Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae
Abstract
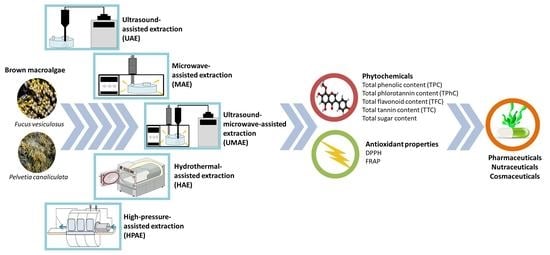
:1. Introduction
2. Results and Discussion
2.1. Effect of Technologies on the Yields and Phytochemical Contents of Extracts
2.2. Effect of Novel Technologies on the Antioxidant Capacity of the Extracts
3. Materials and Methods
3.1. Macroalgal Biomass and Processing
3.2. Extraction Procedures
3.3. Phytochemical Analyses
3.3.1. Total Phenolic Content (TPC) and Total Phlorotannin Content (TPhC) Analyses
3.3.2. Total Flavonoid Content (TFC)
3.3.3. Total Sugar Content (TSC)
3.3.4. Total Tannin Content (TTC)
3.4. Antioxidant Analyses
3.4.1. 1,1-Diphenyl-2-Picryl-Hydrazil (DPPH) Radical Scavenging Activity
3.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiry, M.; Guiry, G.; AlgaeBase. World-Wide Electronic Publication. Available online: www.algaebase.org (accessed on 27 April 2021).
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts-the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Fatima, F.; Løvstad, H.S.; Rohan, S.; Pedro, M.; Zhengyong, Y. The Global Statusof Seaweed Production, Trade and Utilization; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Zhu, X.; Healy, L.; Zhang, Z.; Maguire, J.; Sun, D.; Tiwari, B.K. Novel postharvest processing strategies for value-added applications of marine algae. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic content of brown algae (pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal variation of the proximate composition, mineral content, fatty acid profiles and other phytochemical constituents of selected brown macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Qin, Y. Health benefits of bioactive seaweed substances. In Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Qin, Y., Ed.; Academic Press Elsevier: Cambridge, MA, USA, 2018; pp. 179–200. [Google Scholar]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of I rish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.; Cherry, P.; Herford, A.; Mustar, S.; Wheater, H.; Brownlee, I.; Seal, C.; Pearson, J. Inhibitory activity of extracts of Hebridean brown seaweeds on lipase activity. Environ. Boil. Fishes 2016, 28, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural deep eutectic solvents as alternatives for extracting phlorotannins from brown algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press Oxford: Oxford, UK, 2000; Volume 30. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, sargassum fusiforme (Harvey) setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Montero, L.; Sánchez-Camargo, A.D.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. Environ. Boil. Fishes 2017, 29, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.H.; Pham, H.N.T.; Nguyen, T.H. Optimization of ultrasound-assisted extraction conditions for phenolics, antioxidant, and tyrosinase inhibitory activities of Vietnamese brown seaweed (Padina australis). J. Food Process. Preserv. 2021, 45, e15386. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Magnusson, M.; Yuen, A.K.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; De Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Harnedy, P.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.; Shen, J.; Luo, Y.; Qu, H.; Gong, X. Research progress on the ethanol precipitation process of traditional Chinese medicine. Chin. Med. 2020, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Santos-Zea, L.; Cruz-Suárez, L.E. Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae). J. Appl. Phycol. 2020, 32, 1441–1453. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-assisted extraction of phlorotannins from fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Zhao, M.; Senthamaraikannan, R.; Padamati, R.B.; Sun, D.; Tiwari, B.K. Green extraction of soluble dietary fibre from coffee silverskin: Impact of ultrasound/microwave-assisted extraction. Int. J. Food Sci. Technol. 2019, 55, 2242–2250. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019, 289, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochemistry 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [Green Version]
- Lemus, A.; Bird, K.; Kapraun, D.F.; Koehn, F. Agar yield, quality and standing crop biomass of Gelidium serrulatum, Gelidium floridanum and Pterocladia capillacea in Venezuela. Food Hydrocoll. 1991, 5, 469–479. [Google Scholar] [CrossRef]
- Lee, W.-K.; Namasivayam, P.; Ho, C.-L. Effects of sulfate starvation on agar polysaccharides of Gracilaria species (Gracilariaceae, Rhodophyta) from Morib, Malaysia. Environ. Boil. Fishes 2013, 26, 1791–1799. [Google Scholar] [CrossRef]
- Mulchandani, K.; Kar, J.R.; Singhal, R.S. Extraction of lipids from chlorella saccharophila using high-pressure homogenization followed by three phase partitioning. Appl. Biochem. Biotechnol. 2015, 176, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Luo, Z.-C.; Yuan, F.; Yu, X.-B. Combined process of high-pressure homogenization and hydrothermal extraction for the extraction of fucoidan with good antioxidant properties from Nemacystus decipients. Food Bioprod. Process. 2017, 106, 35–42. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. Environ. Boil. Fishes 2015, 28, 533–543. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Xie, C.; Yang, H.; Dias, G.; Kong, L.; Bekhit, A.E.-D. Optimization of extraction parameters of antioxidant activity of extracts from New Zealand and Chinese Asparagus officinalis L root cultivars. Ind. Crop. Prod. 2018, 119, 191–200. [Google Scholar] [CrossRef]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. Correction to an unfractionated fucoidan from ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2012, 75, 1674. [Google Scholar] [CrossRef]
- Calixto, F.D.S. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2017, 53, 174–181. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.-W.; König, G.M. Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Environ. Boil. Fishes 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Liu, S.-C.; Lin, J.-T.; Wang, C.-K.; Chen, H.-Y.; Yang, D.-J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, S.W. Understanding carbohydrate analysis. In Food Carbohydrates: Chemistry, Physical Properties and Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–38. [Google Scholar]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 11 March 2021).
- Friendly, M. Corrgrams: Exploratory displays for correlation matrices. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]




| Macroalgae sp. | Extraction Technologies | TPC (mg GAE/g) | TPhC (mg PGE/g) | TFC (mg QE/g) | TTC (mg ChE/g) | TSC (mg GlcE/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | ||
| F. vesiculosus | Control | 316.4 ± 3.9 d | 293.2 ± 5.9 e ** | 257.7 ± 3.2 d | 238.7 ± 4.9 e ** | 180.4 ± 7.8 e | 129.3 ± 6.4 e ** | 123.2 ± 3.4 d | 91.3 ± 3.8 c *** | 136.1 ± 9.1 c | 116.6 ± 8.9 b ns |
| UAE | 445.0 ± 4.6 a | 413.4 ± 5.1 a ** | 362.9 ± 3.7 a | 337.1 ± 4.2 a ** | 286.3 ± 7.8 a | 285.6 ± 7.7 a ns | 189.1 ± 4.4 a | 168.4 ± 7.1 a * | 130.7 ± 6.2 c | 111.2 ± 9.6 b * | |
| MAE | 391.2 ± 6.0 c | 375.9 ± 5.3 c * | 318.9 ± 4.9 c | 306.4 ± 4.4 c * | 202.6 ± 3.4 d | 198.1 ± 7.1 d ns | 161.0 ± 5.6 bc | 142.4 ± 4.4 b * | 199.9 ± 9.2 b | 162.7 ± 7.9 a ** | |
| UMAE | 431.2 ± 4.5 b | 392.2 ± 7.5 b ** | 351.6 ± 3.7 b | 319.7 ± 6.1 b ** | 268.5 ± 6.4 b | 266.3 ± 1.3 b * | 172.8 ± 7.8 b | 151.3 ± 4.4 b ** | 194.5 ± 9.6 b | 157.6 ± 6.0 a ns | |
| HAE | 433.2 ± 6.2 ab | 375.5 ± 2.1 c *** | 353.3 ± 5.1 ab | 306.1 ± 1.7 c *** | 253.0 ± 5.6 b | 250.0 ± 4.4 b ns | 166.9 ± 6.7 b | 146.9 ± 2.2 b ** | 239.4 ± 2.9 a | 164.4 ± 5.0 a *** | |
| HPAE | 387.9 ± 3.8 c | 356.1 ± 2.4 d *** | 316.2 ± 3.1 c | 290.2 ± 2.0 d *** | 231.5 ± 1.3 c | 228.5 ± 7.1 c ns | 148.4 ± 3.4 c | 138.0 ± 9.7 b ns | 123.7 ± 5.6 c | 81.9 ± 2.9 c *** | |
| P. canaliculata | Control | 174.8 ± 5.5 d | 158.3 ± 7.8 e * | 141.9 ± 4.5 d | 128.3 ± 6.4 e * | 82.6 ± 8.4 b | 80.4 ± 6.8 c ns | 33.6 ± 2.2 d | 32.1 ± 1.3 d ns | 35.7 ± 5.0 d | 58.4 ± 7.2 c ** |
| UAE | 250.6 ± 6.0 a | 182.3 ± 7.8 d *** | 203.9 ± 4.9 a | 148.0 ± 6.4 d *** | 122.6 ± 3.4 a | 108.5 ± 5.6 a * | 79.5 ± 4.6 a | 62.4 ± 2.2 a ** | 59.1 ± 6.1 c | 42.4 ± 8.7 d ns | |
| MAE | 205.3 ± 3.6 c | 192.6 ± 7.1 bd ns | 166.8 ± 2.9 c | 156.4 ± 5.8 bd ns | 93.7 ± 7.8 b | 84.1 ± 6.4 c ns | 35.8 ± 3.8 cd | 32.8 ± 2.6 cd ns | 123.1 ± 6.1 b | 45.1 ± 8.1 d *** | |
| UMAE | 238.4 ± 3.0 ab | 210.4 ± 3.4 a *** | 193.9 ± 2.5 ab | 171.0 ± 2.8 a *** | 113.7 ± 3.4 a | 102.6 ± 6.8 ab ns | 47.6 ± 4.6 b | 41.7 ± 3.4 b * | 109.1 ± 3.1 b | 92.4 ± 7.2 b ns | |
| HAE | 236.2 ± 1.9 b | 202.3 ± 3.2 abc *** | 192.1 ± 1.6 b | 164.4 ± 2.7 abc *** | 113.0 ± 4.6 a | 87.8 ± 6.7 bc ** | 55.0 ± 2.6 b | 41.0 ± 4.6 bc * | 175.7 ± 9.0 a | 123.1 ± 7.0 a ** | |
| HPAE | 226.5 ± 5.7 b | 194.1 ± 3.2 cd ** | 184.2 ± 4.7 b | 157.7 ± 2.6 cd ** | 110.7 ± 5.6 a | 93.0 ± 6.8 abc * | 46.9 ± 5.9 bc | 37.3 ± 3.4 bcd ns | 25.1 ± 3.1 d | 15.7 ± 1.2 d * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J.; Jaiswal, A.K.; Tiwari, B.K.; Rajauria, G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Mar. Drugs 2021, 19, 309. https://doi.org/10.3390/md19060309
Garcia-Vaquero M, Ravindran R, Walsh O, O’Doherty J, Jaiswal AK, Tiwari BK, Rajauria G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Marine Drugs. 2021; 19(6):309. https://doi.org/10.3390/md19060309
Chicago/Turabian StyleGarcia-Vaquero, Marco, Rajeev Ravindran, Orla Walsh, John O’Doherty, Amit K. Jaiswal, Brijesh K. Tiwari, and Gaurav Rajauria. 2021. "Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae" Marine Drugs 19, no. 6: 309. https://doi.org/10.3390/md19060309