Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of TSG1-6 from Extrusion-Pretreated Tilapia Scales
2.2. Physicochemical Properties of TSG2, TSG4, and TSG6
2.3. Structure and Molecular Weights of Extracted Gelatins TSG2, TSG4, and TSG6
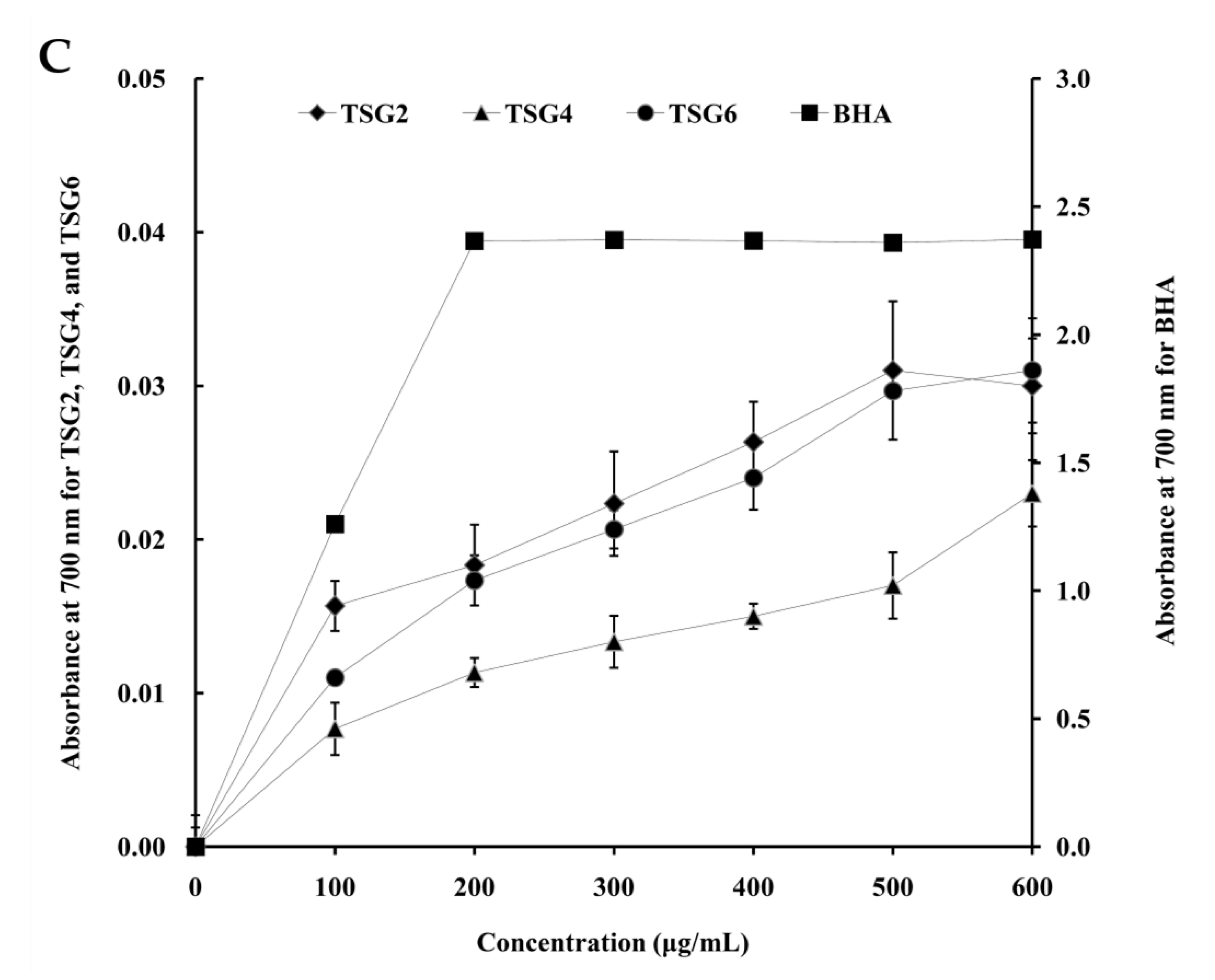
2.4. Antioxidant Activities of TSG2, TSG4, and TSG6
2.5. Preparation of Gelatin Hydrolysates TSGH1, TSGH2, TSGH3, and TSGH4 and Analyses of Antioxidant Activity
2.6. Preparation of Fractionated Products using TSGH4 and Assessment of Their DPPH Scavenging Activities
2.7. Potential Antioxidant Peptides in TSGH4-6-2-b by LC-ESI/MS/MS Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extrusion-Cooking Pretreatment
3.3. Extraction of Gelatin from Non-Extruded Fish Scales and Fish Scale Extrudate
(mL))/(weight of crude protein content of fish scales (g), dry basis)] × 100
3.4. Determination of Protein Concentration
3.5. Color Analysis
3.6. Chemical Composition Analyses
3.7. Determination of Gel Strength
3.8. Differential Scanning Calorimetry (DSC)
3.9. Determination of Viscosity
3.10. Sensory Evaluation
3.11. Fourier Transform Infrared (FTIR) Spectroscopy
3.12. Preparation of Gelatin Hydrolysates
3.13. Molecular Weight Analysis
3.14. Two-Step Separation of Gelatin Hydrolysates by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
3.15. DPPH Radical Scavenging Activity
3.16. ABTS Radical Scavenging Activity
3.17. Reducing Power Assay
3.18. LC-ESI/MS/MS and Data Analyses
3.19. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, K.; Zhang, Y.Y.; Mocan, A.; Zhang, F.; Zhang, J.G.; Wei, Z.J. 1-Deoxynojirimycin, its potential for management of non-communicable metabolic diseases. Trends Food Sci. Technol. 2019, 89, 88–99. [Google Scholar] [CrossRef]
- Wang, J.; Liao, A.M.; Thakur, K.; Zhang, J.G.; Huang, J.H.; Wei, Z.J. Licochalcone B extracted from Glycyrrhiza uralensis fisch induces apoptotic effects in human hepatoma cell HepG2. J. Agric. Food Chem. 2019, 67, 3341–3353. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 161–165. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Fukushima, S.; Haqlwara, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J. Natl. Cancer Inst. 1983, 70, 343–352. [Google Scholar]
- Huang, C.Y.; Kuo, C.H.; Lee, C.H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated Sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Tsai, Y.H.; Hong, Y.H.; Hsieh, S.L.; Huang, R.H. Characterization and antioxidant and angiotensin I-converting enzyme (ACE)-inhibitory activities of gelatin hydrolysates prepared from extrusion-pretreated milkfish (Chanos chanos) scale. Mar. Drugs 2018, 16, 346. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Kuo, C.H.; Wu, C.H.; Kuan, A.W.; Guo, H.R.; Lin, Y.H.; Wang, P.K. Free radical-scavenging, anti-inflammatory, and antibacterial activities of water and ethanol extracts prepared from compressional-puffing pretreated mango (Mangifera indica L.) peels. J. Food Qual. 2018, 2018, 1025387. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of gelatin hydrolysates from grass carp (Ctenopharyngodon idellus) scales by Maillard reaction: Antioxidant activity and volatile compounds. Food Chem. 2019, 295, 569–578. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Xu, Y.Y.; Hwang, W.S.; Wang, J.B. Pretreatment of rice straw using an extrusion/extraction process at bench-scale for producing cellulosic ethanol. Bioresour. Technol. 2011, 102, 10451–10458. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chi, Y.J. Effects of twin-screw extrusion on soluble dietary fibre and physicochemical properties of soybean residue. Food Chem. 2013, 138, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- Fisheries Agency, Council of Agriculture, Executive Yuan, Taiwan. Fisheries Statistical Yearbook. 2019. Available online: https://www.fa.gov.tw/cht/PublicationsFishYear/ (accessed on 21 April 2021).
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Rawdkuen, S.; Chaijan, M.; Benjakul, S.; Osako, K.; Tanaka, M. Chemical compositions and characterisation of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT Food Sci. Technol. 2010, 43, 161–165. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Skin gelatin from bigeye snapper and brownstripe red snapper: Chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocoll. 2006, 20, 1216–1222. [Google Scholar] [CrossRef]
- Cheow, C.S.; Norizah, M.S.; Kyaw, Z.Y.; Howell, N.K. Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chem. 2007, 101, 386–391. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Johnston-Banks, F.A. Gelatine. In Food Gels; Harris, P., Ed.; Elsevier Applied Science: London, UK, 1990; pp. 233–285. [Google Scholar]
- Wangtueai, S.; Noomhorm, A. Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT Food Sci. Technol. 2009, 42, 825–834. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Comparative study on characteristics of gelatin from the skins of brownbanded bamboo shark and blacktip shark as affected by extraction conditions. Food Hydrocoll. 2010, 24, 164–171. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Martins, M.E.O.; Sousa, J.R.; Claudino, R.L.; Lino, S.C.O.; Vale, D.A.D.; Silva, A.L.C.; Morais, J.P.S.; De Souza Filho, M.d.S.M.; De Souza, B.W. Thermal and chemical properties of gelatin from tilapia (Oreochromis niloticus) scale. J. Aquat. Food Prod. Technol. 2018, 27, 1120–1133. [Google Scholar] [CrossRef]
- Rokesh, K.; Nithya, A.; Jeganathan, K.; Jothivenkatachalam, K. A facile solid state synthesis of cone-like ZnO microstructure an efficient solar-light driven photocatalyst for rhodamine B degradation. Mater. Today Proc. 2016, 3, 4163–4172. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Senevirathne, M.; Kim, S.H.; Siriwardhana, N.; Ha, J.H.; Lee, K.W.; Jeon, Y.J. Antioxidant potential of Ecklonia cavaon reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci. Technol. Int. 2006, 12, 27–38. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, L.; Piot, J.M.; Guillochon, D.; Amiot, J. Study of the efficiency of a mobile phase used in size-exclusion HPLC for the separation of peptides from a casein hydrolysate according to their hydrodynamic volume. Chromatographia 1991, 32, 499–504. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolome, B.; Lopez-Fandino, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Hsu, K.C. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010, 122, 42–48. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- De Gobba, C.; Espejo-Carpio, F.J.; Skibsted, L.H.; Otte, J. Antioxidant peptides from goat milk protein fractions hydrolysed by two commercial proteases. Int. Dairy J. 2014, 39, 28–40. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- AOAC. Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- BSI. Methods for Sampling and Testing Gelatin (Physical and Chemical Methods); BSI (British Standards Institution): London, UK, 1975. [Google Scholar]
- Songchotikunpan, P.; Tattiyakul, J.; Supaphol, P. Extraction and electrospinning of gelatin from fish skin. Int. J. Biol. Macromol. 2008, 42, 247–255. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Chen, P.W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2018, 23, 78. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Wu, S.J.; Yang, W.N.; Kuan, A.W.; Chen, C.Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, T.C.; Hsieh, S.L.; Tsai, Y.H.; Yeh, C.W.; Huang, C.Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef]




| Pretreatments | TSG1 | TSG2 | TSG3 | TSG4 | TSG5 | TSG6 |
|---|---|---|---|---|---|---|
| Pretreatment solvent | — | — | — | — | 0.1 N NaOH | 0.1 N NaOH |
| Variables of Extrusion | TSG1 | TSG2 | TSG3 | TSG4 | TSG5 | TSG6 |
| Preconditioning solvent | 1.26% citric acid | 1.26% citric acid | 9.37% acetic acid | 9.37% acetic acid | 1.26% acitric acid | 1.26% citric acid |
| Feed moisture (%) | — | 27 | — | 27 | — | 27 |
| Feed rate (kg/h) | — | 11.4 | — | 11.4 | — | 11.4 |
| Barrel temperature (°C) | — | 135 | — | 135 | — | 135 |
| Screw speed (rpm) | — | 360 | — | 360 | — | 360 |
| Die diameter (mm) | — | 3 | — | 3 | — | 3 |
| Variables of Water Extraction | TSG1 | TSG2 | TSG3 | TSG4 | TSG5 | TSG6 |
| Extraction temperature (°C) | 50 | 50 | 50 | 50 | 50 | 50 |
| Extraction time (h) | 1 | 1 | 1 | 1 | 1 | 1 |
| Extraction Yield of Gelatins | TSG12 | TSG22 | TSG32 | TSG42 | TSG52 | TSG62 |
| Extraction yield (%) 1 | 2.10 ± 0.03 a | 22.2 ± 1.0 d | 2.18 ± 0.09 a | 19.3 ± 0.4 c | 2.13 ± 0.03 a | 13.0 ± 0.2 b |
| Physicochemical Properties | TSG2 1 | TSG4 1 | TSG6 1 |
|---|---|---|---|
| L | 82.3 ± 0.0 b | 74.5 ± 0.1 a | 93.2 ± 0.1 c |
| a | 0.63 ± 0.03 b | 1.52 ± 0.03 c | −1.39 ± 0.04 a |
| b | 9.27 ± 0.09 b | 10.0 ± 0.0 c | 5.42 ± 0.02 a |
| Whiteness index | 80.0 ± 0.0 b | 72.5 ± 0.0 a | 91.2 ± 0.0 c |
| Ash (%) | 1.75 ± 0.06 a | 3.58 ± 0.27 b | 3.33 ± 0.09 b |
| pH | 7.2 | 6.2 | 9.9 |
| Bloom gel strength (g) | 185 ± 5 b | 157 ± 5 a | 202 ± 0 c |
| Viscosity (cps) | 9.77 ± 0.12 b | 8.10±0.24 a | 9.53 ± 0.17 b |
| Tmax1 (°C) | 79.0 | 57.9 | 62.6 |
| Tmax2 (°C) | 176 | 182 | 179 |
| H1 (J/g) | 1.40 | 8.01 | 3.37 |
| H2 (J/g) | 116 | 166 | 152 |
| Odor (in solution) 2 | 5.21 ± 1.68 b | 4.04 ± 2.07 a | 5.38 ± 1.60 b |
| Odor (in powder) 2 | 6.08 ± 1.15 b | 3.17 ± 1.97 a | 6.46 ± 1.08 b |
| Overall acceptability 2 | 6.58 ± 1.55 b | 5.08 ± 1.85 a | 5.42 ± 1.35 a |
| Process Variables | TSGH1 | TSGH2 | TSGH3 | TSGH4 |
|---|---|---|---|---|
| Gelatin source | TSG2 | TSG2 | TSG2 | TSG2 |
| Enzyme used | Pepsin | Pepsin + Pancreatin | Pepsin | Pepsin + Pancreatin |
| Digestion conditions | pH 2.0, 37 °C, 4 h | pH 2.0, 37 °C, 4 h (for Pepsin); pH 7.0, 37 °C, 4 h (for Pancreatin) | pH 2.0, 37 °C, 4 h | pH 2.0, 37 °C, 4 h (for Pepsin); pH 7.0, 37 °C, 4 h (for Pancreatin) |
| Ultrafiltration condition | >3000 Da | >3000 Da | <3000 Da | <3000 Da |
| Molecular Weight (MW) | TSGH1 | TSGH2 | TSGH3 | TSGH4 |
| Peak MW 1 (Da) | 24,012 | 18,006 | 131 | 1315 |
| MW interval (Da) | 2883–37,466 | 1800–37,466 | 96–1184 | 74–21,625 |
| Antioxidant Activities1 | TSGH12 | TSGH2 | TSGH3 | TSGH4 |
| DPPH scavenging activity (%) | 43.3 ± 1.1 a 3 | 44.4 ± 3.6 a | 84.0 ± 0.3 c | 71.8 ± 2.5 b |
| TEAC (μM) | 12.7 ± 0.1 a | 29.7 ± 0.4 c | 27.6 ± 0.2 b | 29.2 ± 0.3 c |
| Absorbance (700 nm) | 0.15 ± 0.02 a | 0.24 ± 0.01 b | 0.20 ± 0.05 a | 0.23 ± 0.01 b |
| Samples | Peptide Content (μg/μL) 1 | DPPH Scavenging Activity (Vit C ppm/μg Peptide) 1 |
|---|---|---|
| TSGH4 separated by Superdex peptide column | ||
| TSGH4-1 | 0.13 ± 0.00 | 0.12 ± 0.03 |
| TSGH4-2 | 0.08 ± 0.00 | 0.00 ± 0.00 |
| TSGH4-3 | 0.08 ± 0.00 | 0.00 ± 0.00 |
| TSGH4-4 | 0.15 ± 0.00 | 0.11 ± 0.06 |
| TSGH4-5 | 0.13 ± 0.00 | 0.43 ± 0.06 |
| TSGH4-6 | 0.16 ± 0.05 | 0.97 ± 0.03 |
| TSGH4-7 | 0.31 ± 0.00 | 0.23 ± 0.02 |
| TSGH4-8 | 0.15 ± 0.00 | 0.53 ± 0.03 |
| TSGH4-9 | 0.13 ± 0.00 | 0.00 ± 0.00 |
| TSGH4-10 | 0.69 ± 0.01 | 0.35 ± 0.01 |
| TSGH4-11 | 0.15 ± 0.00 | 0.48 ± 0.02 |
| TSGH4-12 | 0.14 ± 0.00 | 0.00 ± 0.00 |
| TSGH4-6 separated by RP-HPLC C18 column | ||
| TSGH4-6-1 | 0.097 ± 0.003 | 1.17 ± 0.05 |
| TSGH4-6-2 | 0.085 ± 0.002 | 1.17 ± 0.06 |
| TSGH4-6-1 separated by RP-HPLC C18 column | ||
| TSGH4-6-1-a | 0.086 ± 0.003 | 1.22 ± 0.14 |
| TSGH4-6-1-b | 0.089 ± 0.004 | 1.35 ± 0.15 |
| TSGH4-6-1-c | 0.082 ± 0.003 | 0.80 ± 0.08 |
| TSGH4-6-2 separated by RP-HPLC C18 column | ||
| TSGH4-6-2-a | 0.086 ± 0.004 | 1.13 ± 0.19 |
| TSGH4-6-2-b | 0.090 ± 0.004 | 1.60 ± 0.22 |
| TSGH4-6-2-c | 0.092 ± 0.003 | 1.39 ± 0.09 |
| Protein Origin | Molecular Mass (+1) | Peptide Sequence |
|---|---|---|
| Collagen alpha-2(I) chain | 440.24 | GPLGP |
| 442.26 | GPVGL | |
| 470.25 | LPGSP | |
| 511.28 | GPAGPL | |
| 543.27 | GELGPA | |
| 568.30 | GPAGPLG | |
| 592.27 | GPSGFAG | |
| 646.23 | GYDEY | |
| 778.40 | GLPGPPGPS | |
| 1194.57 | ADGNTGPAGPAGPL | |
| 1251.59 | GADGNTGPAGPAGPL | |
| 1268.59 | GPAGARGADGNTGPA | |
| 1349.60 | GSPGPDGNNGPAGPVG | |
| Collagen type I alpha 1 | 440.24 | GPIGP |
| 470.25 | LPGPS | |
| 511.28 | GPAGIP | |
| 543.27 | GEIGPA | |
| 568.30 | QPGLPG | |
| 568.30 | GPAGIPG | |
| 610.31 | VGPPGPS | |
| 994.50 | GPAGASGPAGPR | |
| 1075.51 | GAPGPPGPSGPQG | |
| 1307.62 | GETGPAGVPGPAGPSG | |
| 1330.67 | PGPAGATGAPGPQGPV | |
| 1538.70 | EPGKSGEQGAPGEAGAP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiao, W.-C.; Wu, T.-C.; Kuo, C.-H.; Tsai, Y.-H.; Tsai, M.-L.; Hong, Y.-H.; Huang, C.-Y. Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Mar. Drugs 2021, 19, 275. https://doi.org/10.3390/md19050275
Shiao W-C, Wu T-C, Kuo C-H, Tsai Y-H, Tsai M-L, Hong Y-H, Huang C-Y. Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Marine Drugs. 2021; 19(5):275. https://doi.org/10.3390/md19050275
Chicago/Turabian StyleShiao, Wei-Cheng, Tien-Chiu Wu, Chia-Hung Kuo, Yung-Hsiang Tsai, Mei-Ling Tsai, Yong-Han Hong, and Chun-Yung Huang. 2021. "Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales" Marine Drugs 19, no. 5: 275. https://doi.org/10.3390/md19050275
APA StyleShiao, W.-C., Wu, T.-C., Kuo, C.-H., Tsai, Y.-H., Tsai, M.-L., Hong, Y.-H., & Huang, C.-Y. (2021). Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Marine Drugs, 19(5), 275. https://doi.org/10.3390/md19050275








