang, C.Y.; Ahmed, A.F.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.F.; Chen, Y.W.; Huang, C.Y.; Tseng, Y.J.; Lin, C.C.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Isolation and structure elucidation of cembranoids from a Dongsha Atoll soft coral Sarcophyton stellatum. Mar. Drugs 2018, 16, 210. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zou, Y.H.; Ge, M.X.; Lou, L.L.; Xu, Y.S.; Ahmed, A.; Chen, Y.Y.; Zhang, J.S.; Tang, G.H.; Yin, S. Biscembranoids and cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2017, 15, 85. [Google Scholar] [CrossRef]
- Lin, W.Y.; Chen, B.W.; Huang, C.Y.; Wen, Z.H.; Sung, P.J.; Su, J.H.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids, sarcocrassocolides P–R, from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Su, J.H.; Lu, Y.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Liang, L.F.; Kurtán, T.; Mándi, A.; Yao, L.G.; Li, J.; Lan, L.F.; Guo, Y.W. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Fekry, M.I.; Al-Hammady, M.A.; Khalil, M.N.; El-Seedi, H.R.; Meyer, A.; Porzel, A.; Westphal, H.; Wessjohann, L.A. Cytotoxic effects of Sarcophyton sp. soft coral–is there a correlation to their NMR fingerprints? Mar. Drugs 2017, 15, 211. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, J.; Shmeuli, U.; Zadock, E.; Kashman, Y.; Néeman, I. Sarcophine, a new epoxy cembranolide from marine origin. Tetrahedron 1974, 30, 2817–2824. [Google Scholar] [CrossRef]
- Kashman, Y. Marine Natural Products Chemistry; Faulkner, D.J., Fenical, W.H., Eds.; Plenum Press: New York, NY, USA, 1977; pp. 17–21. [Google Scholar]
- Frincke, J.M.; McIntyre, D.E.; Faulkner, D.J. Deoxosarcophine from a soft coral, Sarcophyton sp. Tetrahedron Lett. 1980, 21, 735–738. [Google Scholar] [CrossRef]
- Kusumi, T.; Yamada, K.; Ishitsuka, M.O.; Fujita, Y.; Kakisawa, H. New cembranoids from the Okinawan soft coral Sinularia mayi. Chem. Lett. 1990, 19, 1315–1318. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hsieh, P.W.; Duh, C.Y.; Wang, S.K.; Soong, K.; Fang, L.S. Studies on the Formosan soft corals I-cytotoxic cembrane diterpenes from Sarcophyton trocheliophorum. J. Chin. Chem. Soc. 1992, 39, 355–357. [Google Scholar] [CrossRef]
- Li, S.W.; Ye, F.; Zhu, Z.D.; Huang, H.; Mao, S.C.; Guo, Y.W. Cembrane-type diterpenoids from the South China Sea soft coral Sarcophyton mililatensis. Acta Pharm. Sin. B 2018, 8, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, J.K.; van Oeveren, A.; van der Deen, H.; Leung, C.W.; Feringa, B.L. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J. Org. Chem. 1996, 61, 1513–1517. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth andviability in vitro. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Maloney, K.N.; Botts, R.T.; Davis, T.S.; Okada, B.K.; Maloney, E.M.; Leber, C.A.; Alvarado, O.; Brayton, C.; Caraballo-Rodríguez, A.M.; Chari, J.V.; et al. Cryptic species account for the seemingly idiosyncratic secondary metabolism of Sarcophyton glaucum specimens collected in Palau. J. Nat. Prod. 2020, 83, 693–705. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.H.; Tseng, Y.J.; Chen, B.W.; Hwang, T.L.; Chen, H.Y.; Dai, C.F.; Sheu, J.H. Tortuosenes A and B, new diterpenoid metabolites from the Formosan soft coral Sarcophyton tortuosum. Org. Lett. 2014, 16, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Ahmed, A.F.; Su, J.H.; Chao, C.H.; Wu, Y.C.; Chiang, M.Y.; Sheu, J.H. Crassocolides A–F, cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Zhang, Q.; Yang, M.; Gu, Y.C.; Liang, L.F.; Tang, W.; Guo, Y.W. Rare cembranoids from Chinese soft coral Sarcophyton ehrenbergi: Structural and stereochemical studies. J. Org. Chem. 2019, 84, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.F.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sarcophytolides G–L, new biscembranoids from the soft coral Sarcophyton elegans. Helv. Chim. Acta. 2013, 96, 2218–2227. [Google Scholar] [CrossRef]
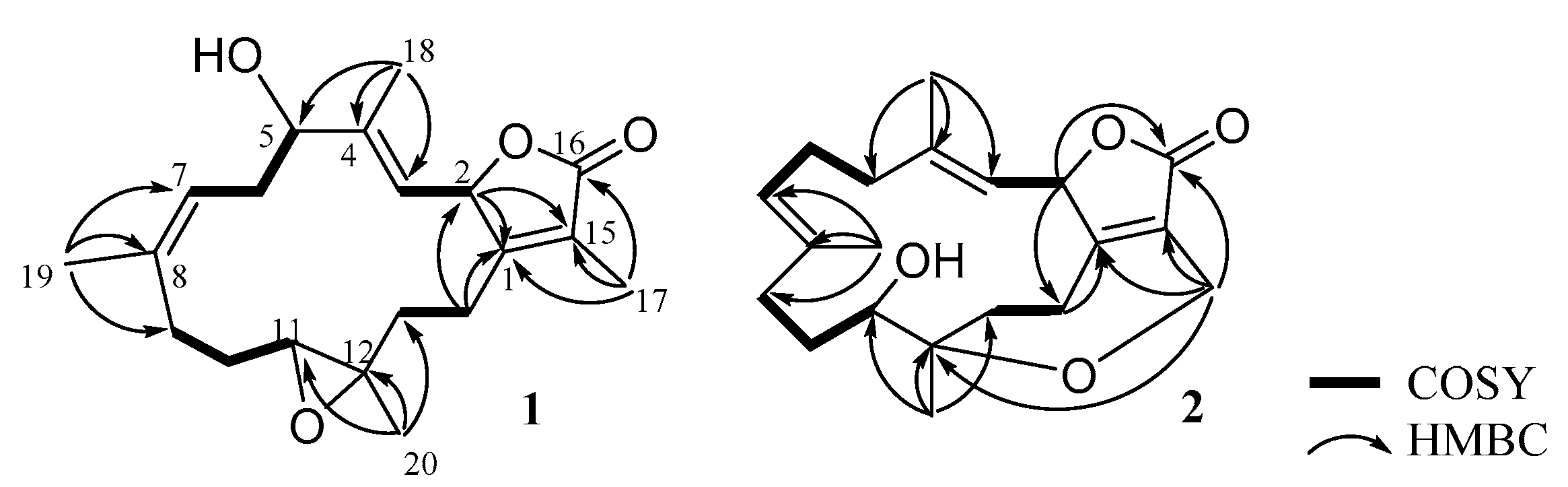

| Position | 1 α | 1 b | 2 c | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 162.4 (C) | 159.9 (C) | 166.1 (C) | |||
| 2 | 78.4 (CH) d | 5.64 d (10.4) e | 77.4 (CH) | 4.99 d (10.5) | 79.2 (CH) | 4.93 d (8.4) |
| 3 | 123.4 (CH) | 4.98 d (10.4) | 123.8 (CH) | 4.55 d (10.0) | 120.7 (CH) | 4.50 d (8.4) |
| 4 | 147.5 (C) | 145.5 (C) | 139.2 (C) | |||
| 5 | 77.5 (CH) | 4.21 dd (10.8, 5.2) | 77.3 (CH) | 3.77 dd (10.5, 5.5) | 36.9 (CH2) | 1.66 m; 1.87 m |
| 6 | 33.8 (CH2) | 2.28 m; 2.52 m | 32.8 (CH2) | 2.23 m; 2.31 m | 24.3 (CH2) | 1.77 m; 2.27 m |
| 7 | 122.5 (CH) | 4.98 d (10.8) | 121.4 (CH) | 4.55 d (10.0) | 127.2 (CH) | 4.76 d (10.4) |
| 8 | 134.9 (C) | 134.4 (C) | 133.8 (C) | |||
| 9 | 37.3 (CH2) | 2.08 m; 2.31 m | 36.9 (CH2) | 1.70 m; 1.98 m | 36.1 (CH2) | 1.92 m; 1.94 m |
| 10 | 24.5 (CH2) | 2.07 m; 1.23 m | 24.2 (CH2) | 1.06 m; 1.90 m | 25.8 (CH2) | 0.95 m; 1.98 m |
| 11 | 62.0 (CH) | 2.45, d (10.8) | 61.1 (CH) | 2.28 dd (10.5, 2.5) | 70.7 (CH) | 3.21 dd (10.4, 6.4) |
| 12 | 61.1 (C) | 59.9 (C) | 79.4 (C) | |||
| 13 | 37.8 (CH2) | 2.05 m; 1.04 t (11.2) | 37.3 (CH2) | 0.77 td (13.5, 2.5); 1.61 dd (13.0, 5.5) | 29.4 (CH2) | 1.70 m; 1.97 m |
| 14 | 24.7 (CH2) | 2.02 m; 2.71 m | 23.8 (CH2) | 1.53 d (13.5); 1.95 m | 24.0 (CH2) | 1.66 m; 2.19 m |
| 15 | 123.5 (C) | 123.8 (C) | 128.1 (C) | |||
| 16 | 174.6 (C) | 173.7 (C) | 172.3 (C) | |||
| 17 | 8.6 (CH3) | 1.78 s | 8.7 (CH3) | 1.67 s | 55.9 (CH2) | 4.31 dd (14.8, 1.6); 4.46 d (14.8) |
| 18 | 10.3 (CH3) | 1.74 s | 9.8 (CH3) | 1.33 s | 18.5 (CH3) | 1.27 s |
| 19 | 14.9 (CH3) | 1.72 s | 14.5 (CH3) | 1.31 s | 15.2 (CH3) | 1.36 s |
| 20 | 15.9 (CH3) | 1.28 s | 15.9 (CH3) | 1.02 s | 22.5 (CH3) | 1.02 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-C.; Huang, T.-Y.; Huang, C.-Y.; Hwang, T.-L.; Sheu, J.-H. Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri. Mar. Drugs 2021, 19, 260. https://doi.org/10.3390/md19050260
Peng C-C, Huang T-Y, Huang C-Y, Hwang T-L, Sheu J-H. Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri. Marine Drugs. 2021; 19(5):260. https://doi.org/10.3390/md19050260
Chicago/Turabian StylePeng, Chia-Chi, Tzu-Yin Huang, Chiung-Yao Huang, Tsong-Long Hwang, and Jyh-Horng Sheu. 2021. "Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri" Marine Drugs 19, no. 5: 260. https://doi.org/10.3390/md19050260
APA StylePeng, C. -C., Huang, T. -Y., Huang, C. -Y., Hwang, T. -L., & Sheu, J. -H. (2021). Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri. Marine Drugs, 19(5), 260. https://doi.org/10.3390/md19050260






