Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications
Abstract
1. Introduction
2. Extraction Technologies
2.1. Solid–Liquid Extraction
2.2. Enzyme-Assisted Extraction
2.3. Pulse-Electric Field Assisted Extraction
2.4. High Hydrostatyc Pressure Extraction
2.5. Ultrasound-Assisted Extraction
2.6. Microwave-Assisted Extraction
3. Protein Purification
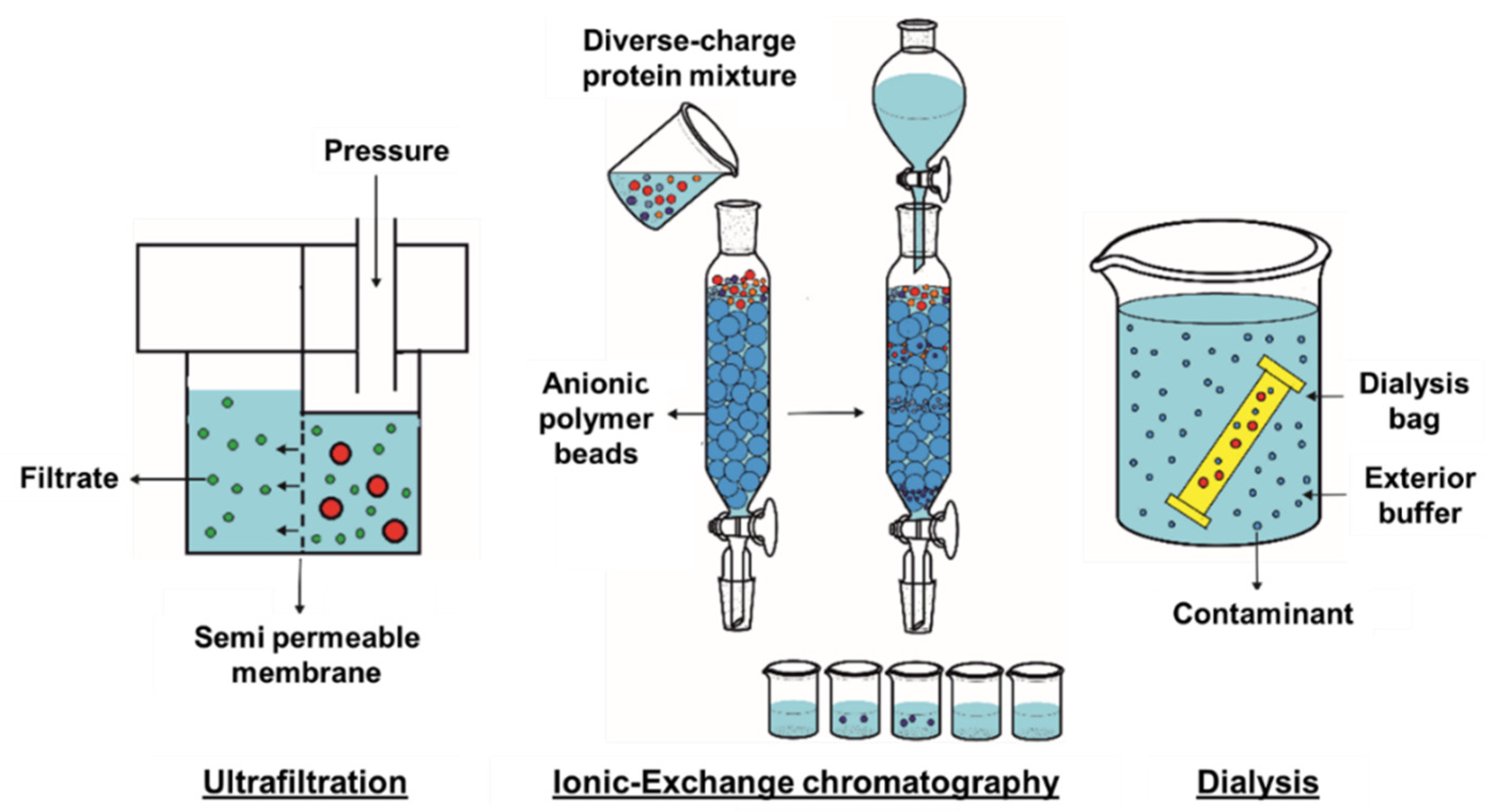
3.1. Ultrafiltration
3.2. Ionic-Exchange Chromatography
3.3. Dyalisis
4. Hydrolysis and Peptide Production
5. Bioactive Properties and Applications of Seaweed Proteins and Derived Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SP | Seaweed proteins |
| dw | Dry weight |
| SLE | Solid–liquid extraction |
| HAE | Heat-assisted extraction |
| EAE | Enzyme-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| MAE | Microwave-assisted extraction |
| PEF | Pulse-electric field |
| HHP | High-hydrostatic pressure |
| HHPE | High-hydrostatic pressure extraction |
| HAE | Heat-assisted extraction |
| EAE | Enzyme-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| MAE | Microwave-assisted extraction |
| IP | Isoelectric point |
| Pr | Precipitation |
| Pu | Purification |
| DI | Dialysis |
| OS | Osmotic shock |
| Epr | Enzymatic pretreatment |
| Upr | Ultrasound pretreatment |
| dW | Deionized water |
| AK | Alkaline |
| MAAs | Mycosporine-like amino acids |
| EAA | Essential amino acids |
| GRAS | Generally recognized as safe |
| RAAS | Renin–angiotensin–aldosterone system |
| IC50 | Half inhibitory concentration |
| DPP-IV | Dipeptidyl peptidase IV |
| MCF-7 | Human breast cancer cell line |
| HepG2 | Human liver cancer cell line |
| SGC-7901 | Human gastric cancer cell line |
| A549 | Human lung cancer cell line |
| HT-29 | Human colon cancer cell line |
| UF | Ultrafiltration |
| IEC | Ionic-exchange chromatography |
| BAP | Bioactive peptide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACE | Angiotensin I converter enzyme |
References
- Carpena, M.; Caleja, C.; Pereira, E.; Pereira, C.; Ćirić, A.; Soković, M.; Soria-Lopez, A.; Fraga-Corral, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; et al. Red Seaweeds as a Source of Nutrients and Bioactive Compounds: Optimization of the Extraction. Chemosensors 2021, 9, 132. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Solutions for the sustainability of the food production and consumption system. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current knowledge and challenges in extraction, characterization and bioactivity of seaweed protein and seaweed-derived proteins. In Advances in Botanical Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 95, pp. 289–326. ISBN 9780081027103. [Google Scholar]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed Proteins, 2nd ed.; Yada, R.Y.B.T.-P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081007297. [Google Scholar]
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 321–343. ISBN 9780124080621. [Google Scholar]
- Batista, S.; Pereira, R.; Oliveira, B.; Baião, L.F.; Jessen, F.; Tulli, F.; Messina, M.; Silva, J.L.; Abreu, H.; Valente, L.M.P. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycol. 2020, 32, 2041–2059. [Google Scholar] [CrossRef]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.Ó.; Friðjónsson, Ó.H.; Jónsdóttir, R. Palmaria palmata as an alternative protein source: Enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor. J. Appl. Phycol. 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.; Yang, Q.; Wang, S. Preparation, characterization of food grade phycobiliproteins from Porphyra haitanensis and the application in liposome-meat system. LWT 2017, 77, 468–474. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; Doran, L.; Auty, M.; Prieto, J.; Hayes, M. Increasing the health benefits of bread: Assessment of the physical and sensory qualities of bread formulated using a renin inhibitory Palmaria palmata protein hydrolysate. LWT-Food Sci. Technol. 2014, 56, 398–405. [Google Scholar] [CrossRef]
- Deniaud, E.; Fleurence, J.; Lahaye, M. Preparation and chemical characterization of cell wall fractions enriched in structural proteins from Palmaria palmata (Rhodophyta). Bot. Mar. 2003, 46, 366–377. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Harnedy-Rothwell, P.A.; Lafferty, R.A.; Sharkey, S.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Macroalgal protein hydrolysates from Palmaria palmata influence the ‘incretin effect’ in vitro via DPP-4 inhibition and upregulation of insulin, GLP-1 and GIP secretion. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Mao, X.; Zhang, X. Novel peptides with anti-proliferation activity from the Porphyra haitanesis hydrolysate. Process Biochem. 2017, 60, 98–107. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from macroalgae and its applications in the cosmetic industry: A circular economy approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A. Functional Protein Concentrates Extracted from the Green Marine Macroalga Ulva sp., by High Voltage Pulsed Electric Fields and Mechanical Press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- O’ Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of Protein from Four Different Seaweeds Using Three Different Physical Pre-Treatment Strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef]
- Mendez, R.L.; Kwon, J.Y. Effect of extraction condition on protein recovery and phenolic interference in Pacific dulse (Devaleraea mollis). J. Appl. Phycol. 2021, 33, 2497–2509. [Google Scholar] [CrossRef]
- Wong, K.; Chikeung Cheung, P. Influence of drying treatment on three Sargassum species 2. Protein extractability, in vitro protein digestibility and amino acid profile of protein concentrates. J. Appl. Phycol. 2001, 13, 51–58. [Google Scholar] [CrossRef]
- Angell, A.R.; Paul, N.A.; de Nys, R. A comparison of protocols for isolating and concentrating protein from the green seaweed Ulva ohnoi. J. Appl. Phycol. 2017, 29, 1011–1026. [Google Scholar] [CrossRef]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal Res. 2020, 47, 101849. [Google Scholar] [CrossRef]
- Veide Vilg, J.; Undeland, I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima—effects of osmotic shock, water volume and temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Lopez-Alonso, M.; Hayes, M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) S. F. Gray. Food Res. Int. 2017, 99, 971–978. [Google Scholar] [CrossRef]
- Wijesekara, I.; Lang, M.; Marty, C.; Gemin, M.P.; Boulho, R.; Douzenel, P.; Wickramasinghe, I.; Bedoux, G.; Bourgougnon, N. Different extraction procedures and analysis of protein from Ulva sp. in Brittany, France. J. Appl. Phycol. 2017, 29, 2503–2511. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, K.; Burlot, A.S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Suwal, S.; Perreault, V.; Marciniak, A.; Tamigneaux, É.; Deslandes, É.; Bazinet, L.; Jacques, H.; Beaulieu, L.; Doyen, A. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng. 2019, 252, 53–59. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Wen, L.; Tan, S.; Zeng, L.; Wang, Z.; Ke, X.; Zhang, Z.; Tang, H.; Guo, H.; Xia, E. Ultrasound-assisted extraction and in vitro simulated digestion of Porphyra haitanensis proteins exhibiting antioxidative and α-glucosidase inhibitory activity. J. Food Meas. Charact. 2020, 14, 3291–3298. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Magnusson, M.; Glasson, C.R.K.; Vucko, M.J.; Angell, A.; Neoh, T.L.; de Nys, R. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Res. 2019, 41, 101555. [Google Scholar] [CrossRef]
- Kandasamy, G.; Karuppiah, S.K.; Rao, P.V.S. Salt- and pH-induced functional changes in protein concentrate of edible green seaweed Enteromorpha species. Fish. Sci. 2012, 78, 169–176. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Fátima Barroso, M.; Simal-Gandara, J.; et al. Antibacterial use of macroalgae compounds against foodborne pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.I.O.; Rastogi, N.K.; Angersbach, A.; Knorr, D. Combined effects of pulsed electric field pre-treatment and partial osmotic dehydration on air drying behaviour of red bell pepper. J. Food Eng. 2003, 60, 89–98. [Google Scholar] [CrossRef]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. Towards marine biorefineries: Selective proteins extractions from marine macroalgae Ulva with pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 37, 194–200. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Park, S.I.; Lee, Y.S. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int. J. Biol. Macromol. 2017, 99, 506–510. [Google Scholar] [CrossRef]
- Janson, J.-C. Protein Purification: Principles, High Resolution Methods, and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 54, ISBN 9780471746614. [Google Scholar]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Chaturvedi, B.K.; Ghosh, A.K.; Ramachandhran, V.; Trivedi, M.K.; Hanra, M.S.; Misra, B.M. Preparation, characterization and performance of polyethersulfone ultrafiltration membranes. Desalination 2001, 133, 31–40. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnolo. Biol. Med. 2015, 11, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.; Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Drago, S.; Sánchez de Medina, F.; Martínez-Augustin, O. Green Alga Ulva spp. Hydrolysates and Their Peptide Fractions Regulate Cytokine Production in Splenic Macrophages and Lymphocytes Involving the TLR4-NFκB/MAPK Pathways. Mar. Drugs 2018, 16, 235. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Levison, P.R. Large-scale ion-exchange column chromatography of proteins. J. Chromatogr. B 2003, 790, 17–33. [Google Scholar] [CrossRef]
- Cutler, P. Protein Purification Protocols, 2nd ed.; Humana Press Inc.: Totowa, NJ, USA, 2003; Volume 244, ISBN 1-59259-655-X. [Google Scholar]
- Rathore, A.S.; Kumar, D.; Kateja, N. Recent developments in chromatographic purification of biopharmaceuticals. Biotechnol. Lett. 2018, 40, 895–905. [Google Scholar] [CrossRef]
- Singh, C.; Sharma, C.S.; Kamble, P.R. Amino acid analysis using ion-exchange chromatography: A review. Int. J. Pharmacogn. 2014, 3, 3559–3567. [Google Scholar] [CrossRef]
- Lenhoff, A.M. Ion-exchange chromatography of proteins: The inside story. Mater. Today Proc. 2016, 3, 3559–3567. [Google Scholar] [CrossRef]
- Ljunglöf, A.; Lacki, K.M.; Mueller, J.; Harinarayan, C.; van Reis, R.; Fahrner, R.; Van Alstine, J.M. Ion exchange chromatography of antibody fragments. Biotechnol. Bioeng. 2007, 96, 515–524. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, Y.H.; Lee, J.I.; Kim, I.H.; Nam, T.J. Antioxidant Activity of Oxygen Evolving Enhancer Protein 1 Purified from Capsosiphon fulvescens. J. Food Sci. 2015, 80, H1412–H1417. [Google Scholar] [CrossRef]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the Nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Tan, Z.; Li, F.; Xu, X.; Xing, J. Simultaneous extraction and purification of aloe polysaccharides and proteins using ionic liquid based aqueous two-phase system coupled with dialysis membrane. Desalination 2012, 286, 389–393. [Google Scholar] [CrossRef]
- Whitford, D. Proteins: Structure and Function; John Wiley & Sons: Chichester, UK, 2005; ISBN 1118685725. [Google Scholar]
- Filoti, D.I.; Shire, S.J.; Yadav, S.; Laue, T.M. Comparative Study of Analytical Techniques for Determining Protein Charge. J. Pharm. Sci. 2015, 104, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and Characterization of Bioactive Pro-Peptides with in Vitro Renin Inhibitory Activities from the Macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Tsugita, A.; Scheffler, J.-J. A Rapid Method for Acid Hydrolysis of Protein with a Mixture of Trifluoroacetic Acid and Hydrochloric Acid. Eur. J. Biochem. 2005, 124, 585–588. [Google Scholar] [CrossRef]
- Provansal, M.M.P.; Cuq, J.L.A.; Cheftel, J.C. Chemical and nutritional modifications of sunflower proteins due to alkaline processing. Formation of amino acid crosslinks and isomerization of lysine residues. J. Agric. Food Chem. 1975, 23, 938–943. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Amid, A.; Alam, Z.; Asiyanbi-H, T.T.; Ramli, N. Enzymatic Hydrolysis of Plants and Algae for Extraction of Bioactive Compounds. Food Rev. Int. 2013, 29, 352–370. [Google Scholar] [CrossRef]
- Castro, H.C.; Abreu, P.A.; Geraldo, R.B.; Martins, R.C.A.; dos Santos, R.; Loureiro, N.I.V.; Cabral, L.M.; Rodrigues, C.R. Looking at the proteases from a simple perspective. J. Mol. Recognit. 2011, 24, 165–181. [Google Scholar] [CrossRef]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a Renin Inhibitory Peptide from the Red Seaweed Palmaria palmata as a Functional Food Ingredient Following Confirmation and Characterization of a Hypotensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; O’Keeffe, M.B.; Fitzgerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; Sánchez De Medina, F.; Martínez-Augustin, O. A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem. 2012, 134, 1982–1990. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Harnedy-Rothwell, P.A.; Fernandes, E.; Alves, R.C.; Oliveira, M.B.P.P.; FitzGerald, R.J. Enzymatic Modification of Porphyra dioica-Derived Proteins to Improve their Antioxidant Potential. Molecules 2020, 25, 2838. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Sun, X.; Sun, S.; Xu, N. Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2585–2596. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In Vitro and In Silico Approaches to Generating and Identifying Angiotensin-Converting Enzyme I Inhibitory Peptides from Green Macroalga Ulva lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a novel ACE-inhibitory peptide from Sargassum maclurei in hypertension and reduction of intracellular endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Wakame (Undaria pinnatifida) and Their Antihypertensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Chen, J.-C.; Wang, J.; Zheng, B.-D.; Pang, J.; Chen, L.-J.; Lin, H.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in Laminaria japonica Hydrolysates by RP-HPLC Method. J. Food Process. Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Chabeaud, A.; Vandanjon, L.; Bourseau, P.; Jaouen, P.; Chaplain-Derouiniot, M.; Guerard, F. Performances of ultrafiltration membranes for fractionating a fish protein hydrolysate: Application to the refining of bioactive peptidic fractions. Sep. Purif. Technol. 2009, 66, 463–471. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Samarakoon, K. Recovery of Proteins and Their Biofunctionalities from Marine Algae; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Akaberi, S.; Gusbeth, C.; Silve, A.; Senthilnathan, D.S.; Navarro-López, E.; Molina-Grima, E.; Müller, G.; Frey, W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res. 2019, 43, 101656. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, T.; Nagai, M.; Maebuchi, M.; Furuta, H.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of peptides in sediments derived from an acidic enzymatic soy protein hydrolysate solution. Food Sci. Technol. Res. 2014, 20, 301–307. [Google Scholar] [CrossRef][Green Version]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; de Souza Lopes, J.L.; de Brito, T.V.; das Chagas Vieira Júnior, F.; Sales, A.B.; Aragão, K.S.; Souza, M.H.L.P.; dos Reis Barbosa, A.L.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef]
- Cian, R.E.; Caballero, M.S.; Sabbag, N.; González, R.J.; Drago, S.R. Bio-accessibility of bioactive compounds (ACE inhibitors and antioxidants) from extruded maize products added with a red seaweed Porphyra columbina. LWT-Food Sci. Technol. 2014, 55, 51–58. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.B.; Katanić Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a Source of Bioactive Compounds to Prevent the Development of Type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]


| Species | Protein (% dw) | EAA Composition (% TAA) | EAA (% prot.) | Digestibility | Reference |
|---|---|---|---|---|---|
| Rhodophyta | |||||
| Gracilaria gracilis | 31–45% | R 1.3, H 0.2, K 1.6, T 1.7, I 2.3, L 1.9, V 3.1, M 0.2, F 1.7, C 0.4, P 1.0, A 1.9, Y 1.3, D 2.6, E 2.4, G 1.1, S 1.6 | 14% | 68% (in vivo) | [8,10] |
| Palmaria palmata | 55% | T 4.1, V 5.4, M 2.0, I 4.3, L 7.2, K 5.7, F 4.7, W 0.9, H 1.7, S 6.2, Q + E 14.9, P 7.9, G 5.8, A 8.8, C 2.5, D + N 9.7, Y 2.7, R 5.6 | 36% | 56% (pancreatin) | [8,11] |
| Porphyra sp. | 31% | D 8.5, T 5.3, S 4.9, E 10.2, G 5.1, A 6.2, V 5.2, I 3.3, L 5.9, Y 3.4, F 3.5, H 2.6, K 5.2, R 5.9, P 3.6, C 1.3, M 1.8, W 0.7 | 51% | 57% (pepsin), 56% (pancreatin), 78% (pronase) | [8,12] |
| Clorophyta | |||||
| Cladophora rupestris | 12% | A 5.5, R 6.5, N 15.3, E 15.3, G 6.7, H 1.4, I 3.6, L 7.0, K 7.4, M 1.8, F 4.5, P 5.7, S 4.3, T 5.1, Y 4.3, V 5.8 | 13.9% | N.A. | [13] |
| Codium fragile | 11% | D 0.8, E 1.1, S 0.5, H 0.1, G 0.5, T 0.6, R 0.4, A 0.6, Y 0.4, V 1.4, M 0.9, C 0.1, I 0.4, L 0.7, F 0.5, L 0.5 | 5.4% | N.A. | [14] |
| Ulva sp. | 27% | D 1.5, E 1.5, S 0.8, H 0.1, G 0.8, T 0.8, R 0.5, A 1.1, Y 0.4, V 0.3, M 0.7, I 0.5, L 1.0, F 1.2, K 0.7 | 12% | 17% (pepsin), 67% (pancreatin), 95% (pronase) | [8,15] |
| Ochrophyta | |||||
| Fucus serratus | 4% | A 6.8, R 4.4, N 14.0, E 1596, G 5.8, H 1.7, I 4.0, L 6.8, K 5.5, M 1.9, F 5.0, P 4.0, S 5.6, T 5.5, Y 3.7, V 5.6 | 4.6% | N.A. | [13] |
| Sargassum fusiformis | 12% | D 9.1, T 4.1, S 5.6, E 18.7, G 4.8, A 4.3, V 4.9, I 4.0, L 6.7, Y 2.8, F 4.6, H 2.6, K 3.1, R 4.5, P 3.8, C 0.9, M 1.6, W 0.4 | 10.9% | N.A. | [12] |
| Undaria pinnatifida | 19.8% | D 8.7, T 4.4, S 4.0, E 14.5, G 5.1, A 4.7, V 5.2, I 4.1, L 7.4, Y 2.9, F 4.7, H 2.5, K 5.6, R 5.2, P 3.6, C 0.9, M 1.7, W 0.7 | 35.5% | 24% (pepsin), 48% (pancreatin), 87% (pronase) | [8,12] |
| Source | Pretreatment | Extraction Method | Precipitation and Purification | Yield (% dw) | Reference |
|---|---|---|---|---|---|
| Rhodophyta | |||||
| Palmaria palmata | Freeze-dried OS, (1: 20), 16 h, 4 °C // EPr, Celluclast + Shearzyme (E:S 4.8 × 103 U/100 g), pH 5, 24 h, 40 °C | SLE ak, 0.12 M NaOH + 0.1 mg/L NAC, 1 h, 25 °C | Pr: IP, pH 4, 1N HCl | 11.57% | [22] |
| Freeze dried | EAE (E:S 0.5) Celluclast 0.2% + Alcalase 0.2%, pH 4.5, 14 h, 50 °C // SLE ak, 0.1 M NaOH + 1 g/L NAC, 1.5 h, 25 °C | Pr: IP, pH 3, 5M HCl | 13.7% | [28] | |
| Dried, milled Hydrated (6%) Tris-HCl, pH 5, 16 h, 4 °C | EAE-HHPE, Hemicellulase (E:S 0.05), pH 4.5, 400 MPa, 20 min 40 °C | - | 6.3% | [36] | |
| Soliera chordalis | Dried, milled Hydrated (6%) Tris-HCl, pH 5, 16 h, 4 °C | EAE-HHPE, Hemicellulase (E:S 0.05), pH 4.5, 400 MPa, 20 min, 40 °C | - | 3.4% | [36] |
| Porphyra dioica | Freeze dried OS, (1: 20), 16 h, 4 °C | SLE ak, NaOH 0.12 M, 1 h, 25 °C | Pr: IP, pH 4.5 1M HCl | 14.28% | [34] |
| Neoporphyra haitanensis | Freeze dried | UAE ak, 400 W, 40 kHz, 0.01% NaOH, 20 min, 35 °C | Pr: (NH4)2SO4, 40%, 4 h, 4 °C | 3.8% | [38] |
| Chondrus crispus | Freeze dried | UAE, dW (1:20), 42 Hz, 1 h, 4 °C | Pr: (NH4)2SO4, 80%, 1 h, 4 °C Pu: DI, 3.5 kDa | ~6.7% | [24] |
| Freeze dried | HHPE, dW (1:20) 600 MPa, 4 min, 4 °C | Pu: Filtered, 100 μm nylon bag | ~3.1% | ||
| Condracanthus chamissoi | Oven dried (60 °C) 0.1 M NaOAc buffer, pH 4.5, 10 min, 50 °C | EAE Cellic CTec3 (E:S 0.1, 1.64 U/mg), pH 4.5, 16 h, 50 °C | Pr: Cold acetone (1: 4), 2 h | 6.35% | [37] |
| Clorophyta | |||||
| Ulva sp. | Oven dried (60 °C), freeze dried, milled | UAE ak (2×), (1:10), 1M NaOH, sonication (Hz non specified), 2 h, 25 °C | Pu: Filtered (0.45 μm) // DI, 2 kDa // IEC, Tris buffer, pH 9.5 // DI 2 kDa | 5.4% | [39] |
| Freeze dried, milled | SLE (1:20), lysis solution (8 M urea, 2% Tween, 1% PVP, 30 mM DTT), 16 h, 4 °C | Pu: DI, 6–8 kDa, 4 °C, 16 h | 11.88% | [31] | |
| Untreated | PFE aq, dW, 50 kV, 50 pulses, 0.5 Hz, 34 kJ // Mechanical press | Pr: DI, 100–500 kDa | 4.7% | [23] | |
| Ulva ohnoi | Oven dried (55 °C), milled | SLE aq, dW (1: 20), 16 h, 30 °C // SLE 1M NaOH, pH 12, 30 °C, 2 h | Pr: IP, pH 2.25, 10% v/v HCl | 12.28% | [27] |
| Fresh, pulped | SLE aq, dW (1:20), 16 h, 30 °C // Filtration (100 μm) // SLE 1M NaOH, pH 12, 30 °C, 2 h // Filtration (100 μm) | Pr: IP, pH 2.25, 10% v/v HCl | 17.13% | ||
| OS (1:10), 30 min, 40 °C // 0.05M HCl, 1 h, 85 °C | MAE aq, dW (1:34), 5 min., 123 °C | Pr: IP, pH 2.25, 10% v/v HCl | 11.3% | [40] | |
| Ulva compressa | Oven dried (60 °C), milled OS (1: 20), 16 h, 35 °C | SLE ak, 1M NaOH, pH 12 + 0.5% 2-mercaptoethanol, 2 h, 25 °C | Pr: (NH4)2SO4 80% Pu: DI (kDa n.s.) | 6.48% | [41] |
| Ochrophyta | |||||
| Ascophyllum nodosum | Oven dried (40 °C) UPr, dW (1: 20), 750 W, 20kHz, 10 min, 4 °C | SLE ak (1:15) 0.4M NaOH 1 h, 4°C // SLE ac (1:15) 0.4M HCl 1 h, 4 °C | Pu: HPSEC, 150–300 Å, 15 min, 40 °C | 4.23% | [33] |
| Alaria esculenta | Freeze dried, milled | HAE aq Autoclave, dW (1:20), 0.101 MPa, 2 × 15 min, 124 °C | Pu: Filtered, 100 μm muslin bag | ~2.4% | [24] |
| Sargassum patens | Freeze dried OS, (1:20), 16 h, 35 °C | SLE ak, 1M NaOH, pH 12 + 0.5% 2-mercaptoethanol, 2 h, 25 °C | Pr: (NH4)2SO4 85% Pu: DI (kDa n.s.) | 8.2% | [26] |
| Macrocystis pyrifera | Oven dried (60 °C) 0.1 M NaOAc buffer, pH 4.5, 10 min, 50 °C | EAE Cellic CTec3 (E:S 0.1, 1.64 U/mg), pH 4.5, 16 h, 50 °C | Pr: Cold acetone (1:4), 2 h | 7.39% | [37] |
| Undaria pinnatifida | Dried, powdered | SLE aq, dW (1:3), 20 min, 93 °C | Pu: HPLC, Develosil ODS-5 column, 25% CH3CN + 0.05% CF3COOH | 12% | [42] |
| Fucus vesiculosus | Freeze dried, milled | UAE aq, dW (1:20), 42 Hz, 1 h, 4 °C | Pr: (NH4)2SO4, 80%, 1 h, 4 °C Pu: DI 3.5 kDa | ~1.8% | [24] |
| Seaweed | Hydrolysis Method | Peptide Sequence | Bioactivity Reported | Reference |
|---|---|---|---|---|
| Rhodophyta | ||||
| Palmaria palmata | Papain, (E:S 20.7), pH 6, 24 h, 60 °C | IRLIIVLMPILMA, NIGK, IR | Renin, DPP IV, PAF-AH inhibition | [65,73,74] |
| Corolase PP (E:S 1), pH 7, 2 h, 50 °C | ILAP, LLAP, MAGVDHI, FITDGNK., NAATIIK, ANAATIIK, SDITRPGGQM, DNIQGITKPA., LITGA., LITGAA., LITGAAQA., LGLSGK., LTLAPK, LTIAPK, ITLAPK ITIAPK, VVPT, QARGAAQA | Antioxidant, DPP IV inhibition | [75,76] | |
| Pyropia columbina | Fungal protease concentrate (E: S 5) pH 4.3, 3 h, 55 // Flavourzyme (E:S 2), pH 7, 4 h, 55 °C | N.A. | Antitumor, anti-inflammatory, antioxidant | [77] |
| Neopyropia yezoensis | Pepsin (E:S 0.025), 5 h, 45 °C | NMEKGSSSVVSSRM | Anticoagulant | [61] |
| Porphyra dioica | Alcalase + Flavourzyme (E:S 1), pH 7, 4 h, 50 °C | DYYLR, AGFY, YLVA, AFIT, SFLPDLTDQ, MKTPITE, TYIA, LDLW | ACE, DPP IV inhibition | [34] |
| Prolyve 1000, (E:S 1), 2 h, 50 °C | N.A. (higher < 1 kDa peptides proportion) | Antioxidant | [78] | |
| Porphyra sp. | Pepsin (E:S 8), pH 2, 4 h, 37 °C | GGSK, ELS | α-amylase inhibition | [79] |
| Mazzaella japonica | Thermolysin (E:S 1), pH 7, 5 h, 37 °C | YRD, VSEGLD, TIMPHPR, GGPAT, SSNDYPI, SRIYNVKSNG, VDAHY, YGDPDHY, NLGN, DFGVPGHEP | ACE inhibition | [80] |
| Grateulopia lemaneiformis | α-chymotrypsin (E:S 4), pH 8, 2 h, 37 °C | ELWKTF | Antioxidant | [81] |
| Trypsin (E:S 4), pH 8, 8 h, 37 °C | QVEY | ACE inhibition | [82] | |
| Chlorophyta | ||||
| Ulva°C lathrata | Alcalase (E:S 5), pH 7.6, 90 min, 25 °C // 10 min, 100 °C | PAFG | ACE inhibition | [83] |
| Ulva. intestinalis | Trypsin + Pepsin + Papain (E:S 4), pH 8.42, 5 h, 28.5 °C | FGMPLDR, MELVLR | ACE inhibition | [84] |
| Ulva rigida | Pepsin (E:S 1), pH 2, 20 h, 37 °C // Bromelain (E:S 1), pH 7, 20 h, 37 °C | IP, AFL | ACE, Renin inhibition | [53] |
| Ulva lactuca | Papain (E:S 1), pH 6, 24 h, 60 °C | Total of 58 non-allergenic, ACE inhibitory peptides identified | ACE inhibition | [85] |
| Ulva spp | Purazyme + Flavourzyme // Alkaline protease-Protex 6L + Flavourzyme | N.A. | Anti-inflammatory (IL10 expression & TNF-α inhibition) | [52] |
| Ochrophyta | ||||
| Sargassum maclurei | Pepsin (80 U/g S), pH 2, 2 h, 37 °C // Papain (60 U/g S), pH 7, 3 h, 50 °C | RVLSAAFNTR, IMNILEK, GGVQAIR, KAALMEK, GVFDGPCGT, SGVFDGPCGT, QNIGDPR, AYSSGVSFK, RWDISQPY, LVYIVQGR, KPGGSGR, LGLSAKNYGR, KEAWLIEK, REVADDK, ENFFFAGIDK, QEMVDK, EEEEEEQQQ | Antyhypertensive (ACE & ET-1 inhibition) | [86] |
| Undaria pinnatifida | Protease S “Amano” (E:S 0.01), pH 8, 18 h, 70 °C | VY, IY, AW, FY, VW, IW, LW | Antihypertensive (ACE inhibition & in vivo) | [87] |
| dW (3:20), 20 min, 93 °C | YH, KY, FY, IY | Antihypertensive (ACE inhibition & in vivo) | [42] | |
| Saccharina longicruris | Trypsin (E:S 0.05), pH 7, 24 h, 30 °C | TITLDVEPSDTIDGVK, ISGLIYEETR, MALSSLPR, ILVLQSNQIR, ISAILPSR, IGNGGELPR, LPDAALNR, EAESSLTGGNGCAK, QVHPDTGISK | Antimicrobial | [88] |
| Saccharina japonica | Alcalase + Papain + Trypsin (E:S n.s.), time n.s., pH 7.5, 55 °C | KY, GKY, STKY, AKY, AKYSY, KKFY, FY, KFKY | ACE inhibition | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; H. Avdović, E.; Radulović, M.; Xiao, J.; A. Prieto, M.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. https://doi.org/10.3390/md19090500
Echave J, Fraga-Corral M, Garcia-Perez P, Popović-Djordjević J, H. Avdović E, Radulović M, Xiao J, A. Prieto M, Simal-Gandara J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Marine Drugs. 2021; 19(9):500. https://doi.org/10.3390/md19090500
Chicago/Turabian StyleEchave, Javier, Maria Fraga-Corral, Pascual Garcia-Perez, Jelena Popović-Djordjević, Edina H. Avdović, Milanka Radulović, Jianbo Xiao, Miguel A. Prieto, and Jesus Simal-Gandara. 2021. "Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications" Marine Drugs 19, no. 9: 500. https://doi.org/10.3390/md19090500
APA StyleEchave, J., Fraga-Corral, M., Garcia-Perez, P., Popović-Djordjević, J., H. Avdović, E., Radulović, M., Xiao, J., A. Prieto, M., & Simal-Gandara, J. (2021). Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Marine Drugs, 19(9), 500. https://doi.org/10.3390/md19090500











