In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fucoidan Compositions
2.2. Anti-Inflammatory Activity
2.2.1. The Antiradical and Total Antioxidant Activities
2.2.2. Determination of Mixture Effect
2.2.3. Inhibition of Proteins Denaturation and Membrane Stabilizing Effect
3. Materials and Methods
3.1. Materials
3.2. Extraction Procedures
3.3. Analysis of Fucoidan Composition
3.4. Determination of Antiradical/Antioxidant Activity
3.5. Determination of Mixture Effect
3.6. Inhibition of Proteins Denaturation
3.7. HRBC Membrane Stabilization
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macrom. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Phar. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Monsur, H.A. Anti-inflammatory compounds of macro algae origin: A review. J. Med. Plant Res. 2011, 5, 7146–7154. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Bilan, M.I. Fucoidans—Sulfated polysaccharides of brown algae. Rus. Chem. Rev. 2009, 78, 785. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from fucoidan: New developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; Le Visage, C. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2015, 5, 187–197. [Google Scholar] [CrossRef]
- Qu, Y.; Cao, Z.; Wang, W.; Wang, N.A.; Li, X.; Pan, J. Monthly variations of fucoidan content and its composition in the farmed brown alga Saccharina sculpera (Laminariales, Phaeophyceae). J. Appl. Phycol. 2019, 31, 2623–2628. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.R.S.; Ulber, R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of total phenolic content, in–vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef]
- Ananthi, S.; Gayathri, V.; Malarvizhi, R.; Bhardwaj, M.; Vasanthi, H.R. Anti-arthritic potential of marine macroalgae Turbinaria ornata in Complete Freund’s Adjuvant induced rats. Exp. Toxicol. Pathol. 2017, 69, 672–680. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.S.; Lee, W.W.; Sanjeewa, K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungău, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ. Sci. Pollut. Res. 2020, 27, 11554–11564. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.-W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.-J. In Vitro and in vivo anti-inflammatory effects of sulfated polysaccharides isolated from the edible brown seaweed, Sargassum fulvellum. Mar. Drugs 2021, 19, 277. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Jayawardena, T.U.; Jeon, Y.-J. Anti-fine dust effect of fucoidan extracted from Ecklonia maxima laves in macrophages via inhibiting inflammatory signaling pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Formulation, optimization and in vivo evaluation of fucoidan-based cream with anti-inflammatory properties. Mar. Drugs 2021, 19, 643. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural diversity of fucoidans and their radioprotective effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Minina, S.A. Development of extraction technology and characterization of extract from wrack algae grist. Pharm. Chem. J. 2004, 38, 323–326. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Bilan, M.I.; Shashkov, A.S. Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Bioorg. Khim. 1998, 24, 382–389. [Google Scholar]
- Okada, F. Inflammation and free radicals in tumor development and progression. Redox Rep. 2002, 7, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Nurhidayati, L.; Fitriani, Y.; Abdillah, S.; Mumpuni, E.; Rafi, M. Physicochemical properties and antioxidant activities of crude fucoidan extracted from Sargassum cinereum. J. Ilmu Kefarmasian Indones. 2020, 18, 68–74. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Vasarri, M.; Degl’Innocenti, D. Antioxidant and anti-inflammatory agents from the sea: A molecular treasure for new potential drugs. Mar. Drugs 2022, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: Synergistic and antagonistic effects. J. Am. Oil Chem. Soc. 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Kosman, V.M.; Selezneva, A.I.; Urakova, I.N.; Makarova, M.N.; Makarov, V.G. Immunomodulatory and antioxidants properties of fixed combination of fish oil with plant extracts. Synergy 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Airanthi, M.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jonsdottir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Gangadarappa, S.K.; Vishwanath, M.; Razdan, R.; Jamwal, R.; Bhadri, N.; Inamdar, M.N. Antioxidant potential and ability of phloroglucinol to decrease formation of advanced glycation end products increase efficacy of sildenafil in diabetes-induced sexual dysfunction of rats. Sex. Med. 2016, 4, e106–e114. [Google Scholar] [CrossRef] [PubMed]
- Belaya, N.I.; Belyi, A.V.; Tikhonova, G.A.; Udalov, Y.S.; Andriyenko, G.O. Synergistic effect of binary quercetin–monosaccharide mixtures in the reaction with free radicals. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2019, 62, 38–42. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, Y.Y.; Chen, C.Y.O. Contributions of phenolics and added vitamin C to the antioxidant capacity of pomegranate and grape juices: Synergism and antagonism among constituents. Int. J. Food Sci. Technol. 2013, 48, 2650–2658. [Google Scholar] [CrossRef]
- Parker, T.L.; Miller, S.A.; Myers, L.E.; Miguez, F.E.; Engeseth, N.J. Evaluation of synergistic antioxidant potential of complex mixtures using oxygen radical absorbance capacity (ORAC) and electron paramagnetic resonance (EPR). J. Agric. Food Chem. 2010, 58, 209–217. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Laura, A.; Alvarez-Parrilla, E. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Matus-Basto, A.J.; Acereto-Escoffié, P.; Segura-Campos, M.R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric. Immunol. 2017, 28, 1424–1437. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Maramsa, N.; Basak, M.; Deka, S.; Dey, B.K. In vitro anti-inflammatory activity of Amaranthus caudatus L. leaves. Indian J. Nat. Prod. Resour. 2015, 6, 326–329. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Grant, N.H.; Alburn, H.E.; Kryzanauskas, C. Stabilization of serum albumin by anti-inflammatory drugs. Biochem. Pharmacol. 1970, 19, 715–722. [Google Scholar] [CrossRef]
- Silvestrini, B.; Guglielmotti, A.; Saso, L.; Cheng, C.Y. Changes in concanavalin A-reactive proteins in inflammatory disorders. Clin. Chem. 1989, 35, 2207–2211. [Google Scholar] [CrossRef]
- Pramitha, V.S.; Sree Kumari, N. Anti-inflammatory, anti-oxidant, phytochemical and GC-MS analysis of marine brown macroalga, Sargassum wighti. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 7–15. [Google Scholar]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. Available online: http://www.darujps.com/content/20/1/76 (accessed on 18 August 2022). [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-inflammatory activity of fucoidan extracts in vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Yang, H.-W.; Choi, C.S.; Jeon, Y.-J. Anti-inflammatory mechanisms of fucoidans to treat inflammatory diseases: A review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Dutot, M.; Grassin-Delyle, S.; Salvator, H.; Brollo, M.; Rat, P.; Fagon, R.; Naline, E.; Devillier, P. A marine-sourced fucoidan solution inhibits Toll-like-receptor-3-induced cytokine release by human bronchial epithelial cells. Int. J. Biol. Macromol. 2019, 130, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sonia, K.; Meena, K.S.; Rajesh, D. Structural characterization, biological evaluation and molecular docking studies of fucoidan isolated from brown marine algae. J. Adv. Sci. Res. 2021, 12, 90–100. [Google Scholar] [CrossRef]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Usha, S.K.; Arumugam, M. Bioactive and thermostable sulfated polysaccharide from Sargassum swartzii with drug delivery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar] [CrossRef]
- Vijayraja, D.; Jeyaprakash, K. Phytochemical analysis, in vitro DPPH free radical scavenging, anti-hemolysis and anti-inflammatory activities of Turbinaria ornata (Turner) J. Agardh. World J. Pharm. Pharm. Sci. 2016, 5, 1231–1238. [Google Scholar]
- Béress, A.; Wassermann, O.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, V.V.; Bushneva, O.A.; Ovodova, R.G.; Shashkov, A.S.; Chizhov, A.O.; Ovodov, Y.S. Structural study of bergenan, a pectin from Bergenia crassifolia. Bioorg. Khim. 2007, 33, 47–56. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Silvestri, L.J.; Hurst, R.E.; Simpson, L.; Settine, J.M. Analysis of sulfate in complex carbohydrates. Anal. Biochem. 1982, 123, 303–309. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea antarctica. Dry. Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant capacity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Jangle, S.N. Evaluation of in-vitro anti-inflammatory activity of herbal preparation, a combination of four medicinal plants. Int. J. Basic Appl. Med. Sci. 2012, 2, 109–116. [Google Scholar]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. Pharmaceutical potential of a fucoidan-like sulfated polysaccharide isolated from Halodule pinifolia. Int. J. Biol. Macromol. 2013, 62, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Shafay, S.E.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, antidiabetic, anti-inflammatory and anticancer potential of some seaweed extracts. Food Sci. Technol. 2021, 42, e20521. [Google Scholar] [CrossRef]
- Atya, M.E.; El-Hawiet, A.; Alyeldeen, M.A.; Ghareeb, D.A.; Abdel-Daim, M.M.; El-Sadek, M.M. In vitro biological activities and in vivo hepatoprotective role of brown algae-isolated fucoidans. Environ. Sci. Pollut. Res. 2021, 28, 19664–19676. [Google Scholar] [CrossRef]
- Sonia, K.; Meena, K.S.; Bai, S.A. In vitro cytotoxic activity of a sulphated polysaccharide ulvan against human breast and glioblastoma cell line. Indo Glob. J. Pharm. Sci. 2022, 12, 122–127. [Google Scholar] [CrossRef]

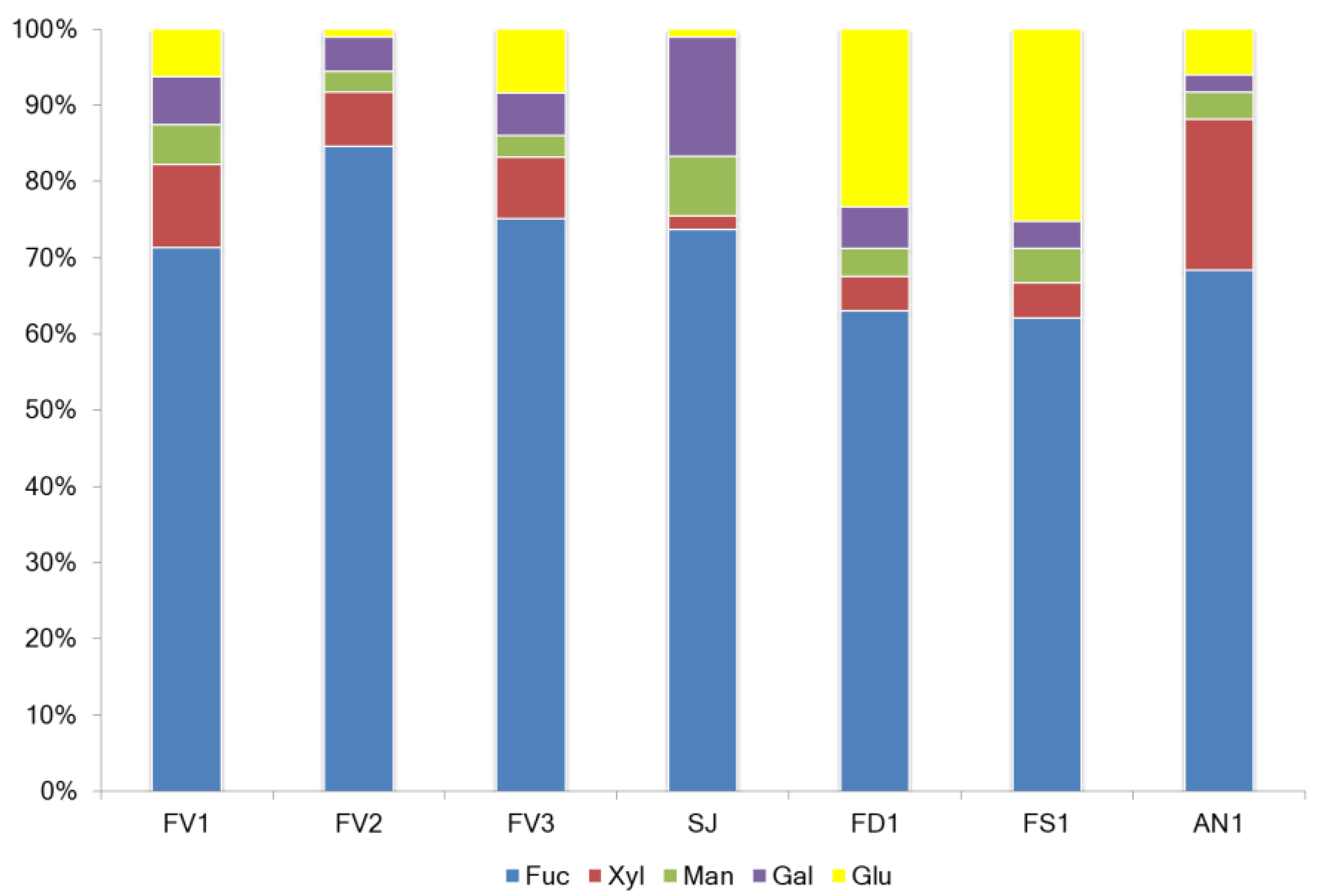
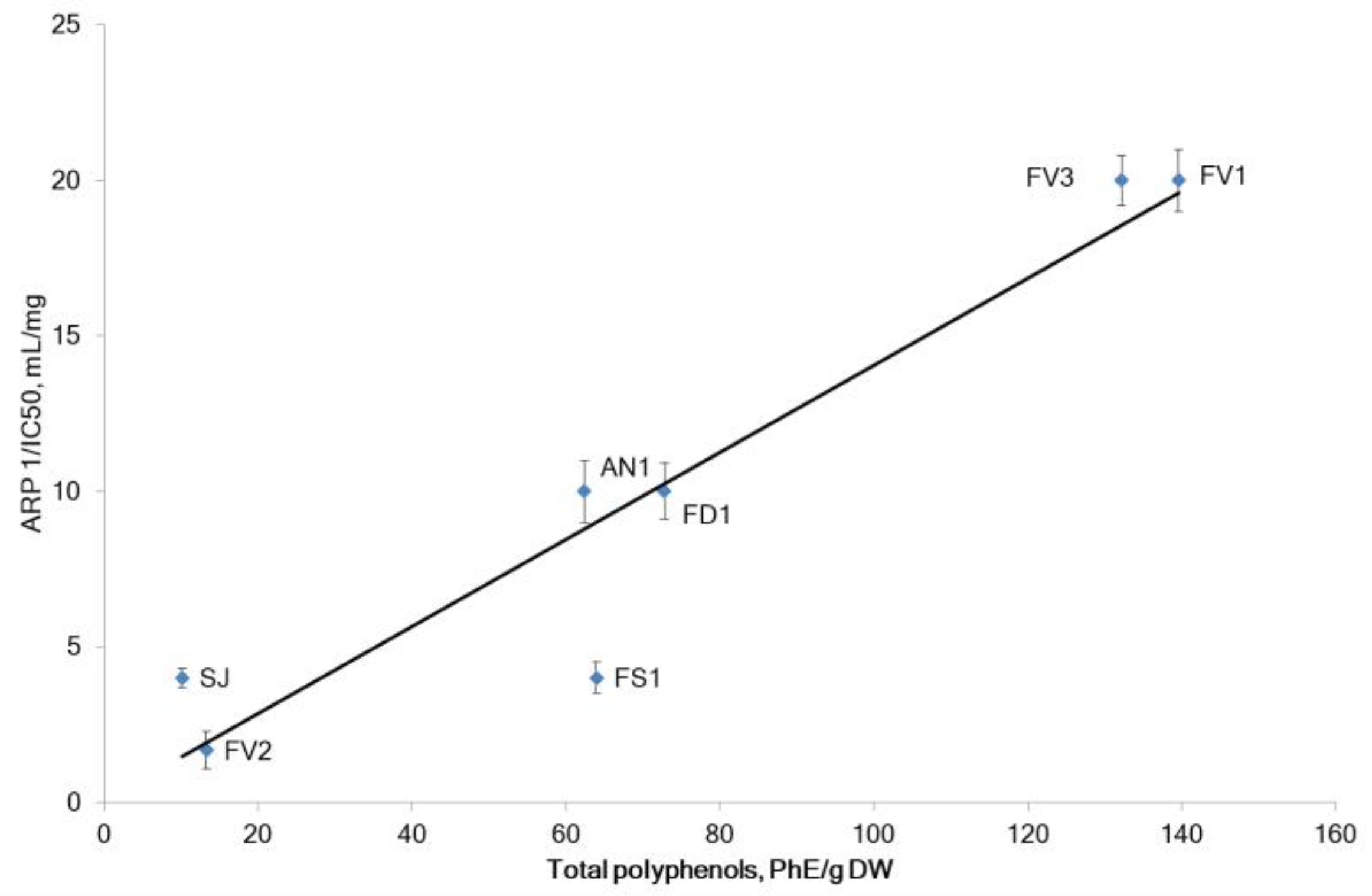


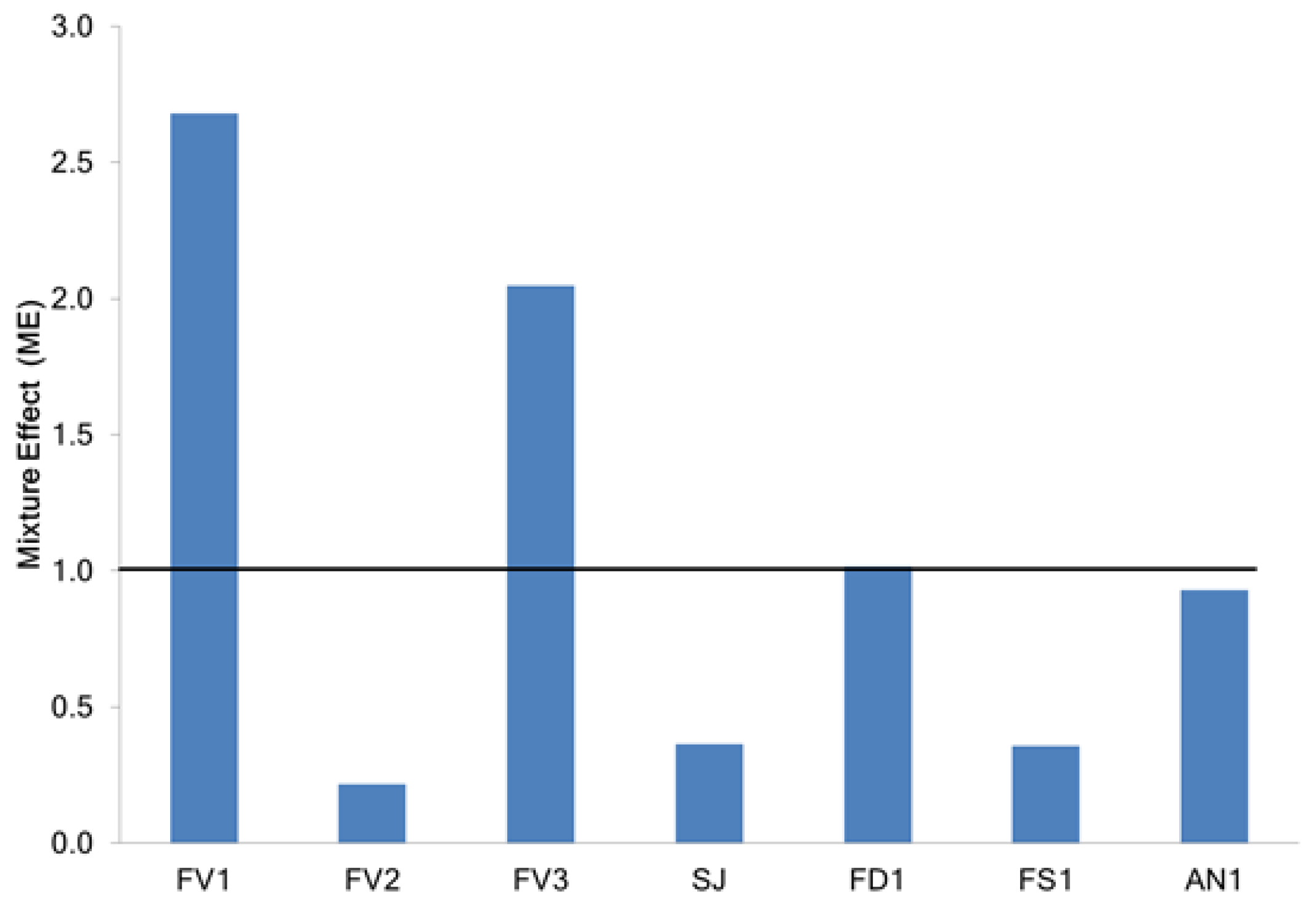


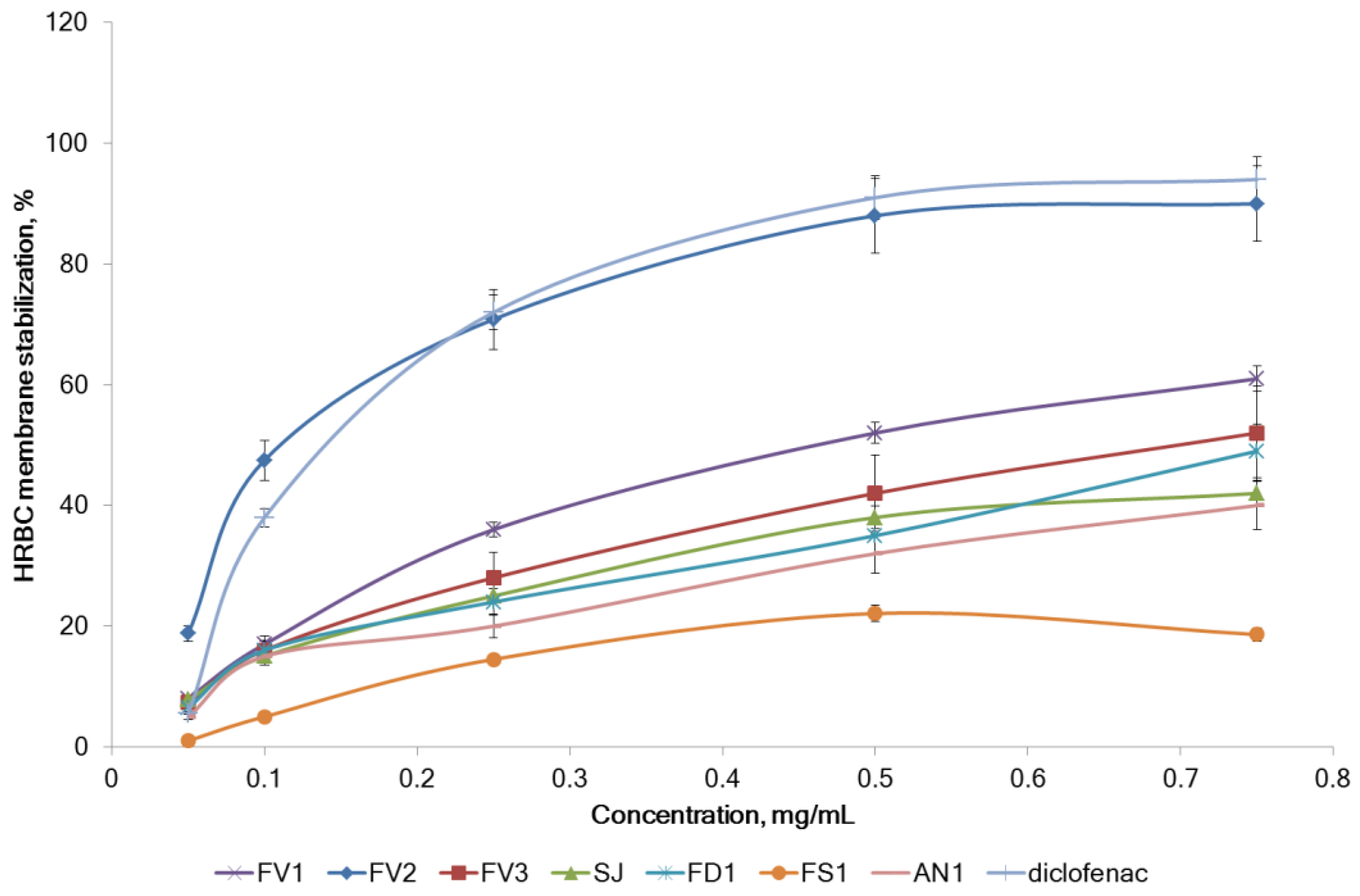
| Seaweed Source | Sample Code | Method of Extraction | Yield (% DW) | Total Neutral Carbohydrate (g/100 g DW) | Sulfate (g/100 g DW) | Total Polyphenols Content (PhE/g DW) |
|---|---|---|---|---|---|---|
| Saccharina japonica | SJ | - | - | 48.4 ± 2.5 | 28.1 ± 0.2 | 10.1 ± 0.1 |
| F. vesiculosus | FV1 | I | 22.3 | 64.1 ± 0.6 | 25.9 ± 2.6 | 139.6 ± 0.7 |
| F. vesiculosus | FV2 | II | 6.6 | 62.6 ± 0.4 | 25.4 ± 1.0 | 13.3 ± 0.3 |
| F. vesiculosus | FV3 | III | 8.2 | 53.7 ± 0.6 | 20.9 ± 1.5 | 132.2 ± 0.7 |
| F. distichus | FD1 | I | 15.4 | 50.7 ± 1.1 | 17.6 ± 0.3 | 72.8 ± 0.6 |
| F. serratus | FS1 | I | 13.6 | 45.5 ± 1.5 | 15.1 ± 0.1 | 64.0 ± 0.4 |
| A. nodosum | AN1 | I | 16.1 | 49.9 ± 0.1 | 19.2 ± 0.1 | 62.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. https://doi.org/10.3390/md20100606
Obluchinskaya ED, Pozharitskaya ON, Shikov AN. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Marine Drugs. 2022; 20(10):606. https://doi.org/10.3390/md20100606
Chicago/Turabian StyleObluchinskaya, Ekaterina D., Olga N. Pozharitskaya, and Alexander N. Shikov. 2022. "In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds" Marine Drugs 20, no. 10: 606. https://doi.org/10.3390/md20100606
APA StyleObluchinskaya, E. D., Pozharitskaya, O. N., & Shikov, A. N. (2022). In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Marine Drugs, 20(10), 606. https://doi.org/10.3390/md20100606






