Abstract
Four new streptoglycerides E–H (1–4), with a rare 6/5/5/-membered ring system, were isolated from a marine-derived actinomycete Streptomyces specialis. The structures of 1–4 were elucidated by detailed analysis of HRESIMS, 1D and 2D NMR data and ECD spectra as well as comparison of their spectroscopic data with those reported in literature. Compounds 1–4 showed significant anti-inflammatory activity by inhibiting lipopolysaccharide (LPS)-induced nitric oxide (NO) production in Raw 264.7 cells with IC50 values ranging from 3.5 to 10.9 µM. Especially, 2 suppressed mRNA expression levels of iNOS and IL-6 without cytotoxicity.
1. Introduction
Inflammation is a normal defense mechanism that occurs to cope with tissue damage and microbial infection [1]. When tissues are exposed to LPS, NO and cytokines such as interleukins (IL-6 and IL-1β), and tumor necrosis factor-α (TNF-α) are produced naturally [2,3]. However, the uncontrolled cytokine overproduction promotes a variety of diseases [4]. NO and interleukins play a central role in the pathogenesis of inflammation owing to overproduction under abnormal physiological conditions [5,6]. Therefore, NO and interleukins are considered as a critical indicator for developing drugs for inflammatory diseases [7,8].
Up to now, there have been many reports on marine natural products with anti-inflammatory activity [9,10,11]. Marine organisms inhabiting extreme environments have adopted unique survival strategies for growing and reproducing under hostile conditions, biosynthesizing molecules valuable for pharmaceutical applications [12,13]. Marine Streptomyces are an especially attractive source for exploring marine natural products as they produce various kinds of secondary metabolites with potent biological activities [14,15].
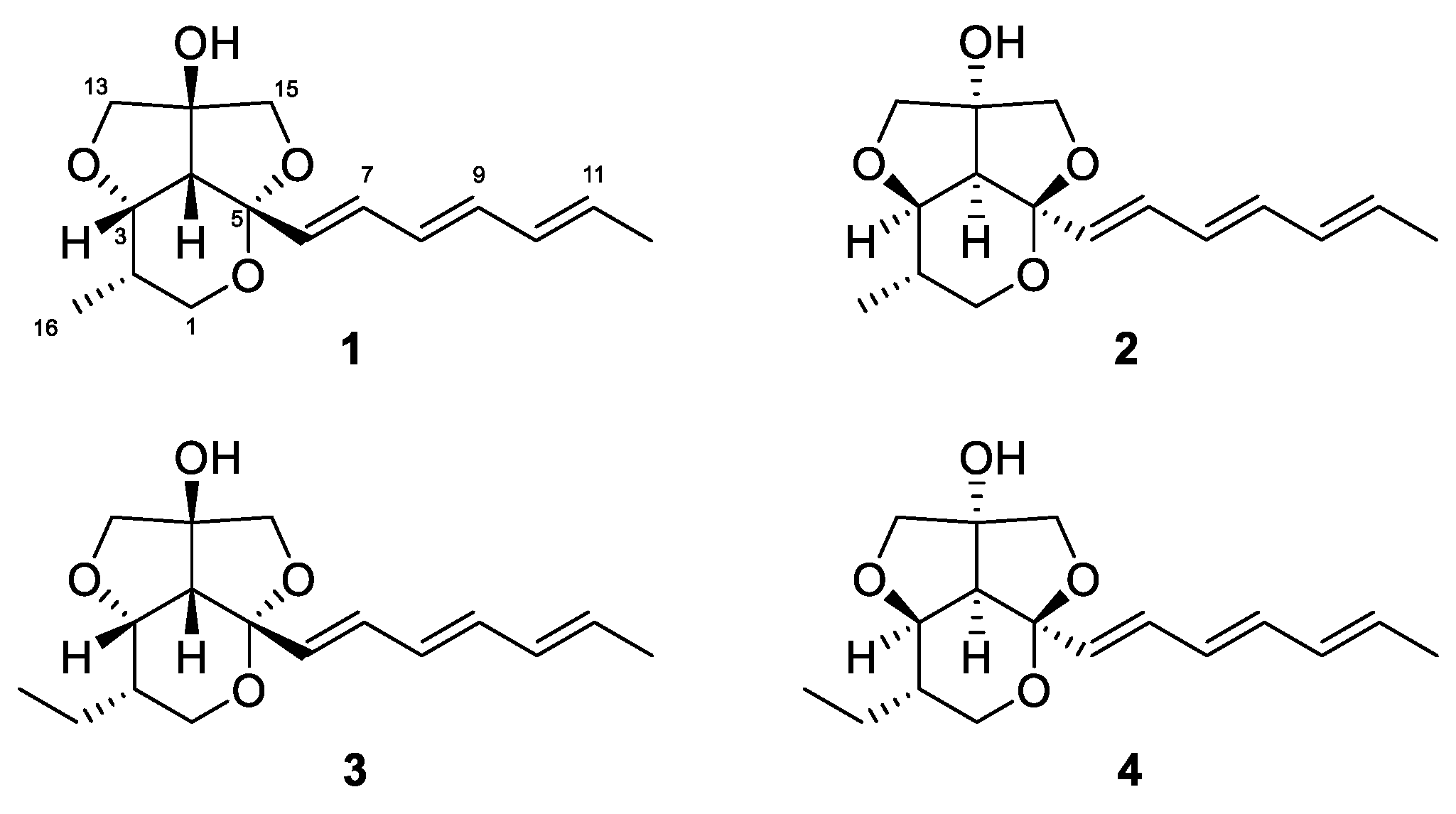
Recently, we reported streptoglycerides A–D with a quite rare 6/5/5 heterocyclic scaffold and anti-inflammatory activity isolated from a strain of marine-derived Streptomyces sp. [16]. During our ongoing investigation of secondary metabolites from marine microorganisms, we encountered another strain, designated as Streptomyces specialis 208DD-067, isolated from a sediment sample collected off Dokdo, South Korea, which produced new derivatives of streptoglycerides (1–4). Herein, we report the isolation, structure elucidation and anti-inflammatory activity of streptoglycerides E–H (1–4) (Figure 1).
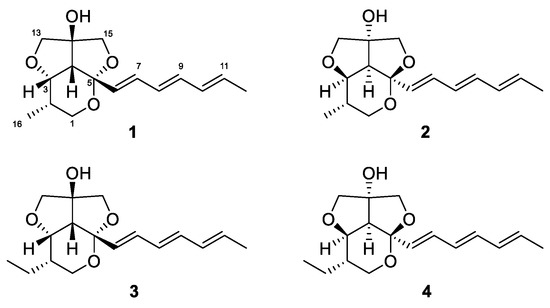
Figure 1.
Structures of streptoglycerides E–H (1–4).
2. Results and Discussion
2.1. Structural Elucidation
Compound 1 was isolated as a white powder. The molecular formula of 1 was determined to be C16H22O4 by combined analysis of HRESIMS (m/z 301.1417 [M+Na]+, calcd. for C16H22O4Na 301.1416) and NMR data. The 1H NMR spectra of 1 displayed signals of two methyl groups at δH 0.94 (d, J = 7.0, H3-16) and 1.76 (d, J = 7.0, H3-12); a methine at δH 2.41 (d, J = 7.0, H-4); six oxygenated methylenes at δH 3.36-4.00; an oxygenated methine at δH 4.11 (dd, J = 7.0, 3.1, H-3) and six olefinic protons at δH 5.66–6.40 (Table 1). The 13C NMR and HSQC spectra of 1 exhibited the presence of two methyl carbons at δC 13.0 and 18.4; three oxygenated methylene carbons at δC 62.3, 78.4 and 79.7; an oxygenated methine carbon at δC 80.0; eight methine carbons at δC 32.1, 54.3, 130.3, 131.5, 131.8, 132.9, 136.0 and 133.6; and two nonprotonated carbons at δC 87.4 and 106.1 (Table 1).

Table 1.
1H and 13C NMR data for 1–4 at 600 MHz and 150 MHz in CD3OD (δ in ppm, J in Hz), respectively.
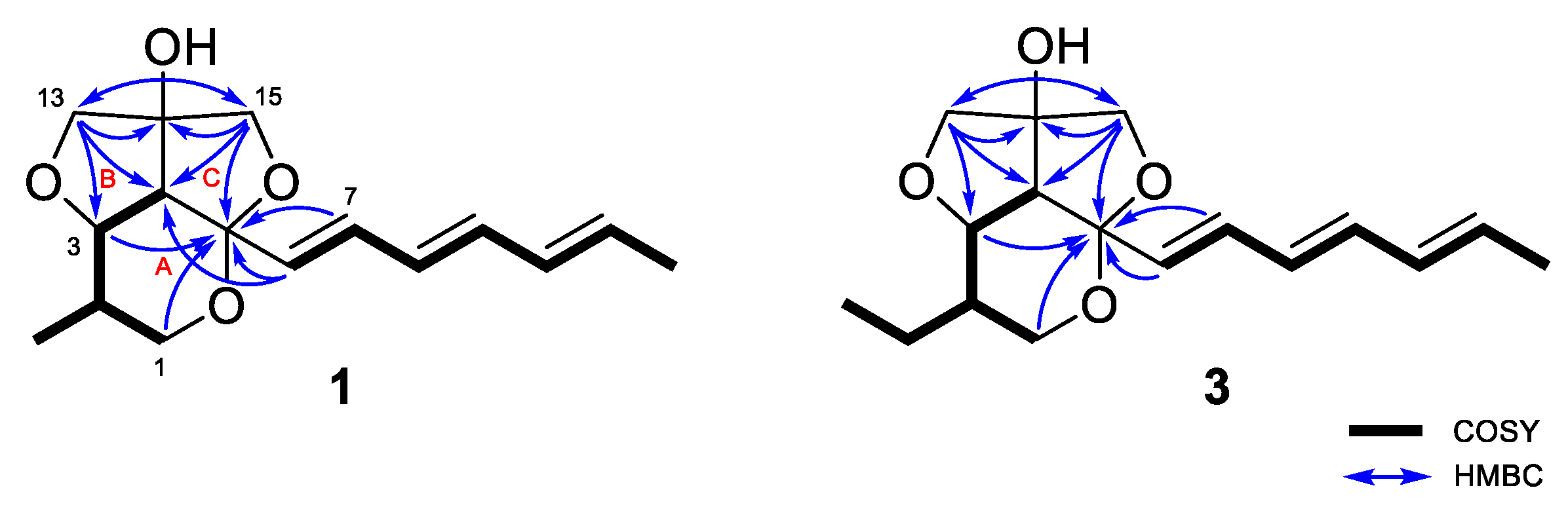
The planar structure of 1 was elucidated by detailed analysis of 1H-1H COSY and HMBC data. The structure of ring A was determined as a 2,3,4-substituted-5-methyl-tetrahydropyran by the continuous COSY correlations from H-1a,b to H-4 through H-2, H-3, and H3-16 and the HMBC correlations from H-1a,b to C-5 (Figure 2). The HMBC correlations from H-13a,b to C-14 and C-15; and H-15a,b to C-13 and C-14 and their chemical shifts of δC 79.7 (C-13), 87.4 (C-14), and 78.4 (C-15) identified a partial structure corresponding to a 2-C-substituted glycerol moiety. The HMBC correlations from H-13a,b to C-3 and C-4 and from H-15a,b to C-4 and C-5 confirmed the connection of the glycerol moiety to the ring A via ether linkages of C-3/C-13 and C-5/C15 and the linkage between C-4 and C-14 was also determined by the HMBC correlations from H-13a,b to C-4 and C-14 and H-15a,b to C-4 and C-14. The presence of a triene side chain was determined by the continuous COSY correlations from H-6 to H3-12 and the connection of the side chain to the ring A at C-5 was confirmed by the HMBC correlations from H-6 to C-4 and C-5 and from H-7 to C-5. Thus, the planar structure of 1 was determined as 2-methylstreptoglyceride B which is closely related to streptoglyceride B, recently isolated from Streptomyces sp. [16].
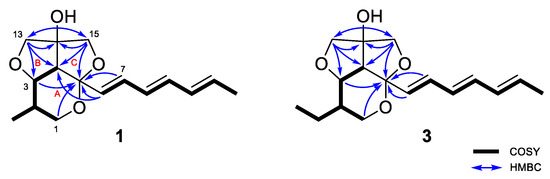
Figure 2.
Key 1H-1H COSY and HMBC correlations for streptoglycerides E (1) and G (3).
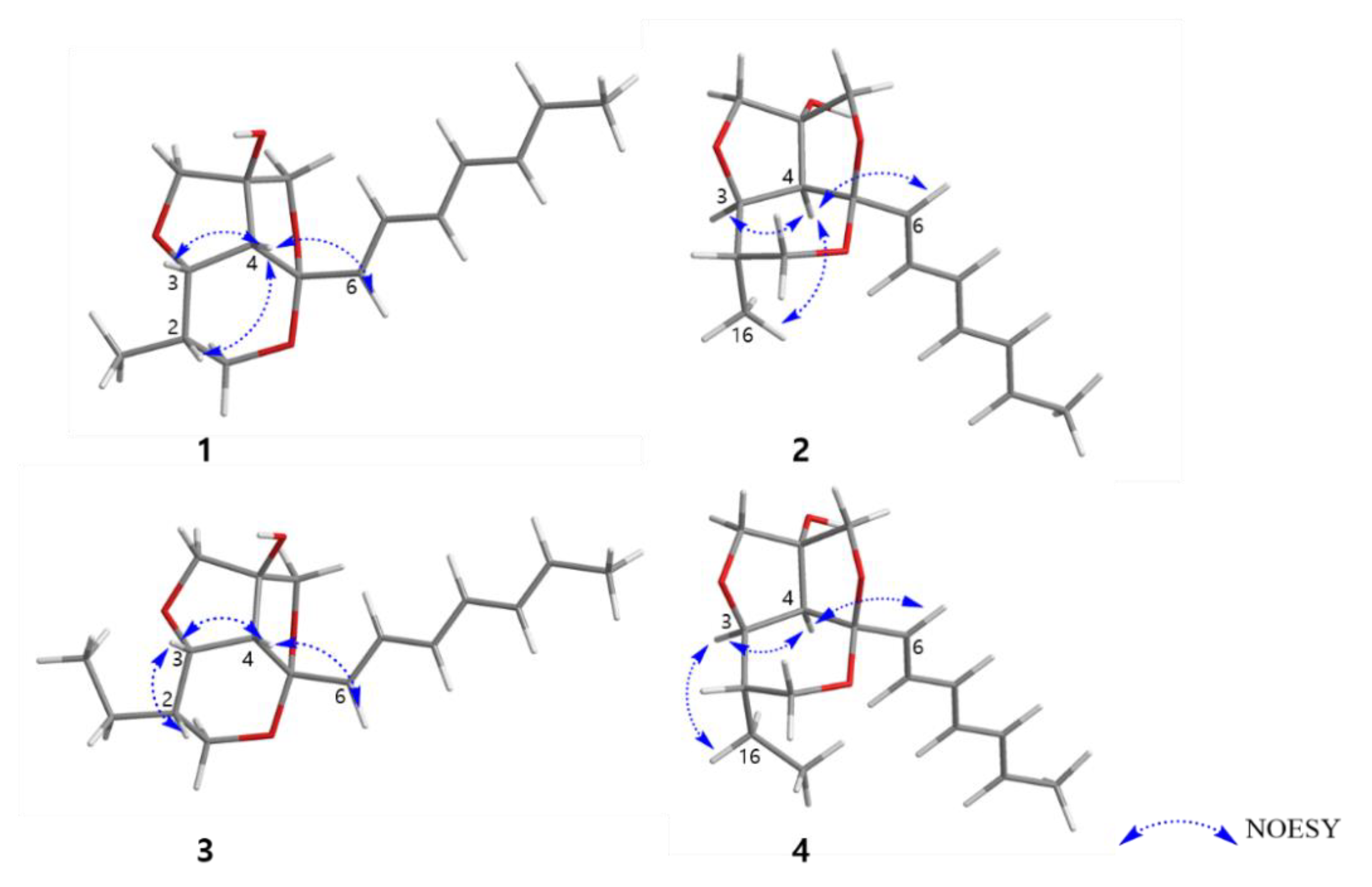
The relative configuration of 1 was determined by NOESY correlations and coupling constant analysis (Figure 3). The geometries of double bonds in the triene chain were deduced as E-configuration on the basis of their large coupling constants (J ≥14 Hz, Table 1). The NOESY correlations from H-4 to H-2, H-3, H-6, and H-7 suggested that these protons had a cofacial relationship. The coupling constant (7.0 Hz) for H-3 and H-4 also indicated these two protons are located as a syn-arrangement.
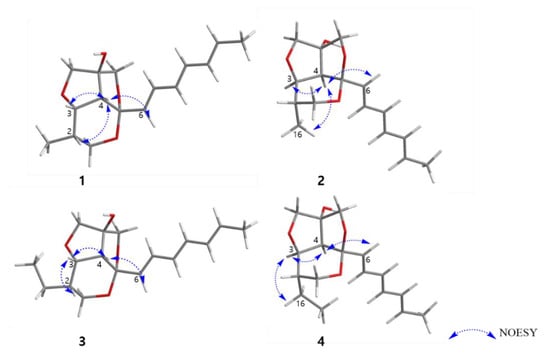
Figure 3.
Key NOESY correlations of 1–4.
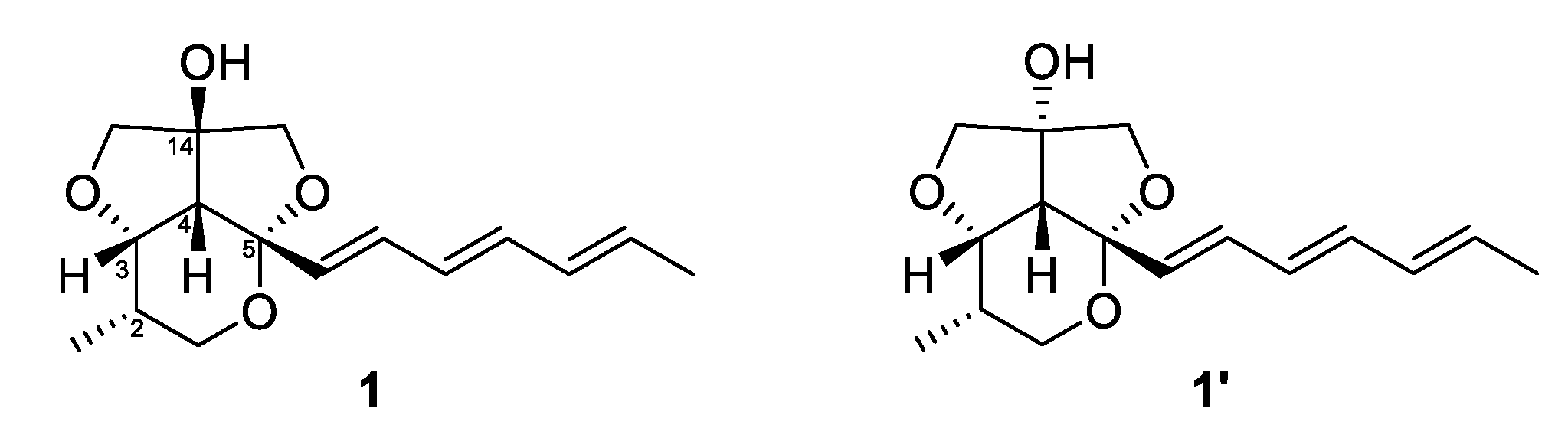
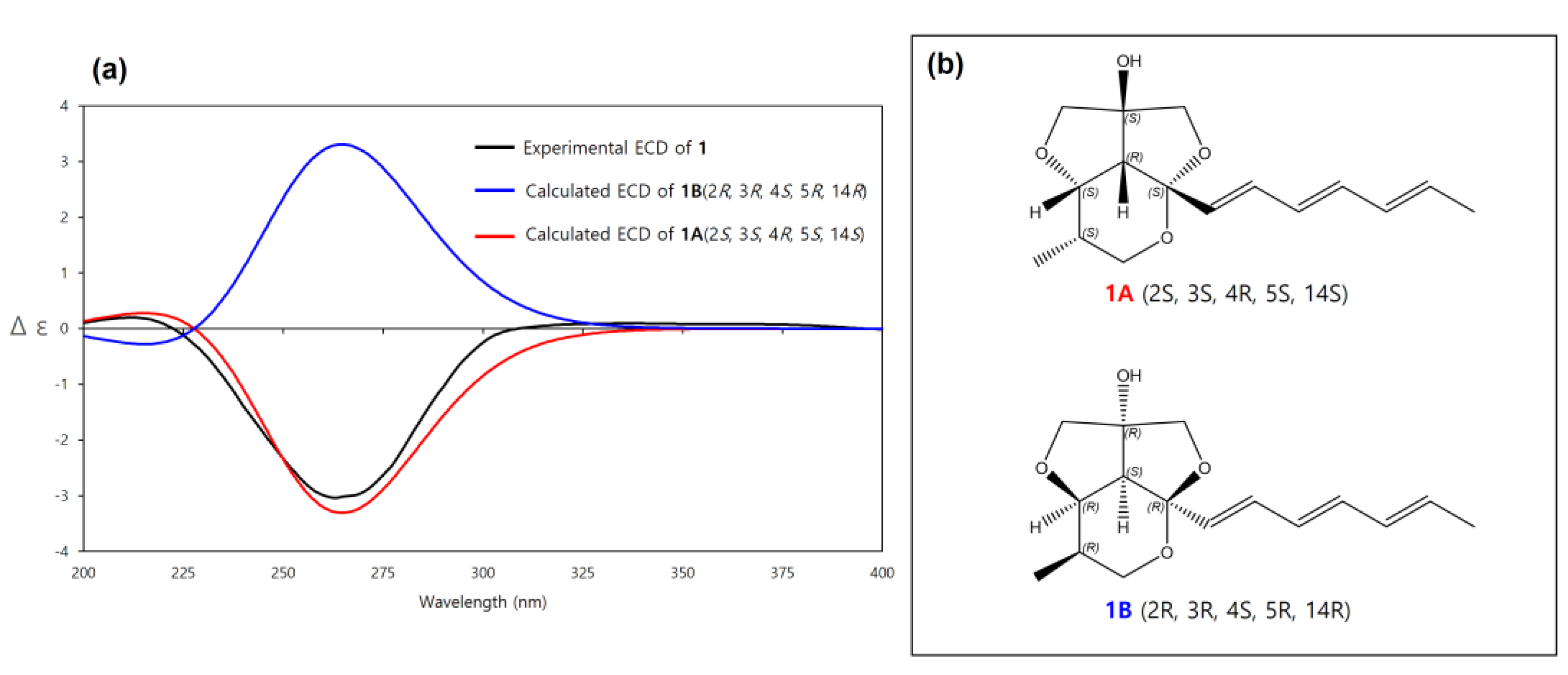
Furthermore, the fact that the furofuran ring system (rings B and C) is a rigid structure and always has a cis-fused relationship, on the basis of general stereochemical considerations, and the lack of NOE correlations from H-6 to H-13b and H-15b allowed us to determine the relative configuration of C-14 (OH-14 and H-4 had a cis-relationship) [17,18]. Therefore, the relative configuration of C-2, C-3, C-4, C-5 was determined as 2S*, 3S*, 4R*, 5S* as depicted in Figure 3. To further confirm this fact, the 3D models and conformational analysis of two possible relative configurations of 1 (2S*, 3S*, 4R*, 5S*, 14S*) and 1’ (2S*, 3S*, 4R*, 5S*, 14R*) were built by Conflex program and their 1H and 13C chemical shifts were calculated by Gaussian software. Using both 1 H and 13C NMR chemical shifts in the DP4+ probability output resulted in a 100% preference for the 1 diastereomer over the 1’ diastereomer (Figure 4, Figure S33 in the supplementary). This result also supported the fact that H-4 and OH-14 are cofacial. Thus, there were only two possible absolute configurations of the 6/5/5 tricyclic ring system for 1 as 1A (2S, 3S, 4R, 5S, 14S) or 1B (2R, 3R, 4S, 5R, 14R).
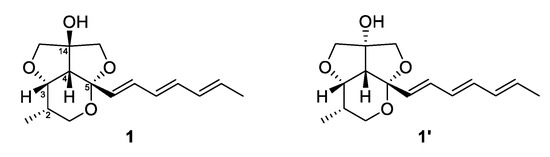
Figure 4.
Structures of 1 and 1’.
The absolute configuration of 1 was determined by ECD spectrum calculations. The theoretical ECD spectra of 1A (2S, 3S, 4R, 5S, 14S) and its enantiomer 1B (2R, 3R, 4S, 5R, 14R) were generated by the Gaussian 16 program. The experimental ECD spectrum of 1 showed a good agreement with the calculated ECD spectrum of 1A (Figure 5). Therefore, the absolute configuration of 1 was determined as 2S, 3S, 4R, 5S, 14S, which was same to other natural products with the 6/5/5 tricyclic ring skeleton (diocollettine A, streptoglycerides A–D, and bafilomycins P-Q) [16,17,19]. Thus, 1 was determined as a new derivative of streptoglyceride B and named streptoglyceride E.
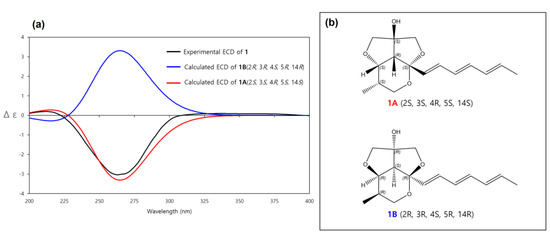
Figure 5.
(a) Experimental and calculated ECD spectra of 1A and 1B. (b) Absolute configurations of 1A (2S, 3S, 4R, 5S, 14S) and its enantiomer 1B (2R, 3R, 4S, 5R, 14R).
Compound 2 was also isolated as a white powder. The molecular formula of 2 was determined to be the same as that of 1, C16H22O4 by HRESIMS data (m/z 301.1417 [M+Na]+, calcd. for C16H22O4Na, 301.1416). The 1D and 2D NMR data of 2 were similar but not identical to those of 1, and by detailed analysis of HMBC and COSY data, the planar structure of 2 was determined to be the same as 1, suggesting that 2 is a diastereomer of 1. Additionally, the optical rotation values of 2 { +10.0 (c 0.1, MeOH)} and 1 { −20.0 (c 0.1, MeOH)} also supported their stereoisomeric relationship.
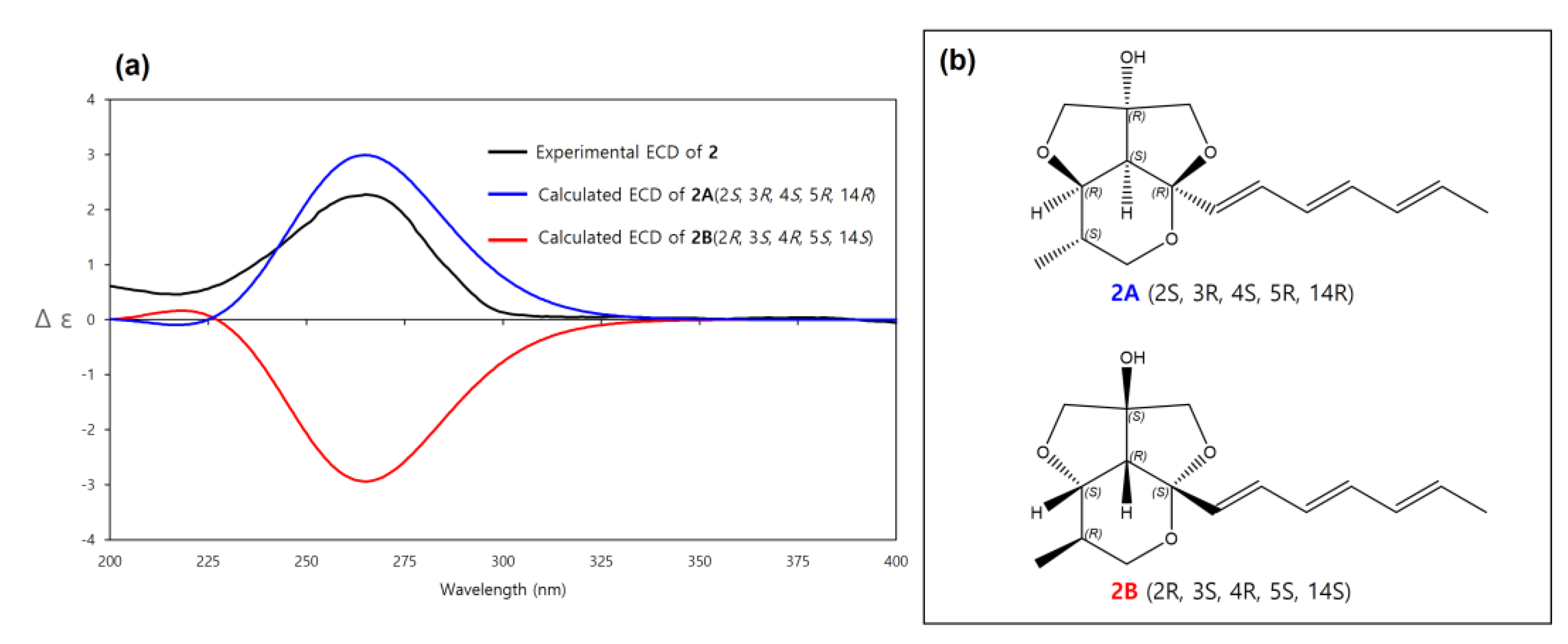
The relative configuration of 2 was also determined by analysis of NOESY data and coupling constants. The strong NOESY correlations between H-4 and H-3, H-6 and H3-16 indicated that these protons were located on the same face of the molecule. The significant difference between 2 and 1 was the methyl group (H3-16) located on the same face with H-4 in 2, while H-4 and H3-16 had a trans-relationship in 1. Furthermore, the fact that H-4 and OH-14 had a co-facial relationship was determined by a similar procedure to that of 1. Therefore, the relative configuration of 2 was determined as 2S*, 3R*, 4S*, 5R*, 14R* (2A) (Figure 3).
The absolute configuration of 2 was determined by comparison of its experimental ECD spectrum with calculated ECD spectra of 2A (2S, 3R, 4S, 5R, 14R) and its enantiomer 2B (2R, 3S, 4R, 5S, 14S). The experimental ECD spectrum of 2 matched well with the calculated ECD spectrum of 2A (Figure 6). Therefore, the absolute configuration of 2 was determined as 2S, 3R, 4S, 5R, 14R. Thus, 2 was a new diastereomer of 1 and named streptoglyceride F.
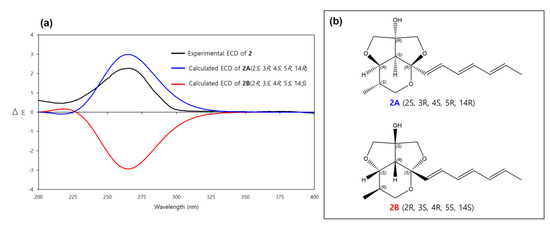
Figure 6.
(a) Experimental and calculated ECD spectra of 2A and 2B. (b) Absolute configurations of 2A (2S, 3R, 4S, 5R, 14R) and its enantiomer 2B (2R, 3S, 4R, 5S, 14S).
Compound 3 was isolated as a white powder. The molecular formula of 3 was determined to be C17H24O4 by HR-ESIMS data (m/z 315.1570 [M+Na]+, calcd. for C17H24O4Na 315.1572), one methylene group (-CH2-) more than that of 1. The 1H and 13C NMR data of 3 were almost identical to that of 1, except for the presence of one more methylene group at δC 21.5 (C-16) and δH 1.31 (H-16a) and 1.40 (H-16b). Furthermore, the continuous COSY correlations from H3-17 (δH 0.97) to H-2 (δH 1.69) via H-16a,b suggested that an ethyl group was substituted at C-2 in 3 instead of a methyl substitution in 1. Thus, the planar structure of 3 was determined as 2-ethylstreptoglyceride B (Figure 2).
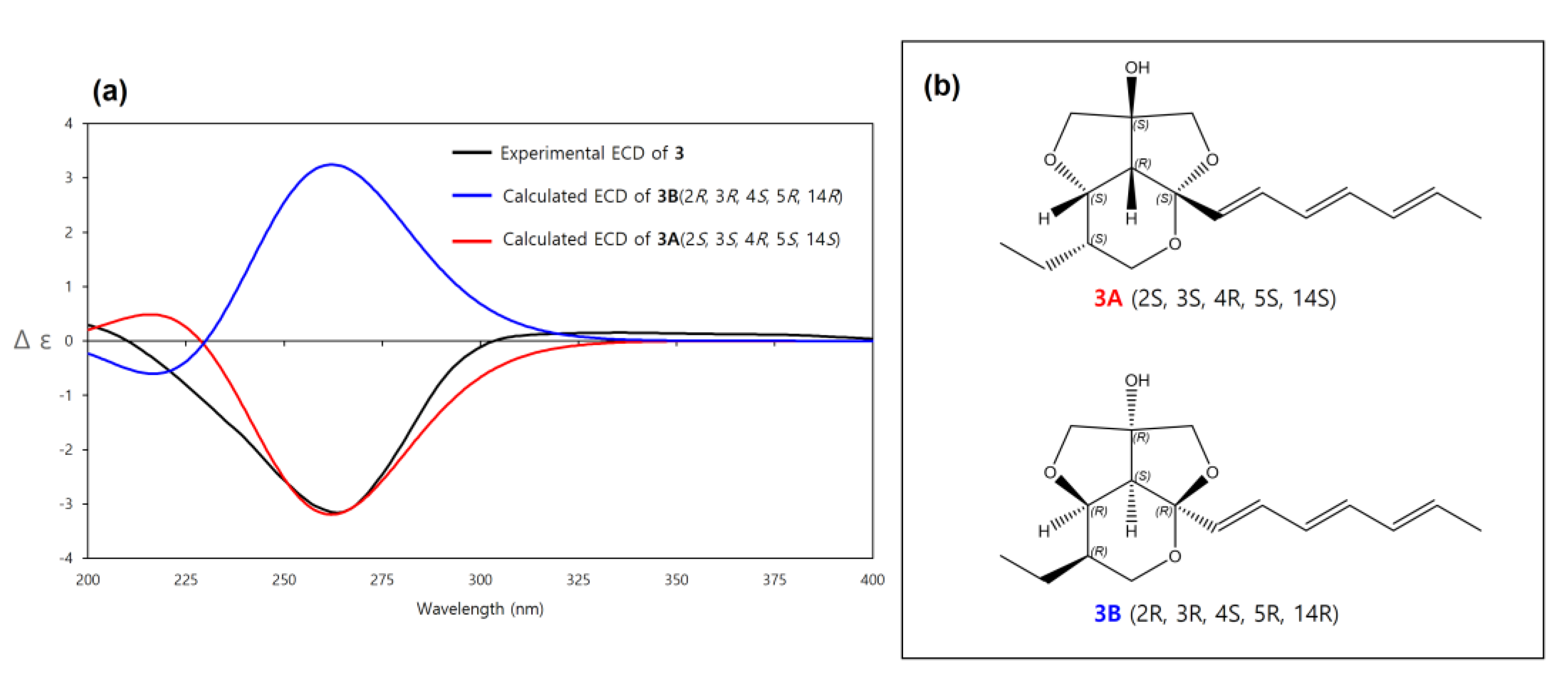
The relative configuration of 3 was determined to be the same as 1 by the same afore-mentioned procedure for 1, (2S*, 3S*, 4R*, 5S*, 14S*, (3A)) (Figure 3), and the experimental ECD spectrum of 3 was compared to the calculated ECD spectra of 3A and its enantiomer 3B. The experimental ECD spectrum of 3 was well-matched to the calculated ECD spectrum of 3A (Figure 7). Therefore, the absolute configuration of 3 was determined as 2S, 3S, 4R, 5S, 14S. Thus, the structure of 3 was determined as a new derivative of streptoglyceride B and named streptoglyceride G.
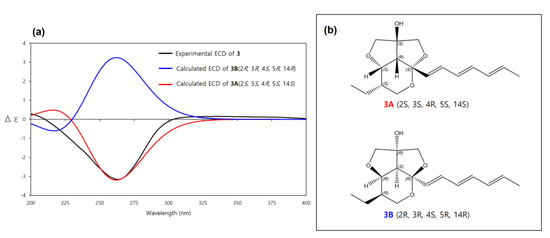
Figure 7.
(a) Experimental and calculated ECD spectra of 3A and 3B. (b) Absolute configurations of 3A (2S, 3S, 4R, 5S, 14S) and its enantiomer 3B (2R, 3R, 4S, 5R, 14R).
Compound 4 was isolated as a white powder. The molecular formula of 4 was determined to be the same as that of 3, C17H24O4 by HR-ESIMS data (m/z 315.1573 [M+Na]+, calcd. for C17H24O4Na, 315.1572). The 1D and 2D NMR data of 4 were also almost identical to those of 3 and by detailed analysis of HMBC and COSY data, the planar structure of 4 was determined to be the same as 3, (2-ethylstreptoglyceride B). However, the optical rotation values of 4 { +30.0 (c 0.1, MeOH)} and 3 { −40.0 (c 0.1, MeOH)} suggested that they had a stereoisomeric relationship.
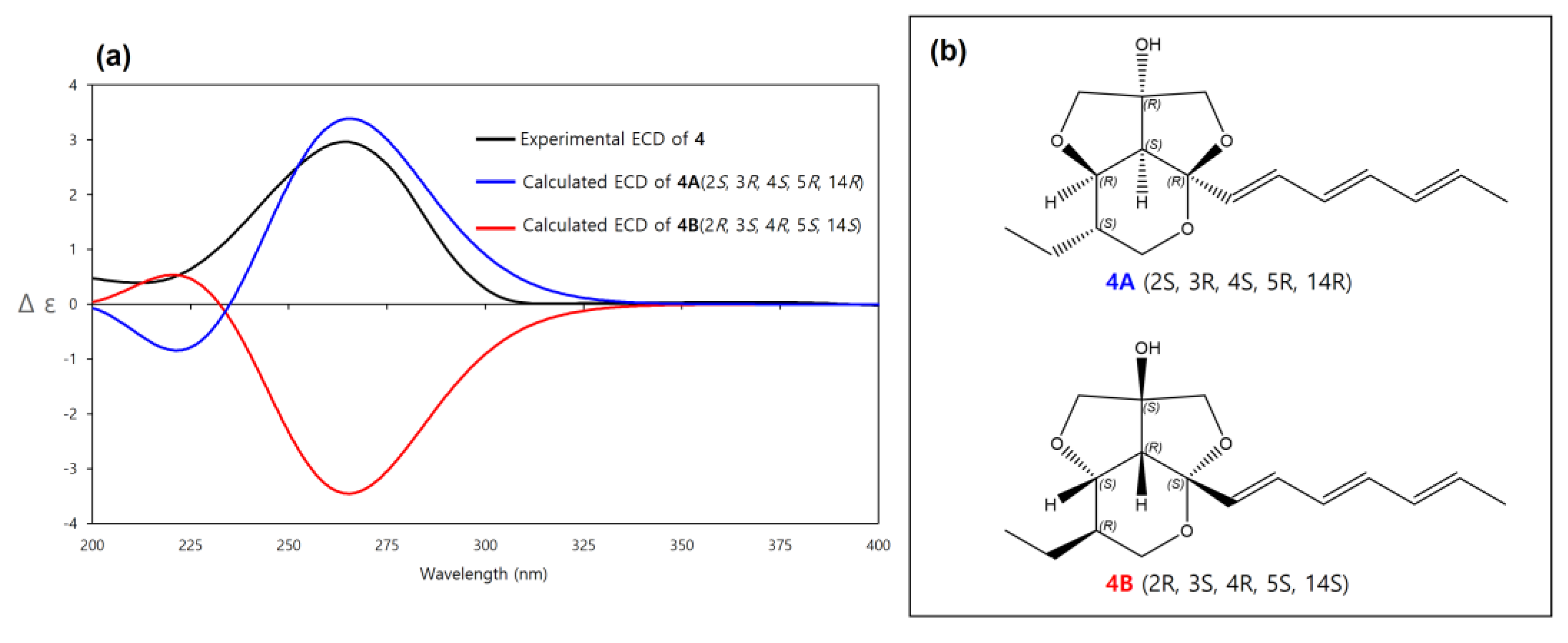
The relative configuration of 4 was determined to be the same as 2 by the same above-mentioned procedure for 2 (2S*, 3R*, 4S*, 5R*, 14R*, (4A)) and the experimental ECD spectrum of 4 was compared to the calculated ECD spectra of 4A (2S, 3R, 4S, 5R, 14R) and its enantiomer 4B (2R, 3S, 4R, 5S, 14S). The experimental ECD spectrum of 4 was well matched to the calculated ECD spectrum of 4A (Figure 8). Therefore, the absolute configuration of 4 was determined as 2S, 3R, 4S, 5R, 14R. Thus, 4 was determined as a new diastereomer of 3 and named streptoglyceride H.
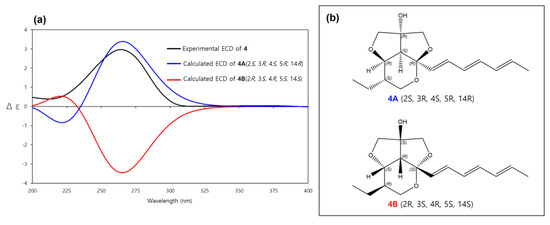
Figure 8.
(a) Experimental and calculated ECD spectra of 4A and 4B. (b) Absolute configurations of 4A (2S, 3R, 4S, 5R, 14R) and its enantiomer 4B (2R, 3S, 4R, 5S, 14S).
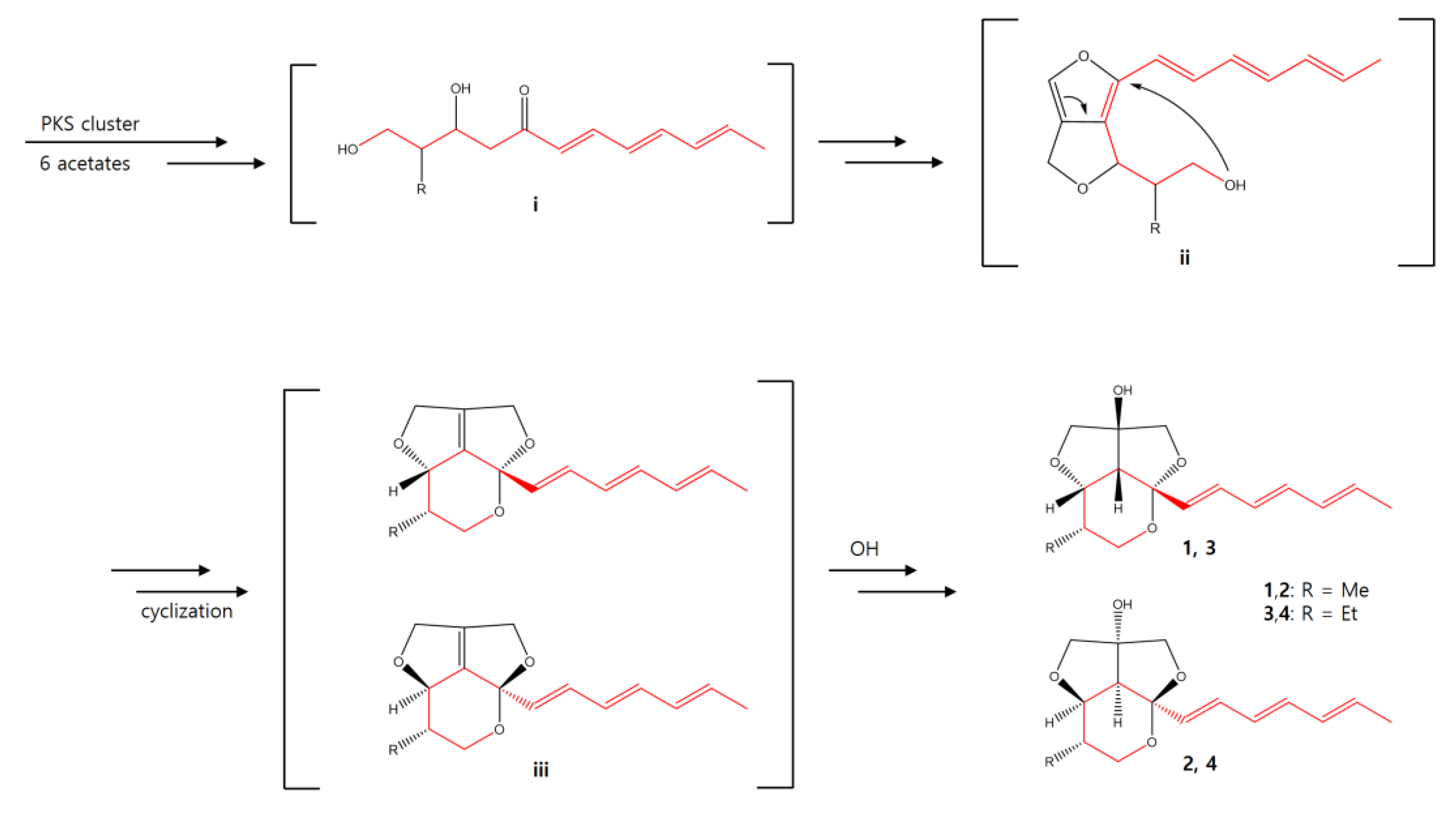
The biosynthetic pathway of streptoglyceride A–D was previously proposed [20]. Similarly, we propose a plausible biosynthetic pathway of 1–4 (Scheme 1). The polyketide synthase (PKS) clusters would lead to the formation of a hexaketide intermediate i as a starting compound. The intermediate i could be converted to ii to form an intermediate with a rare 5/5 furo[3,4-c]furan ring. Afterward, cyclization of ii affords intermediate iii, which could be further converted into streptoglycerides E-H possessing a rare 6/5/5 ring system.
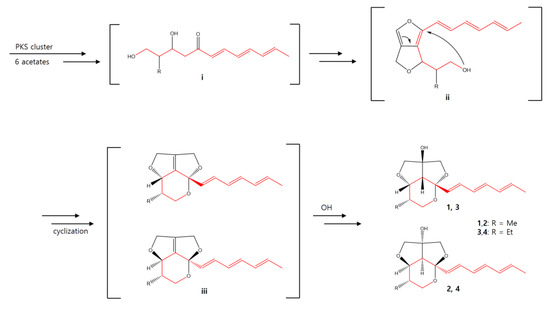
Scheme 1.
Plausible biosynthesis pathway of 1–4.
2.2. Bioactivities
Compounds 1–4 were screened for their effects on the production of NO in LPS-stimulated RAW 264.7 mouse macrophage cell line. All four compounds showed moderate inhibitory effects with IC50 values ranging from 3.5 to 10.9 μM (Table 2). The test was performed four times to confirm the reproducibility and statistical analyses were conducted using a t-test.

Table 2.
Inhibitory effects of 1–4 on LPS-induced NO production in RAW 264.7 cells.
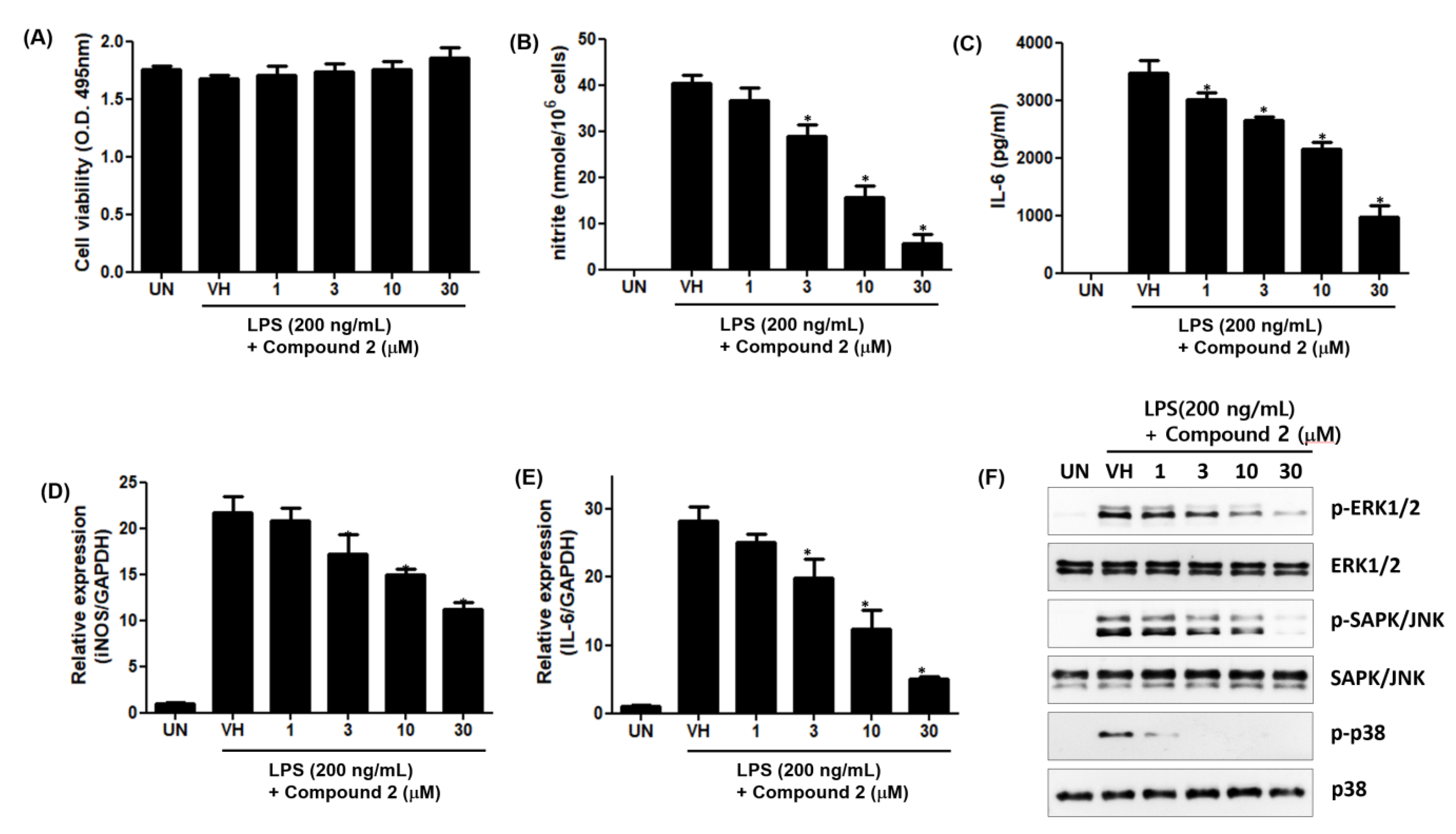
To further investigate the anti-inflammatory effects of 2, we examined the effect of 2 on LPS-induced production of inflammatory mediators, including NO and Interleukin-6 (IL-6), in RAW 264.7 cells. As shown in Figure 9A,B, the treatment of RAW 264.7 cells with LPS increased the accumulation of nitrite and IL-6, and 2 dose-dependently inhibited LPS-induced production of nitrite and IL-6 in LPS-stimulated RAW 264.7 cells. To further investigate whether the effects of 2 were due to its effects on the mRNA expression of cognate genes, we examined the effect of 2 on the mRNA expression of inducible nitric oxide synthase (iNOS) and IL-6 by qPCR. The mRNA levels of iNOS and IL-6 were induced by LPS treatment, and this induction was suppressed by 2 in a dose-dependent manner (Figure 9D,E).
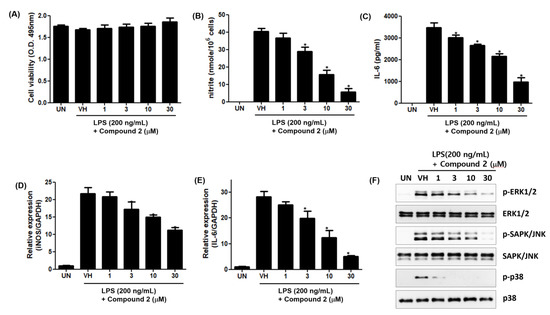
Figure 9.
Inhibitory effects of 2 on LPS-induced nitrite production and IL-6 secretion in RAW 264.7 cells. RAW 264.7 cells were pretreated with the indicated concentrations of 2 for 1 h and stimulated with LPS (200 ng/mL) for 24 h. (A) Cell viability was determined by XTT assay. The levels of nitrite (B) and IL-6 (C) in culture supernatants were determined by Griess reaction and ELISA, respectively. The mRNA levels of iNOS (D) and IL-6 (E) were examined by qPCR. Data are represented as mean ± S.D. of quadruplicate determinations. * denotes that the response is significantly different from vehicle-treated group as determined by Dunnett’s multiple comparison test at p < 0.05. (F) MAPK activation was tested by Western Immunoblot Analysis. The results shown are representatives of more than two independent experiments.
The concentrations of 2 used in this study had no cytotoxic effect on the viability of RAW 264.7 cells. Additionally, the mitogen-activated protein kinase (MAPK) activation study showed that phosphorylation of extracellular signal-regulated protein kinases (ERK), c-Jun N-terminal kinase (JNK) and p38 proteins was inhibited by 2 (Figure 9). The ERK, JNK and p38 proteins belonging to the MAPK superfamily are phosphorylated in the cytoplasm of stimulated cells by LPS. The activated p-ERK, p-JNK and p38 proteins activate transcription factors related with inflammation in their nucleus. Thus, 2 is considered to have anti-inflammatory activity by inhibiting the activation of the MAPK pathway.
3. Materials and Methods
3.1. General Experimental Procedures
The 1D and 2D NMR spectra were acquired on a Bruker 600 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). UV-VIS spectra were acquired by a Shimadzu UV-1650PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan). IR spectra were acquired on a JASCO FT/IR-4100 spectrophotometer (JASCO Corporation, Tokyo, Japan). Optical rotations were recorded on a Rudolph Research Analytical (Autopol III) polarimeter. High-resolution ESIMS experiments were performed on a hybrid ion-trap time-of-flight mass spectrometer (Shimadzu LC/MS-IT-TOF, Kyoto, Japan). HPLC was performed on a RI-101(Shodex). Semi-preparative HPLC was conducted using an ODS column (YMC-Pack-ODS-A, 250 × 10 mm i.d, 5 µm).
3.2. Isolation and Cultivation of the Strain 208DD-067
The strain 208DD-067 was isolated from a sediment sample collected in Dokdo, South Korea in August 2020. The strain was identified as Streptomyces specialis on the basis of 16S rRNA gene sequence analysis (GenBank accession number OL691077). The seed and mass cultures of the strain 208DD-067 were performed in Bennett’s medium (BN broth, 1% glucose, 0.2% tryptone, 0.1% yeast extract, 0.1% beef extract, 0.5% glycerol, 3.2% sea salt, pH 7.0 before sterilization). A single colony of the strain from the agar plate was inoculated aseptically into a 2 L flask filled with 1 L of BN broth. After that, the strain was incubated at 24 °C for 7 days on a rotary shaker at 120 rpm and then the culture broth was transferred to a 100 L fermenter filled with 70 L of BN broth. The mass culture was incubated for 21 days at 28 °C and then harvested.
3.3. Extraction and Isolation of Metabolites
The culture broth (70 L) was centrifuged (60,000 rpm), and the supernatant was extracted twice with EtOAc (70 L). The EtOAc soluble part was evaporated to yield a crude extract (7.0 g). The extract was subjected to ODS open column chromatography followed by a stepwise gradient elution with MeOH in H2O (1:4, 2:3, 3:2, 4:1 and 100:0, v/v). The fraction eluted with 60% MeOH in H2O was purified by a semi-preparative reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 10 mm i.d, 5 µm, flow rate 2.0 mL/min, RI detector) using an isocratic elution with 60% MeOH in H2O to yield 1 (0.5 mg, tR = 36 min) and 2 (0.9 mg, tR = 31 min) and the fraction eluted with 80% MeOH in H2O was purified by a semi-preparative reversed-phase HPLC (YMC-Pack-ODS-A, 250 × 10 mm i.d, 5 µm, flow rate 2.0 mL/min, RI detector) using an isocratic elution with 50% ACN in H2O to yield 3 (0.9 mg, tR = 20 min) and 4 (1.2 mg, tR = 15 min).
Streptoglyceride E (1): a white powder; −20.0 (c 0.1, MeOH); IR νmax 3343, 2960, 2840, 1643, 1465, 1409, 1014 UV (MeOH) λmax (log ε) 202 (3.45), 266 (2.48) nm; HRESIMS m/z 301.1417 [M+Na]+ (calcd for C16H22O4Na, 301.1416); 1H and 13C NMR data (CD3OD, 600 MHz and 150 MHz, respectively), Table 1.
Streptoglyceride F (2): a white powder; +10.0 (c 0.1, MeOH); IR νmax 3386, 2921, 2864, 2364, 2314, 1597, 1412, 1057 UV (MeOH) λmax (log ε) 266 (2.45) nm; HRESIMS m/z 301.1417 [M+Na]+ (calcd for C16H22O4Na, 301.1416); 1H and 13C NMR data (CD3OD, 600 MHz and 150 MHz, respectively), Table 1.
Streptoglyceride G (3): a white powder; −40.0 (c 0.1, MeOH); IR νmax 3356, 2967, 2349, 2311, 1649, 1543, 1066 UV (MeOH) λmax (log ε) 231 (2.45), 265 (2.48) nm; HRESIMS m/z 315.1570 [M+Na]+ (calcd for C17H24O4Na, 315.1572); 1H and 13C NMR data (CD3OD, 600 MHz and 150 MHz, respectively), Table 1.
Streptoglyceride H (4): a white powder; +30.0 (c 0.1, MeOH); IR νmax 3392, 2965, 2929, 2876, 1646, 1596, 1455, 1374, 1212, 1059 UV (MeOH) λmax (log ε) 266 (2.45) nm; HRESIMS m/z 315.1573 [M+Na]+ (calcd for C17H24O4Na, 315.1572); 1H and 13C NMR data (CD3OD, 600 MHz and 150 MHz, respectively), Table 1.
3.4. Cell Viability Assay
Cell viability assay was performed using a Cell Proliferation Kit II (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, the XTT labeling mixture was prepared by mixing 50 volumes of 1 mg/mL sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate with 1 volume of 0.383 mg/ml of N-methyldibenzopyrazine methyl sulfate, added to the cultures and incubated for 2 h at 37 °C. Absorbance was measured at 495 nm with a reference wavelength at 650 nm.
3.5. Computational Methods
Conformational analysis was performed using molecular mechanics force-field (MMFF94s) calculations with a search limit of 3.0 kcal mol−1 in CONFLEX version 8.0 program (CONFLEX Corporation, Tokyo, Japan). The low-energy conformers were further optimized using the density functional theory (DFT) method at the B3LYP/6-311G+(d,p) level. The theoretical ECD spectra were calculated by Gaussian 16 software (Gaussian Inc., Wallingford, CT, USA) using the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-311+G (d,p) level in MeOH with the Polarizable Continuum Model (PCM) utilizing the integral equation formalism variant (IEFPCM) model. The Boltzmann distributions of conformers were calculated based on their Hartrees energy, and the calculated ECD spectra were averaged according to the Boltzmann distributions. NMR chemical shifts were calculated by a gauge-independent atomic orbitals (GIAO) method at the B3LYP/6-311+G(d,P)/IEFPCM level in MeOH.
3.6. Nitrite Quantification
NO2− accumulation was used as an indicator of NO production in the medium. RAW 264.7 cells were plated at 5 × 105 cells/mL and stimulated with LPS (200 ng/mL) in the presence or absence of 2 (1, 3, 10 or 30 μM) for 24 h. The isolated supernatants were mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, and 2% phosphoric acid), and incubated at room temperature for 10 min. NaNO2 was used to generate a standard curve, and nitrite production was determined by measuring the optical density at 540 nm.
3.7. Enzyme-Linked Immunosorbent Assay (ELISA)
RAW 264.7 cells were plated at 5 × 105 cells/mL and stimulated with LPS (200 ng/mL) in the presence or absence of compound 2 (1, 3, 10 or 30 μM) for 24 h. The culture supernatants were collected, and the amount of IL-6 was determined by mouse IL-6 ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
3.8. RNA Isolation and Quantification of mRNA Expression by qPCR
Total cellular RNA was extracted using an RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) with RNase-Free DNase Set (Qiagen) according to the manufacturer’s instructions. cDNA was generated from total RNA by reverse transcription using AccuPower RT PreMix (Bioneer). The resulted cDNA was amplified by qPCR in conjunction with Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA, USA). For qPCR, samples were amplified by 45 cycles of denaturation (95 °C for 15s) and amplification (60 °C for 1 min using ABI 7500 Sequence Detection System (Applied Biosciences, Foster City, CA, USA). The gene expression levels relative to control gene (GAPDH) was calculated by 2−ΔΔCt method.
3.9. Western Immunoblot Analysis
Twenty micrograms of whole-cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred to a nitrocellulose membrane (Amersham Biosciences UK, Ltd., Little Chalfont, Buckinghamshire, UK). Each membrane was pre-incubated for 1 h at room temperature in Tris-buffered saline, pH 7.6, containing 0.05% Tween 20 and 5% nonfat milk. Each nitrocellulose membrane was incubated with specific antibodies against p-ERK1/2, ERK1/2, p-SAPK/JNK, SAPK/JNK, p-p38 and p38 (Cell Signaling Technology, Danvers, MA, USA). Immunoreactive bands were then detected by incubating with secondary IgG antibody conjugated with horseradish peroxidase and visualizing with enhanced chemiluminescence reagents (GE Healthcare, Chicago, IL, USA).
3.10. Statistical Analysis
One-way ANOVA followed by Dunnett’s multiple comparison test was used for statistical analysis using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). The criteria for statistical significance were set at * p < 0.05.
4. Conclusions
We isolated four new streptoglycerides E–H (1–4) from a marine-derived actinomycete Streptomyces specialis. The structures of the new compounds (1–4) were elucidated by detailed analysis of HR-ESI-MS, 1D and 2D NMR data and ECD calculations. All compounds showed good anti-inflammatory activity with IC50 values ranging from 3.5 to 10.9 µM. Furthermore, the results demonstrated that 2 inhibited the mRNA expression of iNOS and IL-6 without cytotoxicity in RAW264.7 cells. According to this study, 2 inhibited the phosphorylation of ERK, JNK and p38 proteins in the MAPK pathway. To the best of our knowledge, this study represents the third example of marine natural products with a rare 6/5/5/-membered ring system and has anti-inflammatory activity. Streptoglycerides E–H (1–4) are a promising series of compounds with good anti-inflammatory activity and the potential for further therapeutic lead identification.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md20010044/s1, Figures S1–S41: The analyzed data of MS, IR, 1D and 2D NMR spectra and Energy-minimized models of conformers of compounds 1–4, Table S1–S9: The calculated ECD data of compounds 1–4.
Author Contributions
H.J.S. was the principal investigator, who proposed ideas for the present work, managed and supervised the whole research work, prepared and corrected the manuscript, and contributed to the structure elucidation of the new compounds. C.-S.H. conducted all experiments for compounds 1–4, including fermentation, isolation, and structure elucidation, and prepared the manuscript. C.V.A. contributed to analyzing data and visualization. J.S.K. and Y.D.Y. evaluated the anti-inflammatory activity of compounds 1–4. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Korea Institute of Ocean Science and Technology (Grant PE99952) and the Ministry of Oceans and Fisheries, Republic of Korea (Grant PG52261).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in the article are available in the Supplementary Materials.
Acknowledgments
The authors express gratitude to Jung Hoon Choi, Korea Basic Science Institute, Ochang, Korea, for providing mass data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herold, K.; Mrowka, R. Inflammation-Dysregulated inflammatory response and strategies for treatment. Acta Physiol. 2019, 226, e13284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, W.J.; Heo, S.J.; Han, S.C.; Lee, H.J.; Kang, G.J.; Kang, H.K.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharmacal Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef]
- Yang, S.H.; Le, B.; Androutsopoulos, V.P.; Tsukamoto, C.; Shin, T.-S.; Tsatsakis, A.M.; Chung, G. Anti-inflammatory effects of soyasapogenol I-αa via downregulation of the MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2018, 113, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Ryan, B.M.; Horikawa, I.; Harris, B.T.; Harris, C.C. Cytokine Storms in Cancer and COVID-19. Cancer Cell 2020, 38, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Isgrò, V.; La Corte, L.; Atzeni, F.; Trifirò, G. Potential Role of Anti-interleukin (IL)-6 Drugs in the Treatment of COVID-19: Rationale, Clinical Evidence and Risks. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Shinde, P.B.; Lee, Y.M.; Hong, J.; Kim, D.K.; Jung, J.H. Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.M.; Kim, D.-C.; Sohn, J.H.; Yim, J.H.; Oh, H. Anti-Inflammatory and Protein Tyrosine Phosphatase 1B Inhibitory Metabolites from the Antarctic Marine-Derived Fungal Strain Penicillium glabrum SF-7123. Mar. Drugs 2020, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.N.; Ji, Y.K.; Kim, N.-H.; Van Tu, N.; Rho, J.-R.; Jeong, E.J. Isoquinolinequinone Derivatives from a Marine Sponge (Haliclona sp.) Regulate Inflammation in In Vitro System of Intestine. Mar. Drugs 2021, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S. Marine Streptomyces as a novel source of bioactive substances. World J. Microbiol. Biotechnol. 2010, 26, 2123–2139. [Google Scholar] [CrossRef]
- Choi, B.-K.; Park, S.-Y.; Choi, D.-K.; Shin, B.; Shin, Y.-H.; Oh, D.-C.; Lee, H.-S.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; et al. Streptoglycerides A–D with a Rare 6/5/5 Tricyclic Ring Skeleton from a Marine Actinomycete Streptomyces species. Org. Lett. 2018, 20, 6037–6040. [Google Scholar] [CrossRef]
- Li, J.-Q.; Zhao, H.-W.; Ma, Z.-J. Cytotoxic bafilomycin analogues 6/5/5 with tricyclic ring system from a marine-derived Streptomyces sp. Tetrahedron Lett. 2020, 61, 151874. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2019, 33, 1357–1373. [Google Scholar] [CrossRef]
- Jing, S.-S.; Wang, Y.; Yan, Y.-M.; Li, X.; Li, X.-J.; Zhao, C.-C.; Sun, J.-C.; Qiu, P.-Y.; Man, S.-L.; Gao, W.-Y. Diocollettines A, an unusual tricyclic diarylheptanoid derivative from the rhizomes of Dioscorea collettii. Tetrahedron Lett. 2016, 57, 3215–3217. [Google Scholar] [CrossRef]
- Choi, B.-K.; Cho, D.-Y.; Choi, D.-K.; Shin, H.J. Miharadienes A–D with unique cyclic skeletons from a marine-derived Streptomyces miharaensis. Org. Chem. Front. 2021, 8, 4845–4852. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).