Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenotypic Characteristics of Strain NJES-13
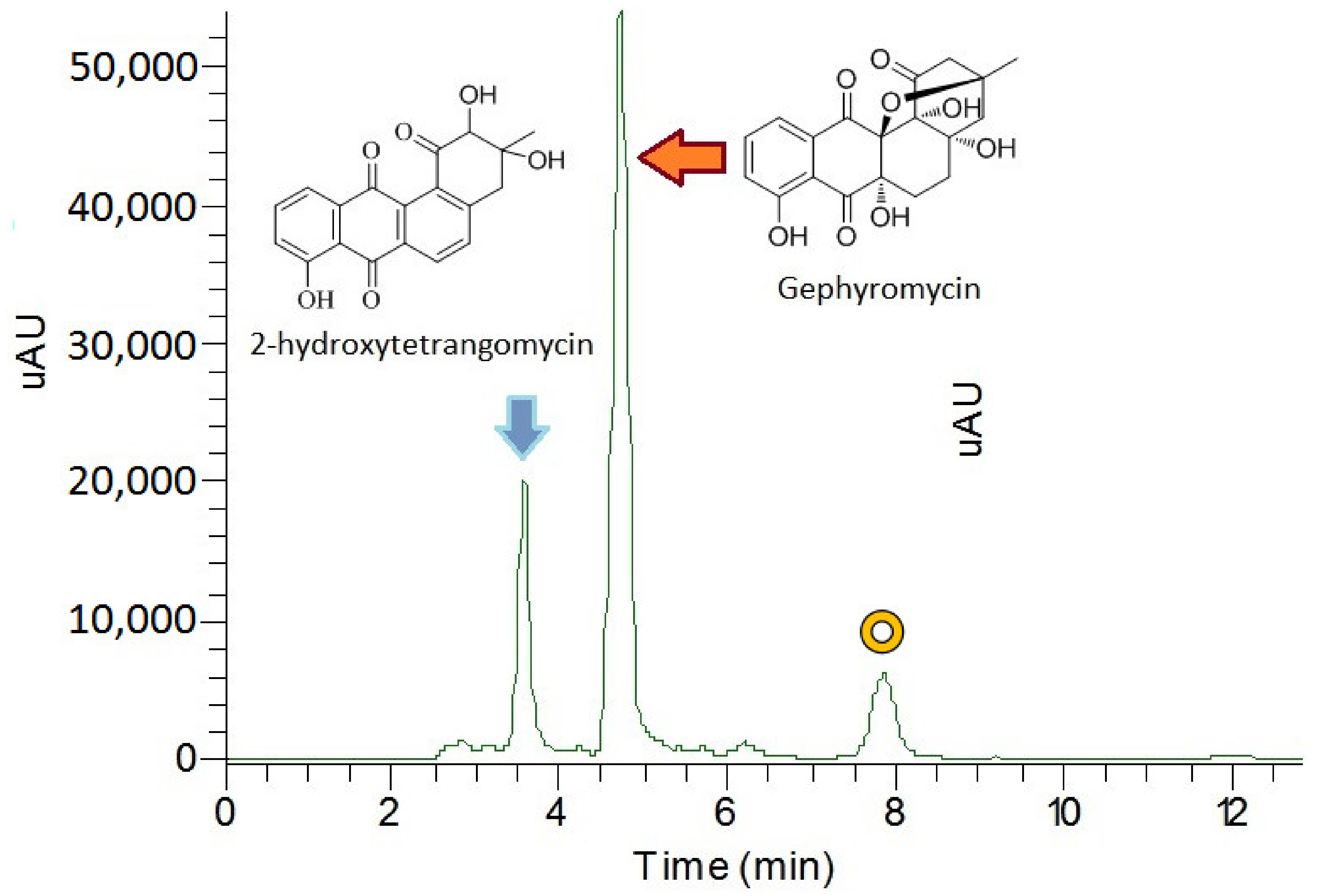
2.2. Gephyromycin Metabolites Characterization
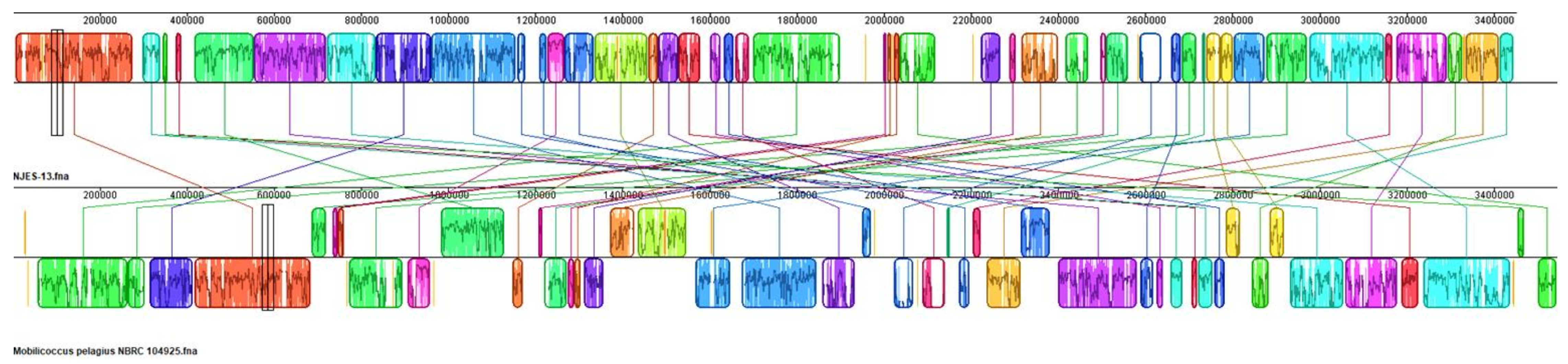
2.3. Whole-Genome Sequencing and Annotation
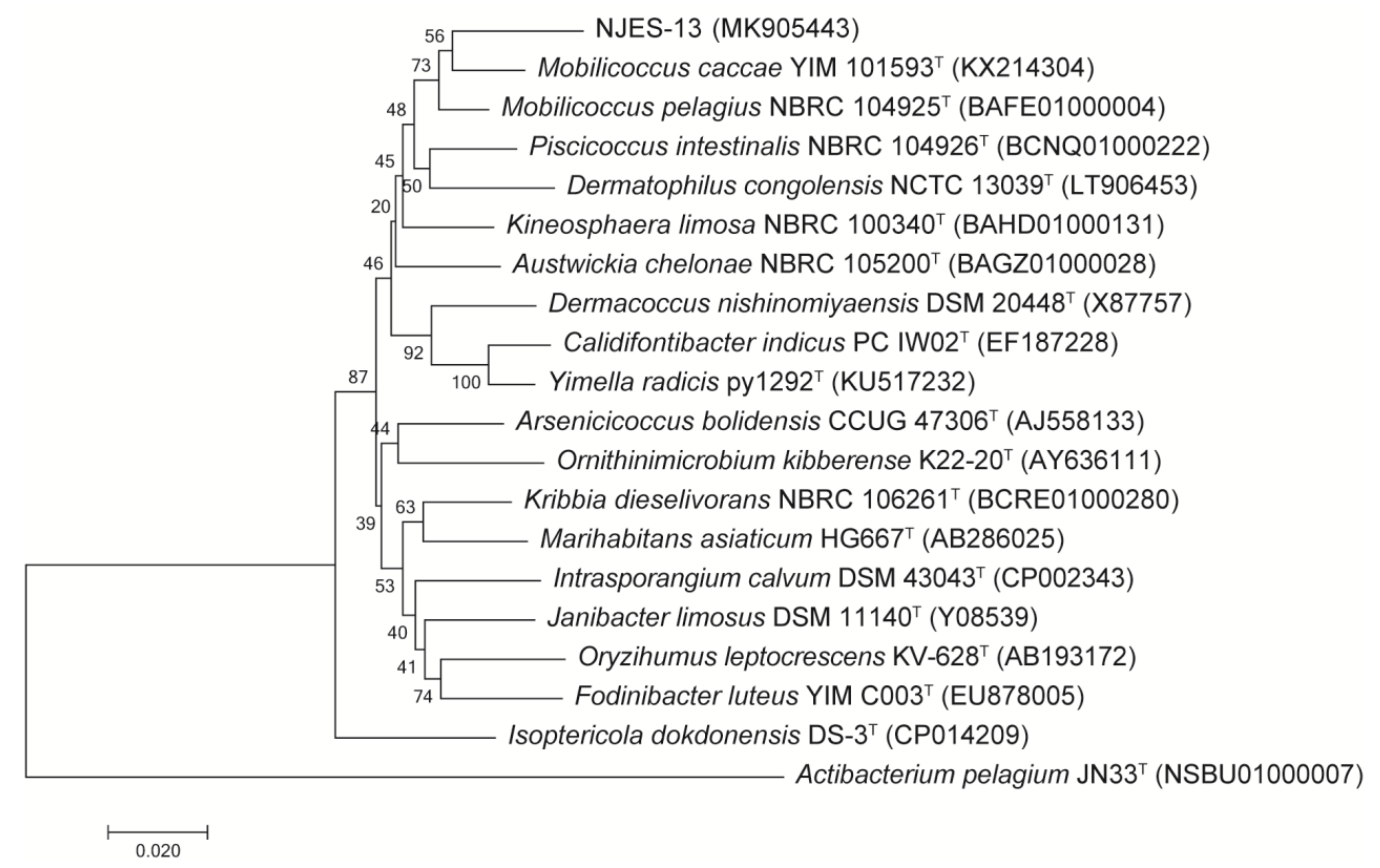
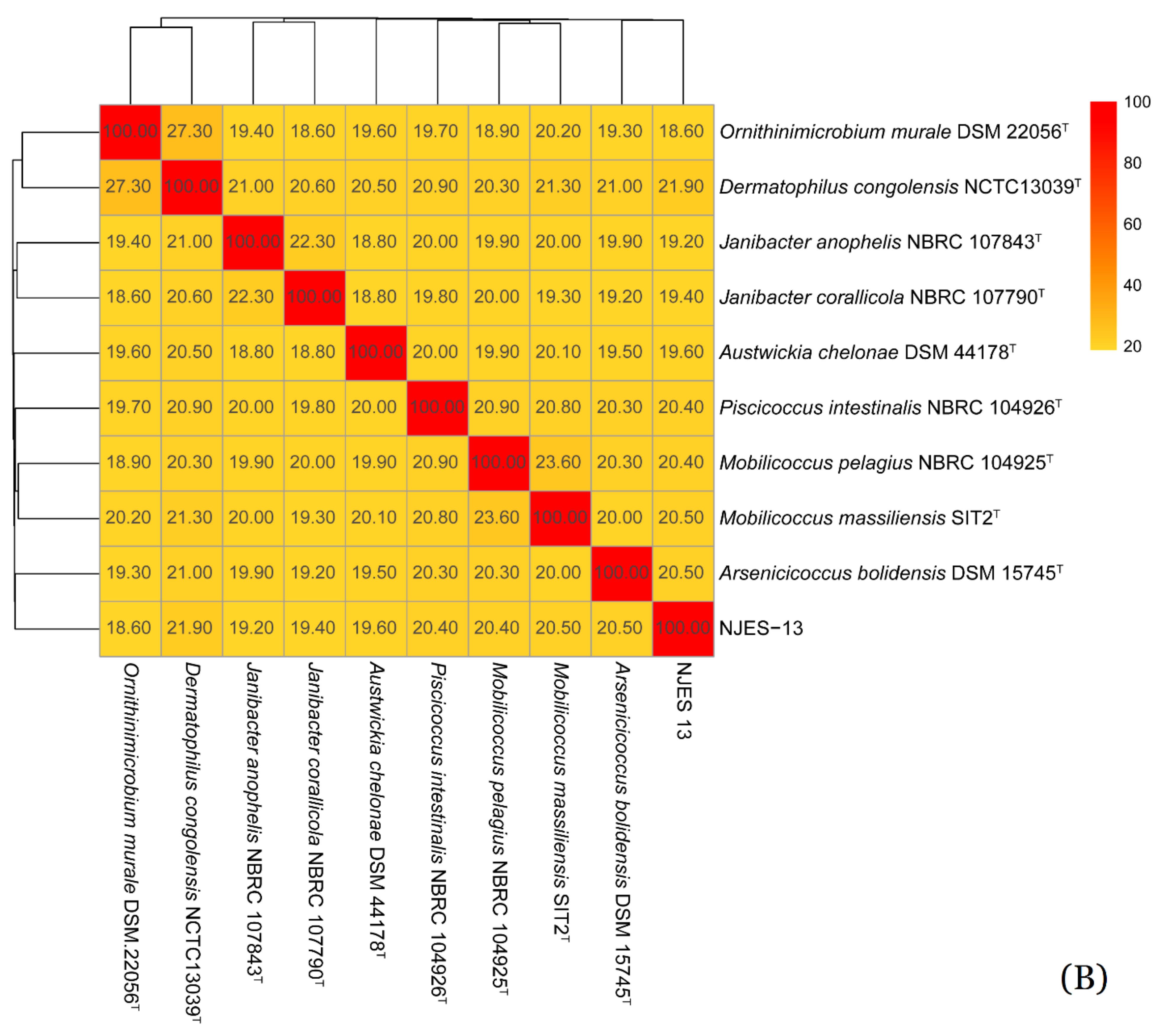
2.4. Phylogenetic Analysis
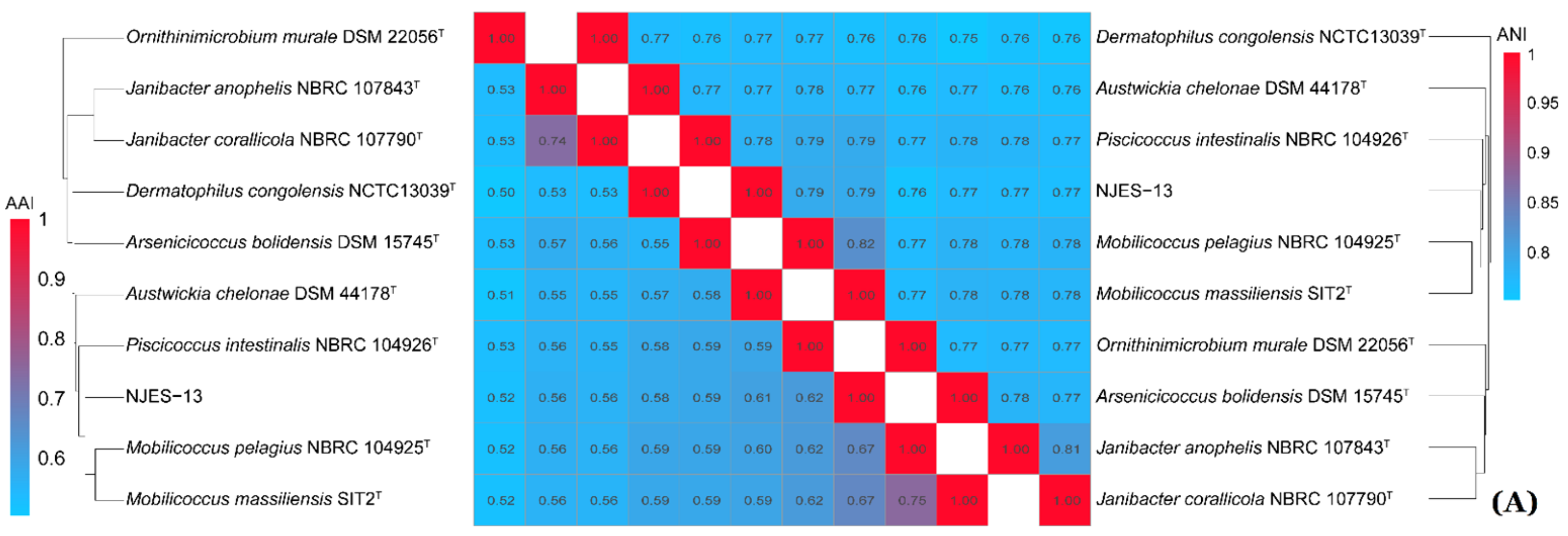
2.5. Phylogenomic Calculations
2.6. Bioflocculanting Potential of Bacterial EPS
2.7. Biosynthetic Genes Responsible for Active Metabolites
3. Materials and Methods
3.1. Bacterial Isolation and Culture
3.2. Phylogenetic Analysis of Bacterial 16S rRNA Genes
3.3. Bacterial Phenotypic Profile
3.4. Characterization of Secondary Metabolites
3.5. Whole-Genome Sequencing, Assembly and Annotation
3.6. Phylogenomic Analysis
3.7. Biosynthetic Gene Clusters Prediction
3.8. Bacterial EPS Bioflocculanting Activity Assay
3.9. GenBank Accession Numbers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiedler, H.P. Abyssomicins-A 20-Year Retrospective View. Mar. Drugs 2021, 19, 299. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Sipkema, D. Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products. Mar. Drugs 2019, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Landry, Z.C.; Vergin, K.; Mannenbach, C.; Block, S.; Yang, Q.; Blainey, P.; Carlson, C.; Giovannoni, S. Optofluidic Single-Cell Genome Amplification of Sub-micron Bacteria in the Ocean Subsurface. Front. Microbiol. 2018, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Jiao, J.Y.; Zhang, X.T.; Li, W.J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355. [Google Scholar] [CrossRef]
- Nouioui, I.; Carro, L.; García-López, M.; Meier-Kolthoff, J.P.; Woyke, T.; Kyrpides, N.C.; Pukall, R.; Klenk, H.P.; Goodfellow, M.; Göker, M. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 2018, 9, 2007. [Google Scholar] [CrossRef]
- Hamada, M.; Iino, T.; Iwami, T.; Harayama, S.; Tamura, T.; Suzuki, K. Mobilicoccus pelagius gen. nov., sp. nov. and Piscicoccus intestinalis gen. nov., sp. nov., two new members of the family Dermatophilaceae, and reclassification of Dermatophilus chelonae (Masters et al. 1995) as Austwickia chelonae gen. nov., comb. nov. J. Gen. Appl. Microbiol. 2010, 56, 427–436. [Google Scholar] [CrossRef]
- Azuma, R.; Ung-Bok, B.; Murakami, S.; Ishiwata, H.; Osaki, M.; Shimada, N.; Ito, Y.; Miyagawa, E.; Makino, T.; Kudo, T.; et al. Tonsilliphilus suis gen. nov., sp. nov., causing tonsil infections in pigs. Int. J. Syst. Evol. Microbiol. 2013, 63, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.A. The genus Dermatophilus. J. Bacteriol. 1964, 88, 509–522. [Google Scholar] [CrossRef]
- Collins, M.D.; Routh, J.; Saraswathy, A.; Lawson, P.A.; Schumann, P.; Welinder-Olsson, C.; Falsen, E. Arsenicicoccus bolidensis gen. nov., sp. nov., a novel actinomycete isolated from contaminated lake sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 605–608. [Google Scholar] [CrossRef][Green Version]
- Liu, W.T.; Hanada, S.; Marsh, T.L.; Kamagata, Y.; Nakamura, K. Kineosphaera gen. nov., sp. nov., a novel Gram-positive polyhydroxyalkanoate-accumulating coccus isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2002, 52, 1845–1849. [Google Scholar]
- Abdelmohsen, U.R.; Yang, C.; Horn, H.; Hajjar, D.; Ravasi, T.; Hentschel, U. Actinomycetes from Red Sea sponges: Sources for chemical and phylogenetic diversity. Mar. Drugs 2014, 12, 2771–2789. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Wingender, J.; Neu, T.R.; Flemming, H.C. What are bacterial extracellular polymer substances? In Microbial Extracellular Polymer Substance; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Bozal, N.; Tudela, E.; Rossello-Mora, R.; Lalucat, J.; Guinea, J. Pseudoalteromonas antartica sp. nov., isolated from an Antarctica coastal environment. Int. J. Syst. Bacteriol. 1997, 47, 345–351. [Google Scholar] [CrossRef]
- Poli, A.; Schiano Moriello, V.; Esposito, E.; Lama, L.; Gambacorta, A.; Nicolaus, B. Exopolysaccharide production by a new Halomonas strain CRSS isolated from saline lake Cape Russell in Antarctica growing on complex and defined media. Biotechnol. Lett. 2004, 26, 1635–1638. [Google Scholar] [CrossRef]
- Poli, A.; Esposito, E.; Orlando, P.; Lama, L.; Giordano, A.; de Appolonia, F.; Nicolaus, B.; Gambacorta, A. Halomonas alkaliantarctica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium. Syst. Appl. Microbiol. 2007, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Liu, R.; Wei, M.; Zhang, J.; Sun, C. EPS364, a Novel Deep-Sea Bacterial Exopolysaccharide, Inhibits Liver Cancer Cell Growth and Adhesion. Mar. Drugs 2021, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Zarandona, I.; Estupiñán, M.; Pérez, C.; Alonso-Sáez, L.; Guerrero, P.; de la Caba, K. Chitosan Films Incorporated with Exopolysaccharides from Deep Seawater Alteromonas Sp. Mar. Drugs 2020, 18, 447. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Jiang, Z.; Wu, Z.; Sheng, Z.; Yang, X.; Sun, J.; Zhang, X.; Yang, Q.; Yu, X.; Yan, J. Limnobacter alexandrii sp. nov., a thiosulfate-oxidizing, heterotrophic and EPS-bearing Burkholderiaceae isolated from cultivable phycosphere microbiota of toxic Alexandrium catenella LZT09. Antonie Van Leeuwenhoek 2020, 113, 1689–1698. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.X.; Ge, Y.M.; Iqbal, M.N.; Yang, X.; Cui, Z.D.; Yang, Q. Sphingopyxis microcytisis sp. nov., a novel bioactive exopolysaccharides-bearing Sphingomonadaceae isolated from the Microcytis phycosphere. Antonie Van Leeuwenhoek 2021, 114, 845–857. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, Y.M.; Iqbal, N.M.; Yang, X.; Zhang, X.L. Sulfitobacter alexandrii sp. nov., a new microalgae growth-promoting bacterium with exopolysaccharides bioflocculanting potential isolated from marine phycosphere. Antonie Van Leeuwenhoek 2021, 114, 1091–1106. [Google Scholar] [CrossRef]
- Gerhard, B.; Gerhard, L.; Kadja, M.; Andreas, H.; Tobias, A.M.G.; Anke, D.; Alan, T.B.; James, E.M.S.; Niko, K.; Werner, E.G.M. Gephyromycin, the first bridged angucyclinone, from Streptomyces griseus strain NTK 14. Phytochemistry 2005, 66, 1366–1373. [Google Scholar]
- Kern, D.L.; Schaumberg, J.P.; Hokanson, G.C.; French, J.C. PD 116,779, a new antitumor antibiotic of the benz[a]anthraquinone class. J. Antibiot. 1986, 39, 469–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, W.J.; Ji, Y.Y.; Jiang, Y.J.; Ying, W.J.; Fang, Z.Y.; Gao, T.T. Gephyromycin C, a novel small-molecule inhibitor of heat shock protein Hsp90, induces G2/M cell cycle arrest and apoptosis in PC3 cells in vitro. Biochem. Biophys. Res. Commun. 2020, 531, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Xie, Z.; Zhang, S.; Wu, Y.; Ge, Y.; Zhang, X. Haliea alexandrii sp. nov., isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1133–1138. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.W.; Zhang, J.; Zhou, X.; Zhang, X.L.; Wang, L.; Yu, T.; Wang, Z.; Bei, J.; Dong, B. Mesorhizobium alexandrii sp. nov., isolated from phycosphere microbiota of PSTs-producing marine dinoflagellate Alexandrium minutum amtk4. Antonie Van Leeuwenhoek 2020, 113, 907–917. [Google Scholar] [CrossRef]
- Zhang, X.L.; Qi, M.; Li, Q.H.; Cui, Z.D.; Yang, Q. Maricaulis alexandrii sp. nov., a novel active bioflocculants-bearing and dimorphic prosthecate bacterium isolated from marine phycosphere. Antonie Van Leeuwenhoek 2021, 114, 1195–1203. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.A.; Jiang, Z.W.; Yang, X.; Zhang, X.L.; Yang, Q. Combined characterization of a new member of Marivita cryptomonadis, strain LZ-15-2 isolated from cultivable phycosphere microbiota of toxic HAB dinoflagellate Alexandrium catenella LZT09. Braz. J. Microbiol. 2021, 52, 739–748. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 12, gkab335. [Google Scholar]
- Martinelli, L.; Redou, V.; Cochereau, B.; Delage, L.; Hymery, N.; Poirier, E.; Le Meur, C.; Le Foch, G.; Cladiere, L.; Mehiri, M.; et al. Identification and Characterization of a New Type III Polyketide Synthase from a Marine Yeast, Naganishia uzbekistanensis. Mar. Drugs 2020, 18, 637. [Google Scholar] [CrossRef] [PubMed]
- Atencio, L.A.; Boya, P.C.A.; Martin, H.C.; Mejía, L.C.; Dorrestein, P.C.; Gutiérrez, M. Genome Mining, Microbial Interactions, and Molecular Networking Reveals New Dibromoalterochromides from Strains of Pseudoalteromonas of Coiba National Park-Panama. Mar. Drugs 2020, 18, 456. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, J.; Li, T.; Li, H.; Liu, Z.; Dong, Y.; Li, W. An Unusual Type II Polyketide Synthase System Involved in Cinnamoyl Lipid Biosynthesis. Angew. Chem. Int. Ed. Engl. 2021, 60, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Prichula, J.; Primon-Barros, M.; Luz, R.C.Z.; Castro, Í.M.S.; Paim, T.G.S.; Tavares, M.; Ligabue-Braun, R.; d’Azevedo, P.A.; Frazzon, J.; Frazzon, A.P.G.; et al. Genome Mining for Antimicrobial Compounds in Wild Marine Animals-Associated Enterococci. Mar. Drugs 2021, 19, 328. [Google Scholar] [CrossRef] [PubMed]
- Simó-Cabrera, L.; García-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Agüero, A.; Monzó Sánchez, F.; Cánovas, V.; et al. Haloarchaea as Cell Factories to Produce Bioplastics. Mar. Drugs 2021, 19, 159. [Google Scholar] [CrossRef]
- Giubilini, A.; Bondioli, F.; Messori, M.; Nyström, G.; Siqueira, G. Advantages of additive manufacturing for biomedical applications of polyhydroxyalkanoates. Bioengineering 2021, 8, 29. [Google Scholar] [CrossRef]
- Yang, Q. Taxonomic Identification and Bioactivity Screening of the Symbiotic Bacteria Strains of Euphausia Superba from Antarctic Ocean. Appl. Mech. Mater. 2013, 295–298, 173–177. [Google Scholar] [CrossRef]
- Yang, Q.; Feng, Q.; Zhang, B.P.; Gao, J.J.; Sheng, Z.; Xue, Q.P.; Zhang, X.L. Marinobacter alexandrii sp. nov., a novel yellow-pigmented and algae growth-promoting bacterium isolated from marine phycosphere microbiota. Antonie Van Leeuwenhoek 2021, 114, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, X.L.; Li, L.; Zhang, R.N.; Feng, L.J.; Mu, J. Ponticoccus alexandrii sp. nov., a novel bacterium isolated from the marine toxigenic dinoflagellate Alexandrium minutum. Antonie Van Leeuwenhoek 2018, 11, 995–1000. [Google Scholar]
- Yang, Q.; Jiang, Z.; Zhou, X.; Xie, Z.; Wang, Y.; Wang, D.; Feng, L.; Yang, G.; Ge, Y.; Zhang, X. Saccharospirillum alexandrii sp. nov., isolated from the toxigenic marine dinoflagellate Alexandrium catenella LZT09. Int. J. Syst. Evol. Microbiol. 2020, 70, 820–826. [Google Scholar] [CrossRef]
- Madritsch, S.; Burg, A.; Sehr, E.M. Comparing de novo transcriptome assembly tools in di-and autotetraploid non-model plant species. BMC Bioinform. 2021, 22, 146. [Google Scholar] [CrossRef]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome. Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, R.; Iqbal, N.M.; Duan, Y.H.; Zhang, X.A.; Wang, L.; Yu, L.Z.; Li, J.Z.; Sun, M.F.; Yang, Q.; et al. Marinobacter shengliensis subsp. alexandrii Subsp. Nov., Isolated from Cultivable Phycosphere Microbiota of Highly Toxic Dinoflagellate Alexandrium catenella LZT09 and Description of Marinobacter shengliensis Subsp. shengliensis Subsp. Nov. Curr. Microbiol. 2021, 78, 1648–1655. [Google Scholar] [PubMed]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Wu, Y.; Lou, L.; Ma, Z.; Wang, D.; Ge, Y.; Zhang, X.; et al. Nioella ostreopsis sp. nov., isolated from toxic dinoflagellate, Ostreopsis lenticularis. Int. J. Syst. Evol. Microbiol. 2020, 70, 759–765. [Google Scholar] [CrossRef]
- Liu, J.Z.; Yang, J.S.; Ge, Y.M.; Yang, Q.; Sun, J.Y.; Yu, X. Acute effects of CH3NH3PbI3 perovskite on Scenedesmus obliquus and Daphnia magana in aquatic environment. Ecotoxicol. Environ. Saf. 2021, 208, 111677. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.-M.; Xie, P.-F.; Zhang, X.-L.; Yang, Q. Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin. Mar. Drugs 2021, 19, 458. https://doi.org/10.3390/md19080458
Gao H-M, Xie P-F, Zhang X-L, Yang Q. Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin. Marine Drugs. 2021; 19(8):458. https://doi.org/10.3390/md19080458
Chicago/Turabian StyleGao, Hui-Min, Peng-Fei Xie, Xiao-Ling Zhang, and Qiao Yang. 2021. "Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin" Marine Drugs 19, no. 8: 458. https://doi.org/10.3390/md19080458
APA StyleGao, H.-M., Xie, P.-F., Zhang, X.-L., & Yang, Q. (2021). Isolation, Phylogenetic and Gephyromycin Metabolites Characterization of New Exopolysaccharides-Bearing Antarctic Actinobacterium from Feces of Emperor Penguin. Marine Drugs, 19(8), 458. https://doi.org/10.3390/md19080458






