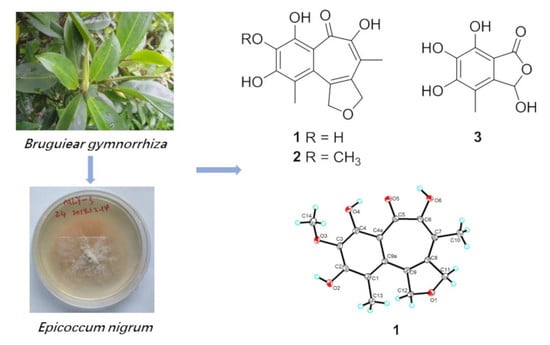
Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3
Abstract
:1. Introduction
2. Results
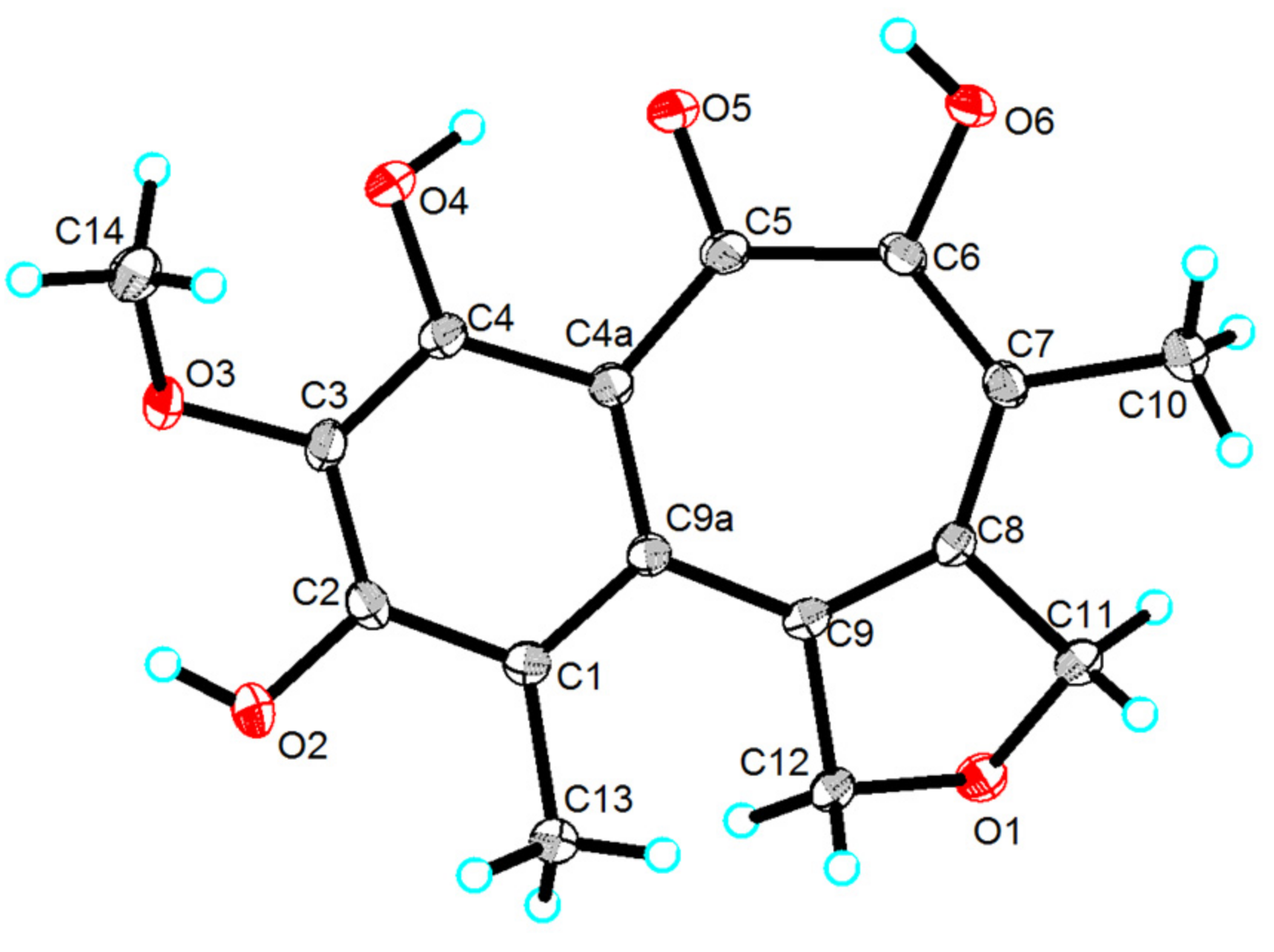
2.1. Structure Elucidation
2.2. Antioxidant Activities In Vitro
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. X-Ray Crystallographic Data
3.5. Antioxidant Activity Analysis
3.5.1. DPPH· (2, 2-diphenyl-1-picrylhydrazyl) Scavenging Activity
3.5.2. ABTS· (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) Radical Cation Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bentley, R. A Fresh Look at Natural Tropolonoids. Nat. Prod. Rep. 2008, 25, 118–138. [Google Scholar] [CrossRef]
- Guo, H.; Roman, D.; Beemelmanns, C. Tropolone natural products. Nat. Prod. Rep. 2019, 36, 1137–1155. [Google Scholar] [CrossRef]
- Zhao, J. Plant Troponoids: Chemistry, Biological Activity, and Biosynthesis. Curr. Med. Chem. 2007, 14, 2597–2621. [Google Scholar] [CrossRef]
- Azegami, K.; Nishiyama, K.; Watanabe, Y.; Suzuki, T.; Yoshida, M.; Nose, K.; Toda, S. Tropolone as a root growth-inhibitor produced by a plant pathogenic Pseudomonas sp. causing seedling blight of rice. Jpn. J. Phytopathol. 1985, 51, 315–317. [Google Scholar] [CrossRef]
- Miller, T.; Belas, R. Dimethylsulfoniopropionate Metabolism by Pfiesteria-Associated Roseobacter spp. Appl. Environ. Microbiol. 2004, 70, 3383–3391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Liu, J.; Liu, Y.; Chen, R.; Li, Y.; Cen, S.; Chen, X.; Guo, S.; Dai, J. Dengratiols A–D, four new bibenzyl derivatives from Dendrobium gratiossimum. Fitoterapia 2021, 152, 104926. [Google Scholar] [CrossRef] [PubMed]
- Fullagar, J.L.; Garner, A.L.; Struss, A.K.; Day, J.A.; Martin, D.P.; Yu, J.; Cai, X.; Janda, K.D.; Cohen, S.M. Antagonism of a zinc metalloprotease using a unique metal-chelating scaffold: Tropolones as inhibitors of P. aeruginosa elastase. Chem. Commun. 2013, 49, 3197–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Lomonosova, E.; Cheng, X.; Moran, E.A.; Meyers, M.; Le Grice, S.F.J.; Thomas, C.J.; Jiang, J.-K.; Meck, C.; Hirsch, D.R.; et al. Hydroxylated Tropolones Inhibit Hepatitis B Virus Replication by Blocking Viral Ribonuclease H Activity. Antimicrob. Agents Chemother. 2014, 59, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and New. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Falcone, E.; Holstein, S.; Anderson, A.; Wright, D.; Wiemer, A. Novel α-substituted tropolones promote potent and selective caspase-dependent leukemia cell apoptosis. Pharmacol. Res. 2016, 113, 438–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuerst, E.P.; Anderson, J.V.; Morris, C.F. Delineating the Role of Polyphenol Oxidase in the Darkening of Alkaline Wheat Noodles. J. Agric. Food Chem. 2006, 54, 2378–2384. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Z.; Chen, Y.; Cai, R.; Chen, G.; She, Z. Secondary Metabolites with α-Glucosidase Inhibitory Activity from the Mangrove Fungus Mycosphaerella sp. SYSU-DZG01. Mar. Drugs 2019, 17, 483. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Jiang, H.; Xiao, Z.; Cao, W.; Yan, T.; Liu, Z.; Lin, S.; Long, Y.; She, Z. (−)- and (+)-Asperginulin A, a Pair of Indole Diketopiperazine Alkaloid Dimers with a 6/5/4/5/6 Pentacyclic Skeleton from the Mangrove Endophytic Fungus Aspergillus sp. SK-28. Org. Lett. 2019, 21, 9633–9636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, P.; Liu, H.; Li, J.; Shao, C.; Yan, T.; Cao, W.; She, Z. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg. Chem. 2019, 88, 102973. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, J.; Huang, X.; Yan, T.; Cao, W.; Liu, H.; Long, Y.; She, Z. Diaporindenes A−D: Four Unusual 2,3-Dihydro 1H indene Analogues with Anti-inflammatory Activities from the Mangrove Endophytic Fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018, 83, 11804–11813. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Huang, Y.; Liu, L.; He, J.; Wang, L.; Yuan, J.; She, Z. Ascomylactams A−C, Cytotoxic 12- or 13-Membered-Ring Macrocyclic Alkaloids Isolated from the Mangrove Endophytic Fungus Didymella sp. CYSK-4, and Structure Revisions of Phomapyrrolidones A and C. J. Nat. Prod. 2019, 82, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yun, Z.; Guan, X.; Kai, T.; Guo, J.; Wang, H.B.; Fu, G. A novel antioxidant isobenzofuranone derivative from fungus cephalosporium sp.AL031. Molecules 2012, 17, 4219–4224. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.H.; Gloer, J.B.; Wicklow, D.T. Isolation of Chromanone and Isobenzofuran Derivatives from a Fungicolous Isolate of Epicoccum purpurascens. Bull. Korean Chem. Soc. 2007, 28, 877–879. [Google Scholar] [CrossRef]
- Bang, S.; Chae, H.-S.; Lee, C.; Choi, H.G.; Ryu, J.; Li, W.; Lee, H.; Jeong, G.-S.; Chin, Y.-W.; Shim, S.H. New Aromatic Compounds from the Fruiting Body of Sparassis crispa (Wulf.) and Their Inhibitory Activities on Proprotein Convertase Subtilisin/Kexin Type 9 mRNA Expression. J. Agric. Food Chem. 2017, 65, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Kemami, W.; Hilaire, V.; Ishida, K.; Hertweck, C. Epicoccalone, a coumarin-type chymotrypsin inhibotor, and isobenzofuran congeners from an Epicoccum sp. Associated with a tree fungus. Eur. J. Org. Chem. 2008, 22, 3781–3784. [Google Scholar] [CrossRef]
- Talontsi, F.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and Antifungal Polyketides from an Endophytic Fungus Epicoccum sp. Associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Ayer, W.A.; Jimenez, L.D. Phomalone, an antifungal metabolite of Phoma etheridgei. Can. J. Chem. 1994, 72, 2326–2332. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Nicholson, J.S. 30. The oxidation products of phenols. Part I. The structure of purpurogallin. J. Chem. Soc. 1948, 2, 116–120. [Google Scholar] [CrossRef]
- Arpin, N.; Favre-Bonvin, J.; Steglich, W. Le fomentariol: Nouvelle benzotropolone isolée de Fomes fomentarius. Phytochemistry 1974, 13, 1949–1952. [Google Scholar] [CrossRef]
- Mesa-Siverio, D.; Estévez-Braun, A.; Ravelo, Á.G.; Murguía, J.R.; Rodríguez-Afonso, A. Novel DNA-Damaging Tropolone Derivatives from Goupia glabra. Eur. J. Org. Chem. 2003, 2003, 4243–4247. [Google Scholar] [CrossRef]
- Klostermeyer, D.; Knops, L.; Sindlinger, T.; Polborn, K.; Steglich, W. ChemInform Abstract: Novel Benzotropolone and 2H-Furo[3,2-b]benzopyran-2-one Pigments from Tricholoma aurantium (Agaricales). Eur. J. Org. Chem. 2010, 31, 603–609. [Google Scholar] [CrossRef]
- Kerschensteiner, L.; Löbermann, F.; Steglich, W.; Trauner, D. Crocipodin, a benzotropolone pigment from the mushroom Leccinum crocipodium (Boletales). Tetrahedron 2011, 67, 1536–1539. [Google Scholar] [CrossRef]
- Bryant, R.; Light, R. Stipitatonic Acid Biosynthesis. Incorporation of [formyl-14C]-3-Methylorcylaldehyde and [14C]Stipitaldehydic Acid, a New Tropolone Metabolite. Biochemistry 1974, 13, 1516–1522. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Takada, S.; Mori, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Nonaka, K.; Masuma, R.; Otoguro, K.; Shiomi, K.; et al. In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A–C, produced by Penicillium sp. FKI-4410. J. Antibiot. 2010, 64, 183–188. [Google Scholar] [CrossRef] [Green Version]
- El Amrani, M.; Lai, D.; Debbab, A.; Aly, A.H.; Siems, K.; Seidel, C.; Schnekenburger, M.; Gaigneaux, A.; Diederich, M.; Feger, D.; et al. Protein Kinase and HDAC Inhibitors from the Endophytic Fungus Epicoccum nigrum. J. Nat. Prod. 2013, 77, 49–56. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Z.; Chen, S.; Huang, X.; Cui, H.; Long, Y.; Lu, Y.; She, Z. Antioxidative Polyketones from the Mangrove-Derived Fungus Ascomycota sp. SK2YWS-L. Sci. Rep. 2016, 6, 36609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
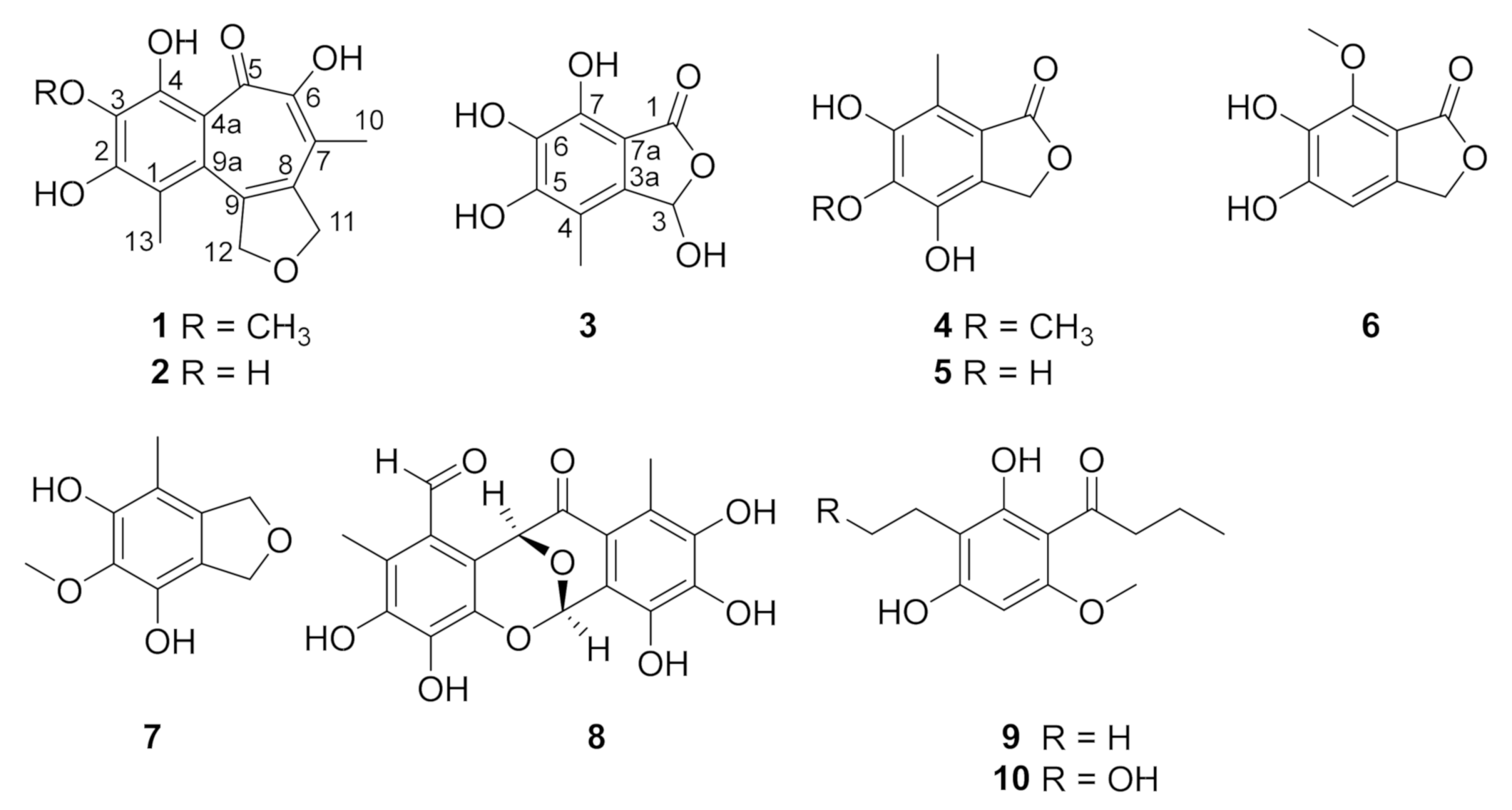
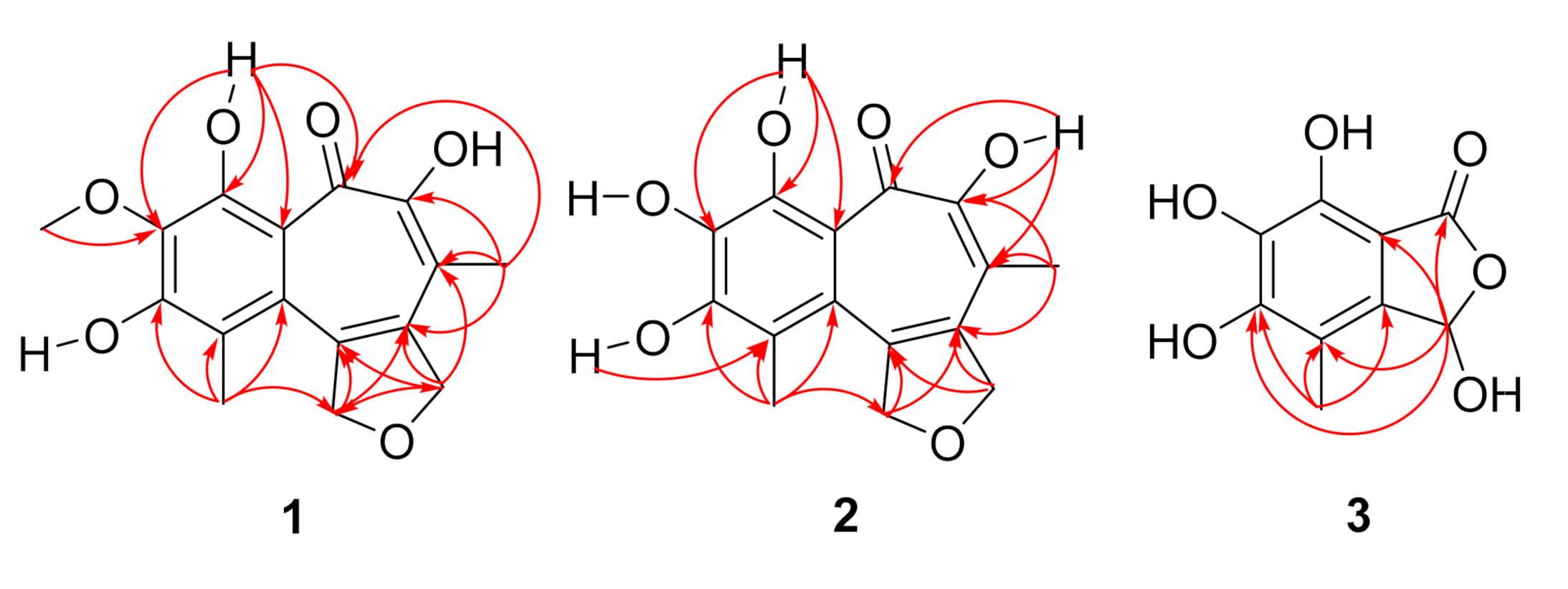
| 1 | 2 | |||
|---|---|---|---|---|
| No. | δC, Type | δH Mult (J in Hz) | δC, Type | δH Mult (J in Hz) |
| 1 | 114.1, C | 114.2, C | ||
| 2 | 154.5, C | 150.6, C | ||
| 3 | 134.1, C | 131.8, C | ||
| 4 | 154.6, C | 149.0, C | ||
| 4a | 117.2, C | 116.6, C | ||
| 5 | 184.3, C | 182.2, C | ||
| 6 | 151.4, C | 152.1, C | ||
| 7 | 119.4, C | 119.9, C | ||
| 8 | 135.9, C | 135.1, C | ||
| 9 | 132.1, C | 132.9, C | ||
| 9a | 131.1, C | 127.5, C | ||
| 10 | 14.7, CH3 | 2.07, s | 14.9, CH3 | 2.10, s |
| 11 | 75.6, CH2 | 4.97, s | 75.5, CH2 | 4.97, s |
| 12 | 78.0, CH2 | 5.10, s | 78.2, CH2 | 5.13, s |
| 13 | 15.1, CH3 | 2.17, s | 15.1, CH3 | 2.19, s |
| 2-OH | 10.22, brs | 10.08, s | ||
| 3-OMe/-OH | 59.8, CH3 | 3.80, s | 9.50, s | |
| 4-OH | 14.71, s | 14.87, s | ||
| 6-OH | 9.46, s | 9.35, s | ||
| 3 | ||
|---|---|---|
| No | δC, Type | δH Mult (J in Hz) |
| 1 | 171.6, C | |
| 3 | 99.1, CH | 6.45, s |
| 3a | 138.5, C | |
| 4 | 114.0, C | |
| 5 | 153.0, C | |
| 6 | 143.8, C | |
| 7 | 135.1, C | |
| 7a | 104.8, C | |
| 7-CH3 | 10.6, CH3 | 2.17, s |
| Compound | DPPH· Scavenging Activity IC50 (μM) | ABTS· Scavenging Activity IC50 (μM) |
|---|---|---|
| 1 | 57.6 ± 1.1 | 46.4 ± 1.6 |
| 2 | 26.5 ± 1.0 | 29.2 ± 0.9 |
| 3 | 29.3 ± 1.5 | 23.7 ± 0.6 |
| 4 | 85.2 ± 4.1 | 43.1 ±1.0 |
| 5 | 16.5 ± 0.9 | 23.3 ± 0.6 |
| 6 | >100 | 93.5 ± 2.0 |
| 7 | 53.1 ± 0.7 | 24.0 ± 0.6 |
| 8 | 14.7 ± 0.4 | 18.8 ± 0.4 |
| 9 | >100 | >100 |
| 10 | >100 | >100 |
| ascorbic acid a | 20.1 ± 0.32 | 33.6 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, G.; Tan, Q.; Chen, Y.; Yang, W.; Zang, Z.; Jiang, H.; Chen, S.; Wang, B.; She, Z. Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3. Mar. Drugs 2021, 19, 395. https://doi.org/10.3390/md19070395
Zou G, Tan Q, Chen Y, Yang W, Zang Z, Jiang H, Chen S, Wang B, She Z. Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3. Marine Drugs. 2021; 19(7):395. https://doi.org/10.3390/md19070395
Chicago/Turabian StyleZou, Ge, Qi Tan, Yan Chen, Wencong Yang, Zhenming Zang, Hongming Jiang, Shenyu Chen, Bo Wang, and Zhigang She. 2021. "Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3" Marine Drugs 19, no. 7: 395. https://doi.org/10.3390/md19070395
APA StyleZou, G., Tan, Q., Chen, Y., Yang, W., Zang, Z., Jiang, H., Chen, S., Wang, B., & She, Z. (2021). Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3. Marine Drugs, 19(7), 395. https://doi.org/10.3390/md19070395