A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration
Abstract
:1. Extracellular Matrix (ECM)
2. Collagens Derived from Marine Sources
3. Identification of the Soft Coral Collagen Fibers
4. The Application of Marine Collagen Fibers as Scaffolding for Tissue Engineering
5. A Tailored Bio-Composite Composed of Natural Marine Components
6. Bio-Composite Production and Its Biocompatibility
7. A Bio-Composite for In Vivo Tissue Regeneration
8. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Exposito, J.Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of Collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef]
- Exposito, J.Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [Green Version]
- Exposito, J.-Y.; Lethias, C. Invertebrate and Vertebrate Collagens. In Biology of Extracellular Matrix; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–72. [Google Scholar]
- Ledger, P.W.; Franc, S. Calcification of the Collagenous Axial Skeleton of Veretillum Cynomorium Pall. (Cnidaria: Pennatulacea). Cell Tissue Res. 1978, 192, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, R.J.; Tsuzaki, M.; Watabe, N.; Mechanic, G.L. Collagen in the Spicule Organic Matrix of the Gorgonian Leptogorgia Virgulata. Biol. Bull. 1990, 179, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Blanquet, R.S.; Phelan, M.A. Collagenaceous, Thiol-Containing Proteins of Cnidarian Nematocysts: A Comparison of the Chemistry and Protein Distribution Patterns in Two Types of Cnidae. Comp. Biochem. Physiol. Part B Biochem. 1993, 106, 115–124. [Google Scholar] [CrossRef]
- Larkman, A.U. An Ultrastructural Study of Oocyte Growth within the Endoderm and Entry into the Mesoglea in Actinia Fragacea (Cnidaria, Anthozoa). J. Morphol. 1983, 178, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Larkman, A.U. The Fine Structure of Mitochondria and the Mitochondrial Cloud during Oogenesis on the Sea Anemone Actinia. Tissue Cell 1984, 16, 393–404. [Google Scholar] [CrossRef]
- Fowler, S.J.; Jose, S.; Zhang, X.; Deutzmann, R.; Sarras, M.P.; Boot-Handford, R.P. Characterization of Hydra Type IV Collagen: Type IV Collagen Is Essential for Head Regeneration and Its Expression Is up-Regulated upon Exposure to Glucose. J. Biol. Chem. 2000, 275, 39589–39599. [Google Scholar] [CrossRef] [Green Version]
- Gambini, C.; Abou, B.; Ponton, A.; Cornelissen, A.J.M. Micro- and Macrorheology of Jellyfish Extracellular Matrix. Biophys. J. 2012, 102, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gosline, J.M. Connective Tissue Mechanics of Metridium Senile. J. Exp. Biol. 1971, 55, 763–774. [Google Scholar] [CrossRef]
- Koehl, M.A.R. Mechanical Diversity of Connective Tissue of the Body Wall of Sea Anemones. J. Exp. Biol. 1977, 69, 107–125. [Google Scholar] [CrossRef]
- Tillet-Barret, E.; France, J.M.; France, S.; Garrone, R. Characterization of Heterotrimeric Collagen Molecules in a Sea-pen (Cnidaria, Octocorallia). Eur. J. Biochem. 1992, 203, 179–184. [Google Scholar] [CrossRef]
- Tillet, E.; Franc, J.M.; Franc, S.; Garrone, R. The Evolution of Fibrillar Collagens: A Sea-Pen Collagen Shares Common Features with Vertebrate Type V Collagen. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 113, 239–246. [Google Scholar] [CrossRef]
- Sella, I. Biological, Biochemical, and Mechanical Properties of Collagen Fibers of the Soft Coral Sarcophyton Ehrenbergi; Tel-Aviv University: Tel Aviv-Yafo, Israel, 2012. [Google Scholar]
- Benayahu, Y.; Benayahu, D.; Kashman, Y.; Rudi, A.; Lanir, Y.; Sella, I.; Raz, E. Coral-Derived Collagen and Methods of Farming Same. U.S. Patent 20110038914A1, 8 October 2011. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea; Australian Institute of Marine Science: Townsville, Australia, 2001. [Google Scholar]
- Benayahu, D.; Sharabi, M.; Pomeraniec, L.; Awad, L.; Haj-Ali, R.; Benayahu, Y. Unique Collagen Fibers for Biomedical Applications. Mar. Drugs 2018, 16, 102. [Google Scholar] [CrossRef] [Green Version]
- Mandelberg, Y.; Benayahu, D.; Benayahu, Y. Octocoral Sarcophyton Auritum Verseveldt & Benayahu, 1978: Microanatomy and Presence of Collagen Fibers. Biol. Bull. 2016, 230, 68–77. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef] [Green Version]
- Orgel, J.P.R.O.; Sella, I.; Madhurapantula, R.S.; Antipova, O.; Mandelberg, Y.; Kashman, Y.; Benayahu, D.; Benayahu, Y. Molecular and Ultrastructural Studies of a Fibrillar Collagen from Octocoral (Cnidaria). J. Exp. Biol. 2017, 220, 3327–3335. [Google Scholar] [CrossRef] [Green Version]
- Parry, D.A.D.; Craig, A.S. Growth and development of collagen fibrils in connective tissue. In Ultrastructure of the Connective Tissue Matrix; Springer: New York, NY, USA, 1984; pp. 34–64. [Google Scholar]
- Haj-Ali, R.; Benayahu, Y.; Benayahu, D.; Sasson-levi, A.; Sharabi, M. Composites Comprising Collagen Extracted from Sarcophyton sp. Coral. U.S. Patent 20150013299A1, 21 November.
- Sharabi, M.; Mandelberg, Y.; Benayahu, D.; Benayahu, Y.; Azem, A.; Haj-Ali, R. A New Class of Bio-Composite Materials of Unique Collagen Fibers. J. Mech. Behav. Biomed. Mater. 2014, 36, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, M.; Benayahu, D.; Benayahu, Y.; Isaacs, J.; Haj-Ali, R. Laminated Collagen-Fiber Bio-Composites for Soft-Tissue Bio-Mimetics. Compos. Sci. Technol. 2015, 117, 268–276. [Google Scholar] [CrossRef]
- Ruggiero, F.; Exposito, J.Y.; Bournat, P.; Gruber, V.; Perret, S.; Comte, J.; Olagnier, B.; Garrone, R.; Theisen, M. Triple Helix Assembly and Processing of Human Collagen Produced in Transgenic Tobacco Plants. FEBS Lett. 2000, 469, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Sharabi, M.; Varssano, D.; Eliasy, R.; Benayahu, Y.; Benayahu, D.; Haj-Ali, R. Mechanical Flexure Behavior of Bio-Inspired Collagen-Reinforced Thin Composites. Compos. Struct. 2016, 153, 392–400. [Google Scholar] [CrossRef]
- Pomeraniec, L.; Benayahu, D. Mesenchymal Cell Growth and Differentiation on a New Biocomposite Material: A Promising Model for Regeneration Therapy. Biomolecules 2020, 10, 458. [Google Scholar] [CrossRef] [Green Version]
- Benayahu, D.; Pomeraniec, L.; Shemesh, S.; Heller, S.; Rosenthal, Y.; Rath-Wolfson, L.; Benayahu, Y. Biocompatibility of a Marine Collagen-Based Scaffold in Vitro and in Vivo. Mar. Drugs 2020, 18, 420. [Google Scholar] [CrossRef]
- Chi-Rosso, G.; Gotwals, P.J.; Yang, J.; Ling, L.; Jiang, K.; Chao, B.; Baker, D.P.; Burkly, L.C.; Fawell, S.E.; Koteliansky, V.E. Fibronectin Type III Repeats Mediate RGD-Independent Adhesion and Signaling through Activated Β1 Integrins. J. Biol. Chem. 1997, 272, 31447–31452. [Google Scholar] [CrossRef] [Green Version]
- Guicheney, P.; Vignier, N.; Zhang, X.; He, Y.; Cruaud, C.; Frey, V.; Helbling-Leclerc, A.; Richard, P.; Estournet, B.; Merlini, L.; et al. PCR Based Mutation Screening of the Laminin A2 Chain Gene (LAMA2): Application to Prenatal Diagnosis and Search for Founder Effects in Congenital Muscular Dystrophy. J. Med. Genet. 1998, 35, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Tatham, A.S.; Shewry, P.R. Elastomeric Proteins: Biological Roles, Structures and Mechanisms. Trends Biochem. Sci. 2000, 25, 567–571. [Google Scholar] [CrossRef]
- In Jeong, S.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-Engineered Vascular Grafts Composed of Marine Collagen and PLGA Fibers Using Pulsatile Perfusion Bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef]
- Mauck, R.L.; Baker, B.M.; Nerurkar, N.L.; Burdick, J.A.; Li, W.J.; Tuan, R.S.; Elliott, D.M. Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration. Tissue Eng. Part B Rev. 2009, 15, 171–193. [Google Scholar] [CrossRef] [Green Version]
- Martina, M.; Subramanyam, G.; Weaver, J.C.; Hutmacher, D.W.; Morse, D.E.; Valiyaveettil, S. Developing Macroporous Bicontinuous Materials as Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 5609–5616. [Google Scholar] [CrossRef]
- Ehrlich, H.; Etnoyer, P.; Litvinov, S.D.; Olennikova, M.M.; Domaschke, H.; Hanke, T.; Born, R.; Meissner, H.; Worch, H. Biomaterial Structure in Deep-Sea Bamboo Coral (Anthozoa: Gorgonacea: Isididae): Perspectives for the Development of Bone Implants and Templates for Tissue Engineering. Mater. Werkst. 2006, 37, 552–557. [Google Scholar] [CrossRef]
- Mor-Yossef Moldovan, L.; Kislev, N.; Lustig, M.; Pomeraniec, L.; Benayahu, D. Biomechanical Stimulation Effects on the Metabolism of Adipocyte. J. Cell. Physiol. 2020, 235, 8702–8713. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In Vivo Comparison of Jellyfish and Bovine Collagen Sponges as Prototype Medical Devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Addad, S.; Exposito, J.-Y.; Ment Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Fu, J. Integrated Micro/Nanoengineered Functional Biomaterials for Cell Mechanics and Mechanobiology: A Materials Perspective. Adv. Mater. 2014, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Methodology Used | |
|---|---|
| Morphology | Text Reference |
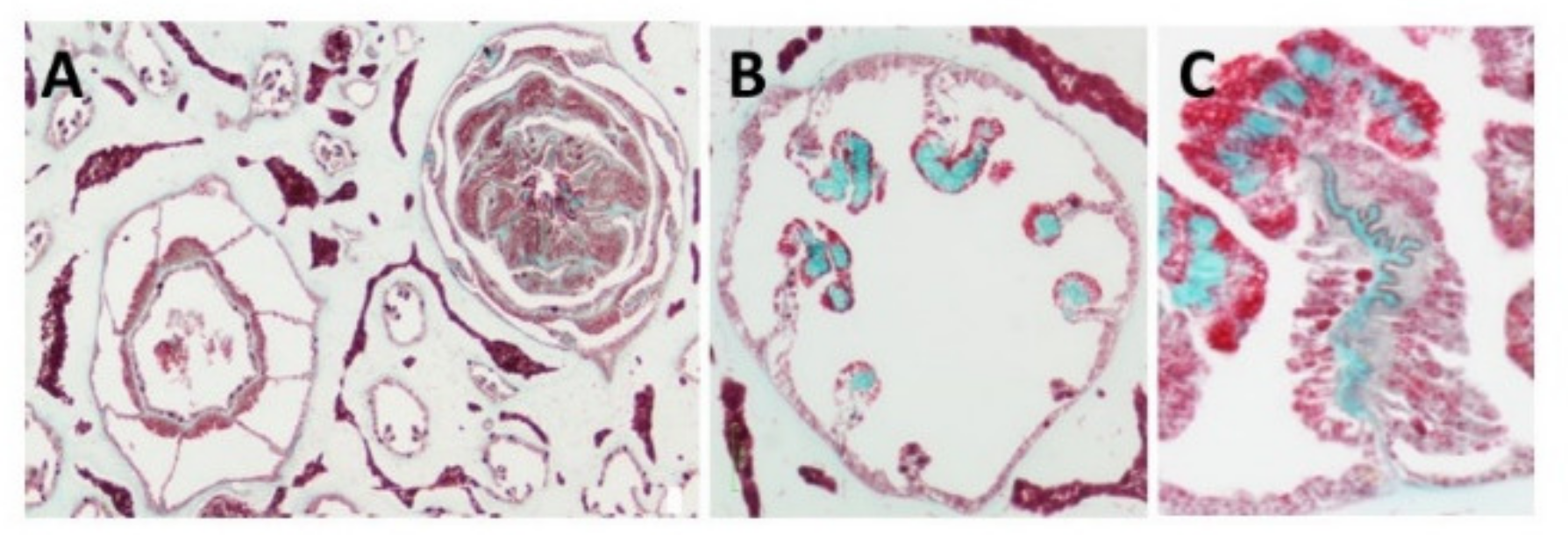
| Light microscopy confirming location and collagenous nature | [15,19] |
| Fluorescent microscopy | [18] |
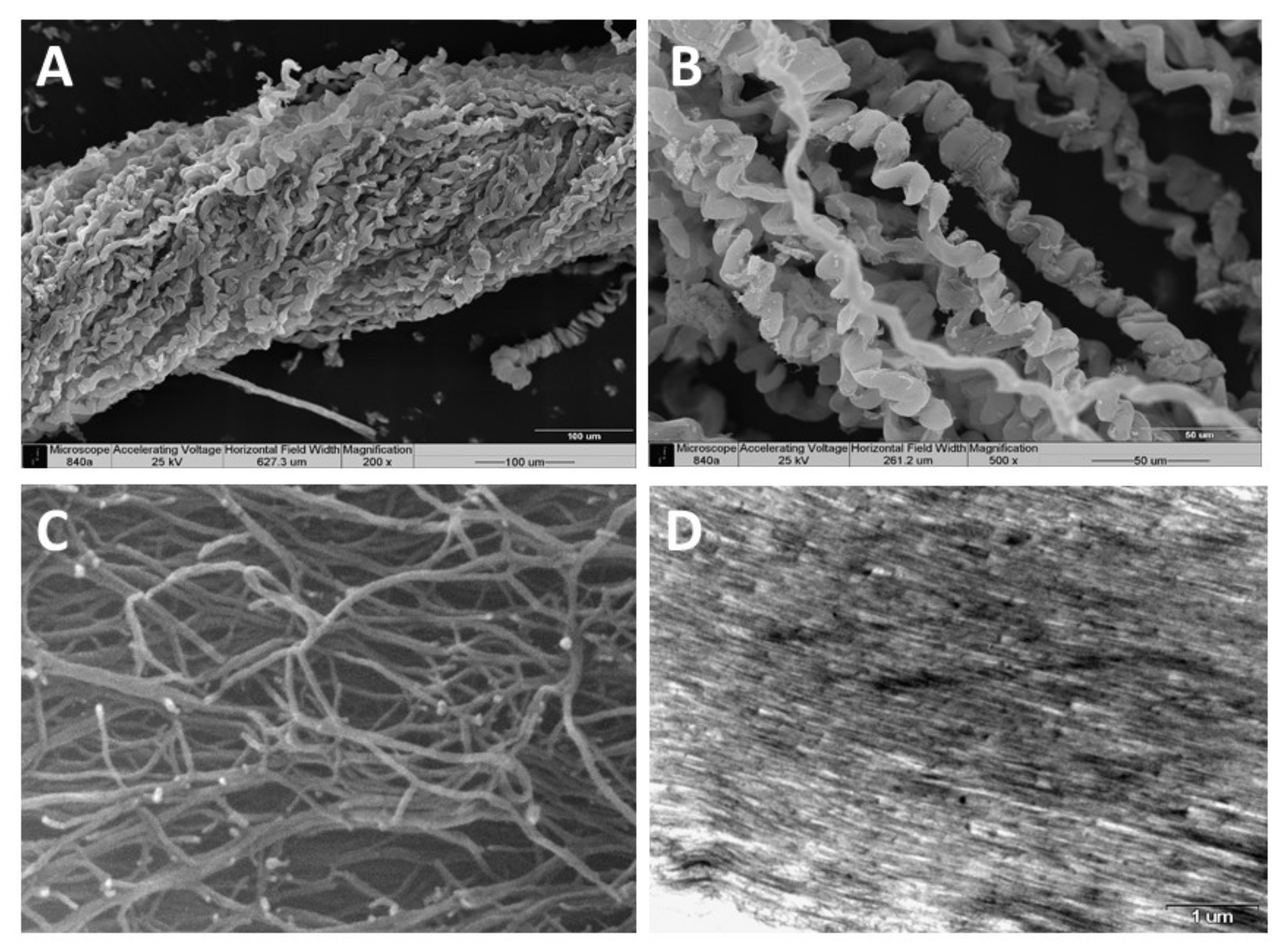
| Scanning Electron Microscopy (SEM) demonstrating fiber coiling | [15,19] |
| Environmental scanning electron microscopy (E–SEM) demonstrating fibrilar structure | [19] |
| Transmission electron microscopy (TEM) demonstrating fibrilar micro structure | [19,21] |
| Physical properties | |
| Fibers diameter 9–25 µm | [15,19] |
| Fibrils diameter 25 nm | [19] |
| DSC 68 °C | [15,16] |
| Molecular properties | |
| Histology staining (Masson Trichrome) | [15,19] |
| Nuclear magnetic resonance (NMR) revealing high levels of Glycine and Hydroxy-proline) | [15,16,21] |
| Maldi–TOF (MS/MS) sequence analysis | [21] |
| X-ray diffraction revealing (periodicity of 66 nm | [21] |
| Cytotoxicity and biocompability | |
| In vitro cell growth | [18,28] |
| In vivo transplanted scaffold | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benayahu, D.; Benayahu, Y. A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration. Mar. Drugs 2021, 19, 419. https://doi.org/10.3390/md19080419
Benayahu D, Benayahu Y. A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration. Marine Drugs. 2021; 19(8):419. https://doi.org/10.3390/md19080419
Chicago/Turabian StyleBenayahu, Dafna, and Yehuda Benayahu. 2021. "A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration" Marine Drugs 19, no. 8: 419. https://doi.org/10.3390/md19080419
APA StyleBenayahu, D., & Benayahu, Y. (2021). A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration. Marine Drugs, 19(8), 419. https://doi.org/10.3390/md19080419






