Antiaging Potential of Peptides from Underused Marine Bioresources
Abstract
:1. Introduction
2. Antiaging Activity of Peptides from the Ocean
2.1. Free Radical Scavenging Activity and Sequence Characteristics of Peptides
Free Radical Scavenging Activity
| Source | Sequences | Activities (EC50) | Ref. |
|---|---|---|---|
| Microorganism | |||
| Chlorella vulgaris | favourzyme hydrolysates | Superoxide3 (0.323 mg/mL), Hydroxyl2 (0.139 mg/mL) | [25] |
| Spirulina sp. | Thr-Met-Glu-Pro-Gly-Lys-Pro | Inhibition of ROS production | [26] |
| Dunaliella salina | Ile-Leu-Thr-Lys-Ala-Ala-Ile-Glu-Gly-Lys Ile-Ile-Tyr-Phe-Gln-Gly-Lys Asn-Asp-Pro-Ser-Thr-Val-Lys Thr-Val-Arg-Pro-Pro-Gln-Arg | DPPH1 | [27] |
| Penicillium brevicompactum | N-cinnamoyl tripeptide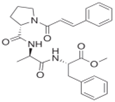 | Hydroxyl2 (equivalent to that of quercetin at 0.1 mM) | [28] |
| Kocuria marina | Phe-Glu, Asp-Ile, Ser-Ser-Gln, Leu-Glu | DPPH1 (0.24 mg/mL) | [29] |
| Marine invertebrates | |||
| Neptunea arthritica cumingii | Tyr-Ser-Gln-Leu-Glu-Asn-Glu-Phe-Asp-Arg | DPPH1 (0.77 mM) | [30] |
| Tyr-Ile-Ala-Glu-Asp-Ala-Glu-Arg | DPPH1 (1.04 mM) | ||
| Tergillarca granosa | Glu-Met-Gly-Pro-Ala | DPPH1 (0.53 ± 0.02 mg/mL), Hydroxyl2 (0.47 ± 0.03 mg/mL), Superoxide3 (0.75 ± 0.04 mg/mL), ABTS4(0.96 ± 0.08 mg/mL), Inhibition of lipid peroxidation | [31] |
| Trp-Pro-Pro-Asp | DPPH1 (0.36 ± 0.02 mg/mL), Hydroxyl1 (0.38 ± 0.04 mg/mL), Superoxide3 (0.46 ± 0.05 mg/mL), ABTS4 (0.54 ± 0.03 mg/mL), Inhibition of lipid peroxidation | ||
| Brachionus rotundiformis | Leu-Leu-Gly-Pro-Gly-Leu-Thr-Asn-His-Ala, | DPPH1 (189.8 µM) | [32] |
| Asp-Leu-Gly-Leu-Gly-Leu-Pro-Gly-Ala-His | DPPH1 (167.7 µM) | ||
| Fish | |||
| Muscle of Scomberomorous niphonius | Pro-Glu-Leu-Asp-Trp | DPPH1 (1.53 mg/mL), Hydroxyl2 (1.12 mg/mL), Superoxide2 (0.85 mg/mL), Inhibition of lipid peroxidation, Protection of plasmid DNA | [33] |
| Trp-Pro-Asp-His-Trp | DPPH1 (0.70 mg/mL). Hydroxyl2 (0.38 mg/mL) Superoxide3 (0.49 mg/mL). Inhibition of lipid peroxidation, Protect plasmid DNA. | ||
| Phe-Gly-Tyr-Asp-Trp-Trp | DPPH1 (0.53 mg/mL), Hydroxyl2 (0.26 mg/mL), Superoxide3 (0.34 mg/mL). Inhibition of lipid peroxidation, | ||
| Tyr-Leu-His-Phe-Trp | DPPH1 (0.97 mg/mL), Hydroxyl2 (0.67 mg/mL), Superoxide3 (1.37 mg/mL), Inhibit lipid peroxidation. | ||
| Skin of Scomberomorous niphonius | Pro-Phe-Gly-Pro-Asp | DPPH1 (0.80 mg/mL), Hydroxyl2 (0.81 mg/mL), Superoxide3 (0.91 mg/mL, ABTS4 (0.86 mg/mL), FRAP and Inhibition of lipid peroxidation | [34] |
| Pro-Tyr-Gly-Ala-Lys-Gly | DPPH1 (3.02 mg/mL), Hydroxyl2 (0.66 mg/mL), Superoxide3 (0.80 mg/mL), ABTS4 (1.07 mg/mL), FRAP and inhibit lipid peroxidation | ||
| Tyr-Gly-Pro-Met | DPPH1 (0.72 mg/mL), Hydroxyl2 (0.88 mg/mL), Superoxide3 (0.73 mg/mL), ABTS4 (0.82 mg/mL), FRAP and inhibit lipid peroxidation | ||
| Cartilage of Dasyatis akajei | Ile-Glu-Glu-Glu-Gln | DPPH1 (4.61 mg/mL), Hydroxyl2 (0.77 mg/mL), Superoxide3 (0.08 mg/mL), ABTS4 (0.15 mg/mL). | [23] |
| Ile-Glu-Pro-His | DPPH1 (1.90 mg/mL,), Hydroxyl2 (0.46 mg/mL), Superoxide3 (0.17 mg/mL), ABTS4 (0.11 mg/mL), Lipid peroxidation inhibition activity. | ||
| Leu-Glu-Glu-Glu-Glu | DPPH1 (3.69 mg/mL), Hydroxyl2 (0.70 mg/mL), Superoxide3 (0.15 mg/mL), ABTS4 (0.19 mg/mL), Fe2+-chelating ability. | ||
| Val-Pro-Arg | DPPH1 (4.01 mg/mL), Hydroxyl2 (1.30 mg/mL), Superoxide3 (0.16 mg/mL), ABTS4 (0.18 mg/mL). | ||
| Head of Katsuwonus pelamis | Trp-Met-Gly-Pro-Tyr | DPPH1 (0.33 mg/mL), Hydroxyl2 (0.43 mg/mL), Superoxide3 (0.38 mg/mL), FRAP and lipid peroxidation inhibition. | [35] |
| Trp-Met-Phe-Asp-Trp | DPPH1 (0.31 mg/mL), Hydroxyl2 (0.30 mg/mL), Superoxide3 (0.56 mg/mL), FRAP and lipid peroxidation inhibition. | ||
| Glu-Met-Gly-Pro-Ala | DPPH1 (0.46 mg/mL), Hydroxyl2 (0.52 mg/mL), Superoxide3 (0.71 mg/mL), FRAP and lipid peroxidation inhibition. | ||
| Salmon gelatin | Gly-Gly-Pro-Ala-Gly-Pro-Ala-Val, Gly-Pro-Val-Ala, Pro-Pro and Gly-Phe | Oxygen radical absorbance capacity (ORAC, 540.94 ± 9.57 µmol TE/g d.w.) | [36] |
| Pacific cod skin gelatin | Leu-Leu-Met-Leu-Asp-Asn-Asp-Leu-Pro-Pro | Scavenging the intracellular ROS | [37] |
| Jumbo squid (Dosidicus gigas, squid) skin gelatin | Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu Asn-Gly-Pro-Leu-Gin-Ala-Gly-Gln-Pro-Gly-Glu-Arg | Inhibition of oxidant stress; Lipid peroxidation inhibition (>Vit. E). | [38] |
| Whole body of Parastromateus niger | Ala-Met-Thr-Gly-Leu-Glu-Ala | DPPH1 (54%), Metal chelating (78.6%) at 1 mg/mL | [24] |
| Smooth hound viscera (sharks) | Protein hydrate containing Gly, Glx, Lys, Asx, Arg, Pro and Ala | DPPH1, Inhibition of linoleic acid oxidation, Hydroxyl2. | [39] |
| Hoki (Johnius belengerii) frame | Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-His-Pro-Ala-Cys-Pro-Asp-Phe-Asn | DPPH1 (41.37 µM), Hydroxyl2 (17.77 µM), Peroxyl radical scavenging (18.99 µM), Superoxide3 (172.10 µM). | [40] |
| Limanda aspera frame | Arg-Pro-Asp-Phe-Asp-Leu-Glu-Pro-Pro-Tyr | Inhibition of linoleic acid autoxidation | [41] |
| Tuna backbone | Val-lys-Ala-Gly-Phe-Ala-Trp-Thr-Ala-Asn-Gln-Gln-Leu-Ser | Inhibited lipid peroxidation, Quenched free radicals (DPPH, hydroxyl and superoxide) | [42] |
| Frame of Theragra chalcogramma | Leu-Pro-His-Ser-Gly-Tyr | Hydroxyl2 (35% at 53.6 µM) | [43] |
2.2. Inhibition of Cell Apoptosis
2.3. Prolonging Lifespan in Model Organisms
2.4. Ameliorating D-Galactose Induced Aging in Mice
2.5. Antiaging Effects in Human Clinical Assays
3. The Mechanisms Underlying Antiaging Activity
3.1. Improvement of Antioxidant Enzyme Activity
3.1.1. Regulation of Keap1/Nrf2-ARE Expression
3.1.2. Regulation of Klotho
3.1.3. Regulation of DAF-16/FOXO SOD-3 Expression
3.2. Inhibition of the Autophagy and Apoptosis
3.2.1. Directly Scavenging Excess Free Radicals
3.2.2. Regulation of p53-Bax/Bcl-2 Pathway
3.2.3. Regulation of mTORC1-mTOR-Bcl/Bax Apoptosis Pathway
3.3. Regulation of the TNF-α-MMPs-ECM Pathway to Suppress MMP-1 Expression
3.4. Regulation of CCT-PTEN Pathway to Protect Mitochondria
3.5. Restoration of Intestinal Homeostasis and Regulation of Aging-Related Metabolic Disorders
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.; Zhu, S.; He, M.; Luo, F.; Fu, C.; Zou, T. Marine peptides as potential agents for the management of Type 2 Diabetes Mellitus—A prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postep. Hig. Med. Dosw. (Online) 2017, 71, 170–175. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Lara, J.; Sherratt, M.J.; Rees, M. Aging and anti-aging. Maturitas 2016, 93, 1–3. [Google Scholar] [CrossRef]
- Son, D.H.; Park, W.J.; Lee, Y.J. Recent advances in anti-aging medicine. Korean J. Fam. Med. 2019, 40, 289–296. [Google Scholar] [CrossRef]
- Jin, Q.; Peng, D.; Zheng, Z. Advances in extracting and understanding the bioactivities of marine organism peptides: A review. J. Food Process Pres. 2021, e15602. [Google Scholar] [CrossRef]
- Dhaval, A.; Yadav, N.; Purwar, S. Potential applications of food derived bioactive peptides in management of health. Int. J. Pept. Res. Ther. 2016, 22, 377–398. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; García, H.S.; Torres-Llanez, M.J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Safety of milk-derived bioactive peptides. Int. J. Dairy Technol. 2017, 70, 16–22. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161. [Google Scholar] [CrossRef] [Green Version]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Chai, M.; Ye, Y.; Chen, V. Separation and concentration of milk proteins with a submerged membrane vibrational system. J. Membr. Sci. 2017, 524, 305–314. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels-A randomized, double-blind, placebo-controlled trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Development of Biomimetic Tilapia collagen nanofibers for skin regeneration through inducing keratinocytes differentiation and collagen synthesis of dermal fibroblasts. ACS Appl. Mater. Inter. 2015, 7, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.N.; Moraes, C.A.P. Bioactive peptides: Applications and relevance for cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.A.; González-Córdov, F.; Vallejo-Cordob, B.A.; Liceaga, M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Schulz, I.; Mahler, H.C.; Boiteux, S.; Epe, B. Oxidative DNA base damage induced by singlet oxygen and photosensitization: Recognition by repair endonucleases and mutagenicity. Mutat. Res. 2000, 461, 145–156. [Google Scholar] [CrossRef]
- Chi, C.; Cao, Z.; Wang, B.; Hu, F.; Li, Z.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, I.A.; Rogero, M.M.; Junqueira, R.M.; Carrapeiro, M.M. 2,2-Diphenyl-1-picrylhydrazil free radical scavenging activity of antioxidant mixtures evaluated by response surface methodology. Int. J. Food Sci. Technol. 2006, 41, 59–67. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Li, L.; Chi, C.; Wang, B. Four antioxidant peptides from protein hydrolysate of red stingray (Dasyatis akajei) cartilages: Isolation, identification, and activity evaluation. Mar. Drugs 2019, 17, 263. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, R.A.; Kumar, N.S.S. Purification and identification of antioxidant peptide from black pomfret, Parastromateus niger (Bloch, 1975) viscera protein hydrolysate. Food Sci. Biotechnol. 2011, 20, 1087–1094. [Google Scholar]
- Zhang, Y.; Jiang, W.; Hao, X.; Tan, J.; Wang, W.; Yu, M.; Zhang, G.; Zhang, Y. Preparation of the Enzymatic hydrolysates from Chlorella vulgaris protein and assessment of their antioxidant potential using Caenorhabditis elegans. Mol. Biotechnol. 2021, 1–9. [Google Scholar] [CrossRef]
- Heo, S.Y.; Ko, S.C.; Kim, C.S.; Oh, G.W.; Ryu, B.; Qian, Z.J.; Kim, G.; Park, W.S.; Choi, I.W.; Phan, T.T.; et al. A heptameric peptide purified from Spirulina sp. gastrointestinal hydrolysate inhibits angiotensin I-converting enzyme- and angiotensin II-induced vascular dysfunction in human endothelial cells. Int. J. Mol. Med. 2017, 39, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and identification of antioxidant peptide from underutilized Dunaliella salina protein: Extraction, gastrointestinal digestion, and fractionation. BioMed Res. Int. 2019, 2019, 6424651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, H.; Mokudai, T.; Higo, M.; Nonaka, K.; Nagano, Y.; Nagahama, T.; Niwano, Y.; Takahashi, Y.; Omura, S.; Nakashima, T. Cipralphelin, a new anti-oxidative N-cinnamoyl tripeptide produced by the deep sea-derived fungal strain Penicillium brevicompactum FKJ-0123. J. Antibiot. 2019, 72, 775–778. [Google Scholar] [CrossRef]
- Tripathi, V.C.; Horam, S.; Singh, A.; Lata, M.; Reddy, T.J.; Arockiaraj, J.; Pasupuleti, M. The discovery of antioxidants in marine microorganisms and their protective effects on the hepatic cells from chemical-induced oxidative stress. Free Rad. Res. 2020, 54, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, L.; Shi, Y.; Li, X.; Zhang, X.; Hou, H.; Lin, H.; Liu, K. Two Novel multi-functional peptides from meat and visceral mass of marine snail neptunea arthritica cumingii and their activities. Mar. Drugs 2018, 16, 473. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Qiu, Y.; Zhao, Y.; Chi, C.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, X.; Wang, Y.; Zhao, Y.; Chi, C.; Wang, B. Antioxidant peptides from the protein hydrolysate of Spanish Mackerel (Scomberomorous niphonius) muscle by gastrointestinal digestion and their activities. Mar. Drugs 2019, 17, 531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, Y.; Wang, Y.; Chi, C.; Wang, B. Eight collagen peptides from hydrolysate fraction of Spanish Mackerel skins: Isolation, identification, and antioxidant activity evaluation. Mar. Drugs 2019, 17, 224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhao, G.; Zhao, Y.; Qiu, Y.; Chi, C.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their an-tioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belenge-rii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Park, P.J.; Jung, W.K.; Kim, S.K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtaine-d from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Ahn, C.B.; Nam, K.H.; Kim, Y.K.; Yoon, N.Y.; Je, J.Y. Amino acid composition, antioxidant, and cytoprotective effect of blue mussel (Mytilus edulis) hydrolysate through the inhibition of caspase-3 activation in oxidative stress-mediated endothelial cell injury. Mar. Drugs 2019, 17, 135. [Google Scholar] [CrossRef] [Green Version]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice bran phenolic compounds regulate genes associated with antioxidant and anti-inflammatory activity in human umbilical vein endothelial cells with induced oxidative stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Cytoprotective role of edible seahorse (Hippocampus abdominalis)-derived peptides in H2O2-induced oxidative stress in human umbilical vein endothelial cells. Mar. Drugs 2021, 19, 86. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Kang, K.; Qian, Z.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother. Res. PTR 2012, 26, 1555–1563. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Lin, P.; Hua, N.; Hsu, Y.C.; Kan, K.W.; Chen, J.H.; Lin, Y.H.; Lin, Y.H.; Kuan, C.M. Oral Collagen Drink for Antiaging: Antioxidation, facilitation of the increase of collagen synthesis, and improvement of protein folding and DNA repair in human skin fibroblasts. Oxid. Med. Cell. Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Jim Wang, Z.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094. [Google Scholar] [CrossRef]
- Jang, J.; Kim, M.; Nam, G.-H.; Kang, S.; Lee, K.-Y.; Park, Y.-J. Antiaging activity of peptide identified from fermented Trapa Japonica fruit extract in human dermal fibroblasts. Evid.-Based Compl. Alt. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, L.; Häkkinen, V.; Uitto, J.; Larjava, H. Cell biology of gingival wound healing. Periodontology 2000, 24, 127–152. [Google Scholar]
- Zou, Y.; Liu, Y.; Ruan, M.; Feng, X.; Wang, J.; Chu, Z.; Zhang, Z. Cordyceps sinensis oral liquid prolongs the lifespan of the fruit fly, Drosophila melanogaster, by inhibiting oxidative stress. Int. Mol. Med. 2015, 36, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, E.; Wiggins, D.; Fielding, B.; Gould, A.P. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 2007, 445, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Wu, L.; Tong, A.; Zhao, L.; Liu, B.; Zhao, C. Physicochemical characterization of polysaccharides from Chlorella pyrenoidosa and its anti-ageing effects in Drosophila melanogaster. Carbohyd. Polym. 2018, 185, 120–126. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins reversed D-Galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Zheng, L.; Zhao, M.; Fan, J.; Liu, S. Preparation of sea cucumber (Stichopus variegates) peptide fraction with desired organoleptic property and its anti-aging activity in fruit flies and D-galactose-induced aging mice. J. Func. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Awasthi, A.; Prasad, B.; Kumar, J.; Madamwar, D. Phycoerythrin extends life span and health span of Caenorhabditis elegans. Age 2014, 36, 9717. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Su, Q.; Shen, T.; Chen, Q.; Wang, Y.; Jia, W. Antioxidant peptides from sepia esculenta hydrolyzate attenuate oxidative stress and fat accumulation in Caenorhabditis elegans. Mar. Drugs 2020, 18, 490. [Google Scholar] [CrossRef]
- Jia, W.; Peng, Q.; Su, L.; Yu, X.; Ma, C.; Liang, M.; Yin, X.; Zou, Y.; Huang, Z. Novel bioactive peptides from Meretrix meretrix protect Caenorhabditis elegans against free radical-induced oxidative stress through the stress response factor DAF-16/FOXO. Mar. Drugs 2018, 16, 444. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chen, S.; Zhang, C.; Huang, J.; Wu, J.; Zhou, H.; Jin, L.; Qian, X.; Jin, J.; Lyu, J. Enhanced ROS production leads to excessive fat accumulation through DAF-16 in Caenorhabditis elegans. Exp. Gerontol. 2018, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring milt protein hydrolysate improves iInsulin resistance in high-fat-diet-induced obese male C57BL/6J mice. Mar. Drugs 2019, 17, 456. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Guralnik, J.M.; Pahor, M.; Corti, M.C.; Havlik, R.J. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 1997, 277, 728–734. [Google Scholar] [CrossRef]
- Stineman, M.G.; Xie, D.; Pan, Q.; Kurichi, J.E.; Saliba, D.; Streim, J. Activity of daily living staging, chronic health conditions, and perceived lack of home accessibility features for elderly people living in the community. J. Am. Geriatr. Soc. 2011, 59, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, Q.; Dong, Y.; Zhu, S.; Song, S.; Sun, S. Supplementation of mussel peptides reduces aging phenotype, lipid deposition and oxidative stress in D-Galactose-induce aging mice. J. Nutr. Health Aging 2017, 21, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, M.; Brejnrod, A.D.; Aadland, E.K.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; et al. The effect of lean-seafood and non-seafood diets on fecal metabolites and gut microbiome: Results from a randomized crossover intervention study. Mol. Nutr. Food Res. 2019, 63, e1700976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.B.; Won, B.; Yang, S.C.; Kim, D.H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021, 98, 289–297. [Google Scholar] [CrossRef]
- DeBacker, C.M.; Putterman, A.M.; Zhou, L.; Holck, D.E.E.; Dutton, J.J. Age-related changes in type-I collagen synthesis in human eyelid skin. Ophthal. Plast. Recons. Surg. 1998, 14, 13–16. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceut. J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Cai, S.; Wang, Y.; Zhao, Y.; Chi, C.; Wang, B. Cytoprotective effect of antioxidant pentapeptides from the protein hydrolysate of Swim bladders of Miiuy Croaker (Miichthys miiuy) against H(2)O(2)-mediated human umbilical vein endothelial cell (HUVEC) injury. Int. J. Mol. Sci. 2019, 20, 5425. [Google Scholar] [CrossRef] [Green Version]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Quarles, L.D. Fibroblast growth factor 23 and α-Klotho co-dependent and independent functions. Curr. Opin. Nephrol. Hypertens. 2019, 28, 16–25. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, L.; He, A.; Liu, Q. Klotho inhibits unilateral ureteral obstruction-induced endothelial-to-mesenchymal transition via TGF-β1/Smad2/Snail1 signaling in Mice. Front. Pharmacol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iurciuc, S.; Cimpean, A.M.; Mitu, F.; Heredea, R.; Iurciuc, M. Vascular aging and subclinical atherosclerosis: Why such a “never ending” and challenging story in cardiology? Clin. Interv. Aging 2017, 12, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.W.; Mukhopadhyay, A.; Dixit, B.L.; Raha, T.; Green, M.R.; Tissenbaum, H.A. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet. 2006, 38, 251–257. [Google Scholar]
- Li, W.; Shi, Y.; Chang, C.; Huang, C.; Hsiu-Chuan Liao, V. Selenite protects Caenorhabditis elegans from oxidative stress via DAF-16 and TRXR-1. Mol. Nutr. Food Res. 2014, 58, 863–874. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Q.; Peng, Q.; Yu, X.; Yin, X.; Liang, M.; Ma, C.; Huang, Z.; Jia, W. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Func. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, 1005–1028. [Google Scholar] [CrossRef] [Green Version]
- Karbowski, M.; Kurono, C.; Wozniak, M.; Ostrowski, M.; Teranishi, M.; Nishizawa, Y.; Usukura, J.; Soji, T.; Wakabayashi, T. Free radical-induced megamitochondria formation and apoptosis. Free Radic. Bio. Med. 1999, 26, 396–409. [Google Scholar] [CrossRef]
- Klaunig, J.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef]
- Burns, T.F.; El-Deiry, W.S. The p53 pathway and apoptosis. J. Cell. Physiol. 1999, 181, 231–239. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.; Zhou, Q.; Jiang, G. Synthetic phenolic antioxidants cause perturbation in Steroidogenesis and. Environ. Sci. Technol. 2018, 52, 850–858. [Google Scholar] [CrossRef]
- Feldman, M.E.; Apsel, B.; Uotila, A.; Loewith, R.; Knight, Z.A.; Ruggero, D.; Shokat, K.M. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009, 7, e38. [Google Scholar] [CrossRef]
- Ekim, B.; Magnuson, B.; Acosta-Jaquez, H.A.; Keller, J.A.; Feener, E.P.; Fingar, D.C. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol. Cell. Biol. 2011, 31, 2787–2801. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Desponds, C.; Eren, R.O.; Quadroni, M.; Thome, M.; Fasel, N. Caspase-mediated cleavage of raptor participates in the inactivation of mTORC1 during cell death. Cell Death Discov. 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, G.H.; Jo, K.J.; Park, Y.S.; Kawk, H.W.; Yoo, J.G.; Jang, J.D.; Kang, S.M.; Kim, S.Y.; Kim, Y.M. Bacillus/Trapa japonica fruit extract ferment filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement Altern. Med. 2019, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- Prost-Squarcioni, C.; Fraitag, S.; Heller, M.; Boehm, N. Functional histology of dermis. Ann. Dermatol. Venereol. 2008, 135, 5–20. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, H. Regulation of collagen gene expression. Prog. Nucleic Acid Res. Mol. Biol. 1989, 37, 67–106. [Google Scholar]
- Kähäri, V.-M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Papaluca, A.; Wagner, J.R.; Saragovi, H.U.; Ramotar, D. UNG-1 and APN-1 are the major enzymes to efficiently repair 5-hydroxymethyluracil DNA lesions in C. elegans. Sci. Rep. 2018, 8, 6860. [Google Scholar]
- Komakula, S.S.B.; Tumova, J.; Kumaraswamy, D.; Burchat, N.; Vartanian, V.; Ye, H.; Dobrzyn, A.; Lloyd, R.S.; Sampath, H. The DNA repair protein OGG1 protects against obesity by altering Mitochondrial Energetics in white adipose tissue. Sci. Rep. 2018, 8, 14886. [Google Scholar] [CrossRef]
- Nong, V.H.; Arahira, M.; Phan, V.C.; Kim, C.S.; Zhang, D.Y.; Udaka, K.; Fukazawa, C. Molecular cloning and characterization of a group II chaperonin delta-subunit from soybean. J. Biochem. 2002, 132, 291–300. [Google Scholar] [CrossRef]
- Pussila, M.; Toronen, P.; Einarsdottir, E.; Katayama, S.; Krjutskov, K.; Holm, L.; Kere, J.; Peltomaki, P.; Makinen, M.J.; Linden, J.; et al. Mlh1 deficiency in normal mouse colon mucosa associates with chromosomally unstable colon cancer. Carcinogenesis 2018, 39, 788–797. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Labachyan, K.E.; Kiani, D.; Sevrioukov, E.A.; Schriner, S.E.; Jafari, M. The impact of Rhodiola rosea on the gut microbial community of Drosophila melanogaster. Gut Pathog. 2018, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef]
- Kallus, S.J.; Brandt, L.J. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef]
- De Bandt, J.P.; Waligora-Dupriet, A.J.; Butel, M.J. Intestinal microbiota in inflammation and insulin resistance: Re-levance to humans. Curr. Opin Clin. Nutr. Metab. Care 2011, 14, 334–340. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox. Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, K.T.; Chung, M.Y.; Cho, D.H.; Park, C.S. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): Role for a metal ion chelating effect. J. Food Sci. 2005, 70, 388–M391. [Google Scholar] [CrossRef]
- Li, W.; Lu, L.; Liu, B.; Qin, S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 2020, 132, 110826. [Google Scholar] [CrossRef]
- Han, K.; Jin, W.; Mao, Z.; Dong, S.; Zhang, Q.; Yang, Y.; Chen, B.; Wu, H.; Zeng, M. Microbiome and butyrate production are altered in the gut of rats fed a glycated fish protein diet. J. Func. Foods 2018, 47, 423–433. [Google Scholar] [CrossRef]
- Wang, S.; Lv, Z.; Zhao, W.; Wang, L.; He, N. Collagen peptide from Walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Func. Foods 2020, 74, 04194. [Google Scholar] [CrossRef]
- Mei, F.; Duan, Z.; Chen, M.; Lu, J.; Zhao, M.; Li, L.; Shen, X.; Xia, G.; Chen, S. Effect of a high-collagen peptide diet on the gut microbiota and short-chain fatty acid metabolism. J. Func. Foods 2020, 75, 104278. [Google Scholar] [CrossRef]
- Durand, R.; Ouellette, A.; Houde, V.P.; Guénard, F.; Varin, T.V.; Marcotte, B.; Pilon, G.; Fraboulet, E.; Vohl, M.C.; Marette, A.; et al. Animal and cellular studies demonstrate some of the beneficial impacts of herring milt hydrolysates on obesity-induced glucose intolerance and inflammation. Nutrients 2020, 12, 3235. [Google Scholar] [CrossRef]
- Wu, J.; Qiao, K.; Lu, H.; Chen, X.; Su, J.; Liu, Z. Abalone viscera protein hydrolysate modulation of gut microbiota in a mouse model of alcohol induced injury. J. Aquat. Food Prod. Technol. 2017, 26, 880–889. [Google Scholar] [CrossRef]
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L. Regulatory efficacy of Spirulina platensis protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet-fed rats. Int. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef] [Green Version]
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella pyrenoidosa protein hydrolysate-calcium chelate on calcium absorption metabolism and gut microbiota composition in low-calcium diet-fed rats. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Lin, L.; Chen, H.; Ge, X.; Huang, Y.; Zheng, Z.; Li, S.; Zeng, F. Anti-fatigue property of the oyster polypeptide fraction and its effect on gut microbiota in mice. Food Func. 2020, 11, 8659–8669. [Google Scholar] [CrossRef] [PubMed]

| Marine Peptides | Test Animal | Improvement of Gut Microbiota | Ref. |
|---|---|---|---|
| Spirulina Phycocianin (Microalgae) | Mice | Increase the relative abundance of Bacteroidetes and Actinobacteria | [113] |
| Glycosylated fish protein | Mice | Increase the abundance of Allobaculum, Akkermansia, Lactobacillus animalis | [114] |
| Walleye Pollock skin | Mice | Upregulation relative abundance of Lactobacillus and Akkermansia, downregulation the abundance of bacteria associated with intestinal inflammation | [115] |
| Skin collagen peptide of Salmon salar and Tilapia nilotica | Male rats | Increased abundance of Lactobacillus | [116] |
| Herring milt hydrolysate (protein: 47–94%) | Mice | Maintain abundant of Lactobacillus decrease metabolites associated with obesity and inflammatory disease | [117] |
| Peptides from tuna roe | Mice | Short-chain fatty acids production in feces and modulating gut microbiota composition | [52] |
| Abalone viscera | Alcohol induced injured mice | Increase in diversity index and the number of Bacilli (class), Lactobacillales (order), Lactobacillaceae (family), and Lactobacillus (genus) levels | [118] |
| Spirulina platensis protease hydrolyzate | High-fat diet (HFD)-fed rats | Enriched the abundance of gut beneficial bacteria | [119] |
| Chlorella pyrenoidosa protein hydrolysate-calcium chelate | Low-calcium diet-fed rats | Improving the abundances of Firmicutes and Lactobacillus | [120] |
| Oyster polypeptide (OP) fraction | Exhaustive fatigue mice | regulate the abundance of gut microbiota and maintain its balance | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, E.; Zhu, X.; Gao, X.; Ni, J.; Guo, H. Antiaging Potential of Peptides from Underused Marine Bioresources. Mar. Drugs 2021, 19, 513. https://doi.org/10.3390/md19090513
Xia E, Zhu X, Gao X, Ni J, Guo H. Antiaging Potential of Peptides from Underused Marine Bioresources. Marine Drugs. 2021; 19(9):513. https://doi.org/10.3390/md19090513
Chicago/Turabian StyleXia, Enqin, Xuan Zhu, Xuebin Gao, Jindong Ni, and Honghui Guo. 2021. "Antiaging Potential of Peptides from Underused Marine Bioresources" Marine Drugs 19, no. 9: 513. https://doi.org/10.3390/md19090513
APA StyleXia, E., Zhu, X., Gao, X., Ni, J., & Guo, H. (2021). Antiaging Potential of Peptides from Underused Marine Bioresources. Marine Drugs, 19(9), 513. https://doi.org/10.3390/md19090513






