Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments
Abstract
:1. Introduction
2. Results
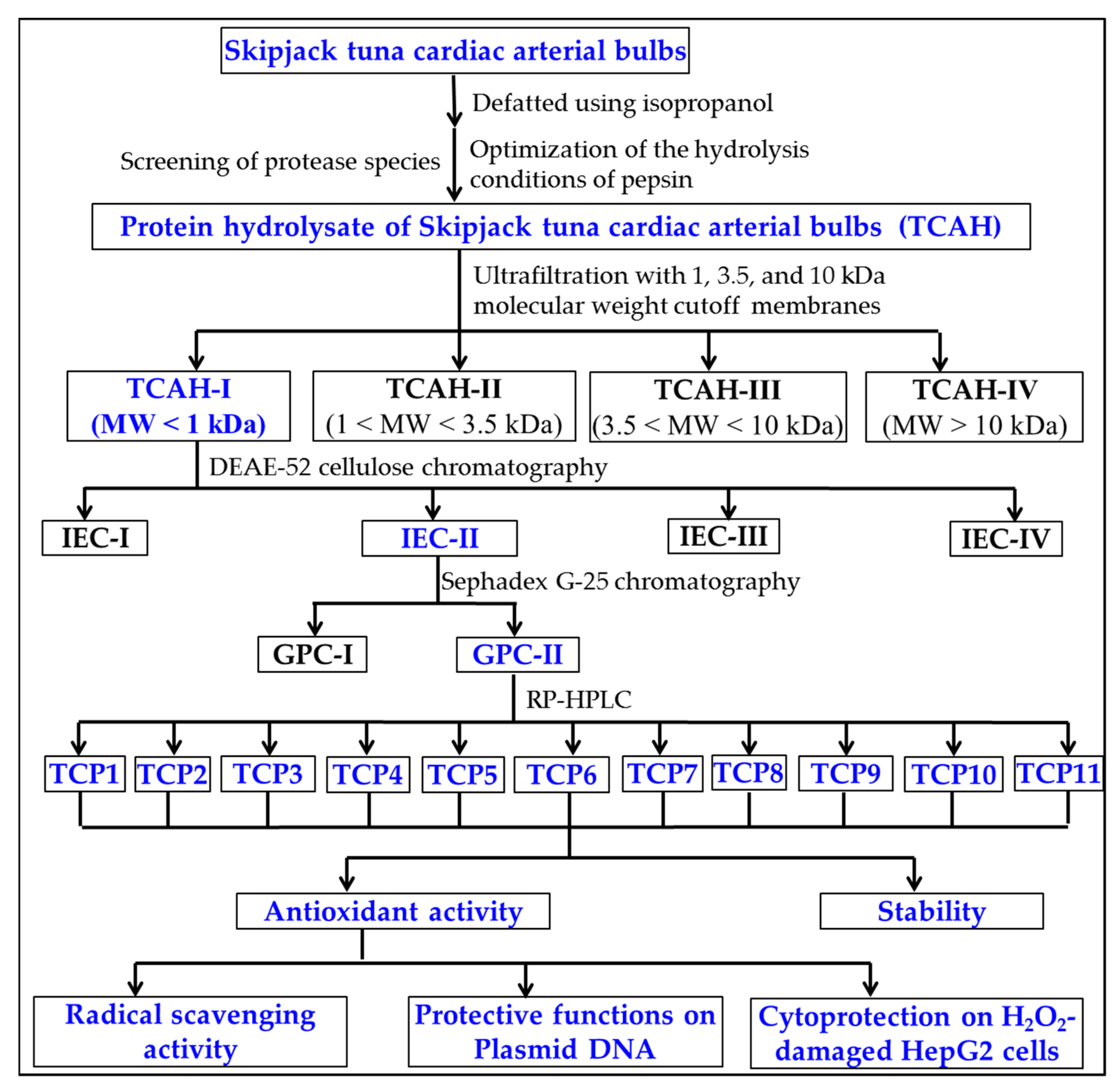
2.1. Preparation of Protein Hydrolysate of Skipjack Tuna Cardiac Arterial Bulbs
2.1.1. Screening of Protease Species
2.1.2. Pepsin Conditions Optimized by Single Factor Experiment
2.1.3. Pepsin Conditions Optimized by Response Surface Experiment
2.2. Preparation of APs from TCAH
2.2.1. Ultrafiltration
2.2.2. Chromatography
2.3. Determination of the Amino Acid Sequences and MWs of TCP1 to TCP11
2.4. Antioxidant Activity of TCP1 to TCP11
2.4.1. Radical Scavenging Activity of TCP1 to TCP11
2.4.2. Protective Effects of TCP3, TCP6, and TCP9 on H2O2-damaged DNA
2.4.3. Cytoprotective Effects of TCP3, TCP6, and TCP9 on H2O2-damaged HepG2 Cells
2.5. Stability of TCP1–TCP11
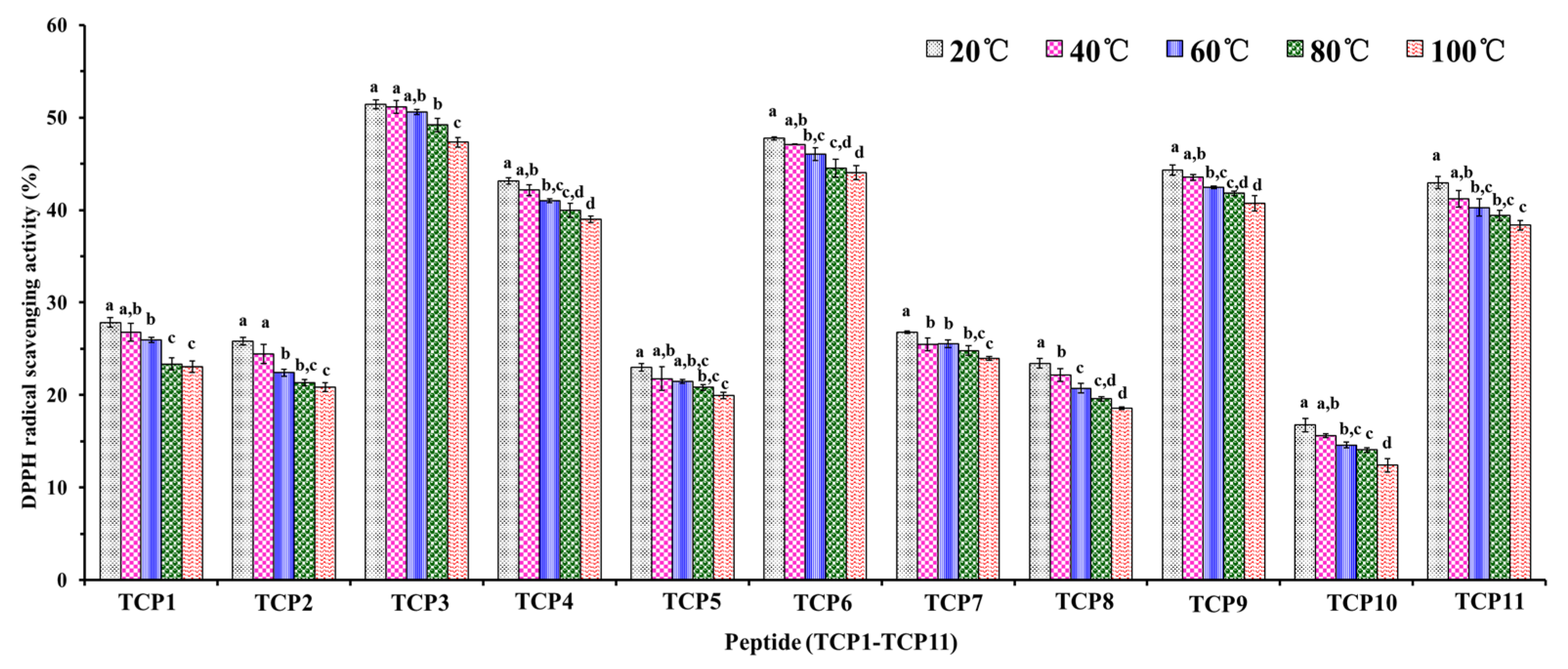
2.5.1. Thermal Stability of TCP1–TCP11
2.5.2. pH Stability of TCP1–TCP11
2.5.3. Stability of TCP1–TCP11 Subjected to Simulated Gastrointestinal (GI) Digestion
3. Discussion
3.1. Preparation of Antioxidant Peptides from Protein Hydrolysate of Tuna Cardiac Arterial Bulbs
3.2. Structure–Activity Relationship of TCP3, TCP6, and TCP9
3.3. Protective Functions of TCP3, TCP6, and TCP9 on H2O2-Damaged DNA and HepG2 Cells
3.4. Stability of TCP1–TCP11
4. Materials and Methods
4.1. Materials and Chemical Reagents
4.2. Preparation of Protein Hydrolysate of Cardiac Arterial Bulbs (TCAH)
4.2.1. Screening of Protease Species
4.2.2. Optimization of Hydrolysis Conditions of Pepsin
4.3. Preparation of APs from TCAH
4.3.1. Ultrafiltration of TCAH
4.3.2. Purification of APs from TCAH-I by Chromatography Methods
4.4. Identification of TCP1 to TCP11
4.5. Antioxidant Activity of TCP1 to TCP11 from Tuna Cardiac Arterial Bulbs
4.5.1. Radical Scavenging Activity
4.5.2. Protective Effects of TCP3, TCP6, and TCP9 on Plasmid DNA
4.5.3. Cytoprotection of TCP3, TCP6, and TCP9 on H2O2-damaged HepG2 Cells
4.6. Stability of TCP1 to TCP11 from Tuna Cardiac Arterial Bulbs
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Liu, C.; Liang, Y.; Lin, Q. Bioactive peptides from foods: Production, function, and application. Food Funct. 2021, 12, 7108–7125. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Suo, S.K.; Zhao, Y.Q.; Wang, Y.M.; Pan, X.Y.; Chi, C.F.; Wang, B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Chi, C.F.; Wang, B. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Wang, Y.M.; Suo, S.K.; Zheng, S.L.; Wang, B. Isolation, identification, molecular docking analysis and cytoprotection of seven novel ACE inhibitory peptides from miiuy croaker byproducts-swim bladders. Front. Mar. Sci. 2022, 9, 977234. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3 T3-F442A cells. PLoS ONE 2015, 10, e0117492. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.M.; Pan, X.; He, Y.; Chi, C.F.; Wang, B. Hypolipidemic activities of two pentapeptides (VIAPW and IRWWW) from miiuy croaker (Miichthys miiuy) muscle on lipid accumulation in HepG2 cells through regulation of AMPK pathway. Appl. Sci. 2020, 10, 817. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Tian, Q.; Fan, Y.; Hao, L.; Wang, J.; Xia, C.; Wang, J.; Hou, H. A comprehensive review of calcium and ferrous ions chelating peptides: Preparation, structure and transport pathways. Crit. Rev. Food Sci. Nutr. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; Food & Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and angiotensin-I-converting enzyme (ACE)-inhibitory peptides from fish as potential cardioprotective compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfifish (Lophius litulon) muscle: Purifification, identifification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.M.; Pan, X.; Chi, C.F.; Wang, B. Antioxidant mechanisms of the oligopeptides (FWKVV and FMPLH) from muscle hydrolysate of miiuy croaker against oxidative damage of HUVECs. Oxid. Med. Cell. Longev. 2021, 2021, 9987844. [Google Scholar] [CrossRef]
- Cai, S.Y.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Cytoprotective effect of antioxidant pentapeptides from the protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy) against H2O2 -mediated human umbilical vein endothelial cell (HUVEC) injury. Int. J. Mol. Sci. 2019, 20, 5425. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.M.; Li, L.Y.; Chi, C.F.; Wang, B. Twelve antioxidant peptides from protein hydrolysate of Skipjack tuna (Katsuwonus pelamis) roe prepared by flavourzyme: Purification, sequence identification, and activity evaluation. Front. Nutr. 2022, 8, 813780. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhao, Y.Q.; Zhao, G.X.; Chi, C.F.; Wang, B. Antioxidant peptides from collagen hydrolysate of redlip croaker (Pseudosciaena polyactis) scales: Preparation, characterization, and cytoprotective effects on H2O2-damaged HepG2 cells. Mar. Drugs. 2020, 18, 156. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-derived proteins and peptides: Promising marine bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth Factor-β/Smad signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 162, 633–640. [Google Scholar] [CrossRef]
- Ye, J.; Tian, X.; Wang, Q.; Zheng, J.; Yang, Y.; Xu, B.; Zhang, S.; Yuan, F.; Yang, Z. Monkfish peptides mitigate high fat diet-induced hepatic steatosis in mice. Mar. Drugs 2022, 20, 312. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Hepatoprotective effect of oyster peptide on alcohol-induced liver disease in mice. Int. J. Mol. Sci. 2022, 23, 8081. [Google Scholar] [CrossRef]
- Artetxe-Arrate, I.; Fraile, I.; Marsac, F.; Farley, J.H.; Rodriguez-Ezpeleta, N.; Davies, C.R.; Clear, N.P.; Grewe, P.; Murua, H. A review of the fisheries, life history and stock structure of tropical tuna (skipjack Katsuwonus pelamis, yellowfin Thunnus albacares and bigeye Thunnus obesus) in the Indian Ocean. Adv Mar Biol. 2021, 88, 39–89. [Google Scholar]
- Suo, S.K.; Zheng, S.L.; Chi, C.F.; Luo, H.Y.; Wang, B. Novel ACE inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study and antioxidant function on H2O2-damaged HUVECs. Front. Nutr. 2022, 9, 957778. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chi, C.F.; Wang, B.; Ding, G.F.; Li, Z.R. Characterization of acid and pepsin soluble collagens from spine and skull of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Luo, Q.B.; Suo, S.K.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Preparation, characterization, and cytoprotective effects on HUVECs of fourteen Novel Angiotensin-I-Converting Enzyme inhibitory peptides from protein hydrolysate of tuna processing by-products. Front. Nutr. 2022, 9, 868681. [Google Scholar] [CrossRef]
- Zheng, S.L.; Luo, Q.B.; Suo, S.K.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Preparation, identification, molecular docking study and protective function on HUVECs of novel ACE inhibitory peptides from protein hydrolysate of skipjack tuna muscle. Mar. Drugs 2022, 20, 176. [Google Scholar] [CrossRef]
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and characterization of gelatin and antioxidant peptides from gelatin hydrolysate of Skipjack tuna (Katsuwonus pelamis) bone stimulated by in vitro gastrointestinal digestion. Mar. Drugs. 2019, 17, 78. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, identification and activity evaluation. Mar. Drugs. 2019, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Li, X.Y.; Wang, J.; He, Y.; Chi, C.F.; Wang, B. Antioxidant peptides from protein hydrolysate of skipjack tuna milt: Purification, identification, and cytoprotection on H2O2 damaged human umbilical vein endothelial cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel antioxidant collagen peptides of Siberian sturgeon (Acipenser baerii) cartilages: The preparation, characterization, and cytoprotection of H2O2-damaged human umbilical vein endothelial cells (HUVECs). Mar. Drugs. 2022, 20, 325. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidant peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionizationmass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and Characterization of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Gao, M.; Wang, Y.Z.; Li, X.R.; Wang, P.; Wang, B. Antioxidant peptides from protein hydrolysate of marine red algae Eucheuma cottonii: Preparation, identification, and cytoprotective mechanisms on H2O2 oxidative damaged HUVECs. Front. Microbiol. 2022, 13, 791248. [Google Scholar] [CrossRef]
- Yang, X.R.; Qiu, Y.T.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar Drugs. 2019, 17, 251. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar Drugs. 2013, 11, 4641–4661. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhao, Y.Q.; Wang, Y.M.; Zhao, W.H.; Wang, P.; Chi, C.F. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 9, 562–569. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, G.X.; Suo, S.K.; Wang, Y.M.; Chi, C.F.; Wang, B. Purification, identification, activity evaluation, and stability of antioxidant peptides from alcalase hydrolysate of Antarctic Krill (Euphausia superba) proteins. Mar. Drugs. 2021, 19, 347. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Sanjukta, S.; Padhi, S.; Sarkar, P.; Singh, S.P. Production, characterization and molecular docking of antioxidant peptides from peptidome of kinema fermented with proteolytic Bacillus Spp. Food Res. Int. 2021, 141, 110161. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hee, C.Y.; Seok, C.Y.; Alam, M.B.; Han, L.S.; Cheol, Y.J. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018, 239, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 319, 126534. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT Food Sci. Technol. 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Zheng, Z.; Si, D.; Ahmad, B.; Li, Z.; Zhang, R. A novel antioxidative peptide derived from chicken blood corpuscle hydrolysate. Food Res. Int. 2018, 106, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Wang, Y.M.; Li, L.; Chi, C.F.; Wang, B. Four antioxidant peptides from protein hydrolysate of red stingray (Dasyatis akajei) cartilages: Isolation, identification, and in vitro activity evaluation. Mar. Drugs. 2019, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and antifreezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Yang, X.R.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant peptides from the protein hydrolysate of Spanish Mackerel (Scomberomorous niphonius) muscle by in vitro gastrointestinal digestion and their in vitro activities. Mar. Drugs. 2019, 17, 531. [Google Scholar] [CrossRef]
- Memarpoor, Y.M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking oxidative stress and DNA damage to changes in the expression of extracellular matrix components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef]
- Møller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef]
- Gonzalez-Hunt, C.P.; Wadhwa, M.; Sanders, L.H. DNA damage by oxidative stress: Measurement strategies for two genomes. Curr. Opin. Toxicol. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nakad, R.; Schumacher, B. DNA damage response and immune defense: Links and mechanisms. Front. Genet. 2016, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, A.; Goulielmaki, E.; Garinis, G.A. DNA Damage: From chronic inflammation to age-related deterioration. Front Genet. 2016, 7, 187. [Google Scholar] [CrossRef]
- Nelson, B.C.; Dizdaroglu, M. Implications of DNA damage and DNA repair on human diseases. Mutagenesis 2020, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar Drugs. 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D.; Hernandez-Escalante, V.M. Bioavailability of bioactive peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis ni-loticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Ong, M.G.L.; Pang, M.J.; Wong, S.J.; Teh, L.K.; Chai, T.T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhao, Y.Q.; Wang, Y.M.; Chi, C.F.; Wang, B. Eight collagen peptides from hydrolysate fraction of Spanish mackerel skins: Isolation, identification, and in vitro antioxidant activity evaluation. Mar. Drugs. 2019, 17, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]

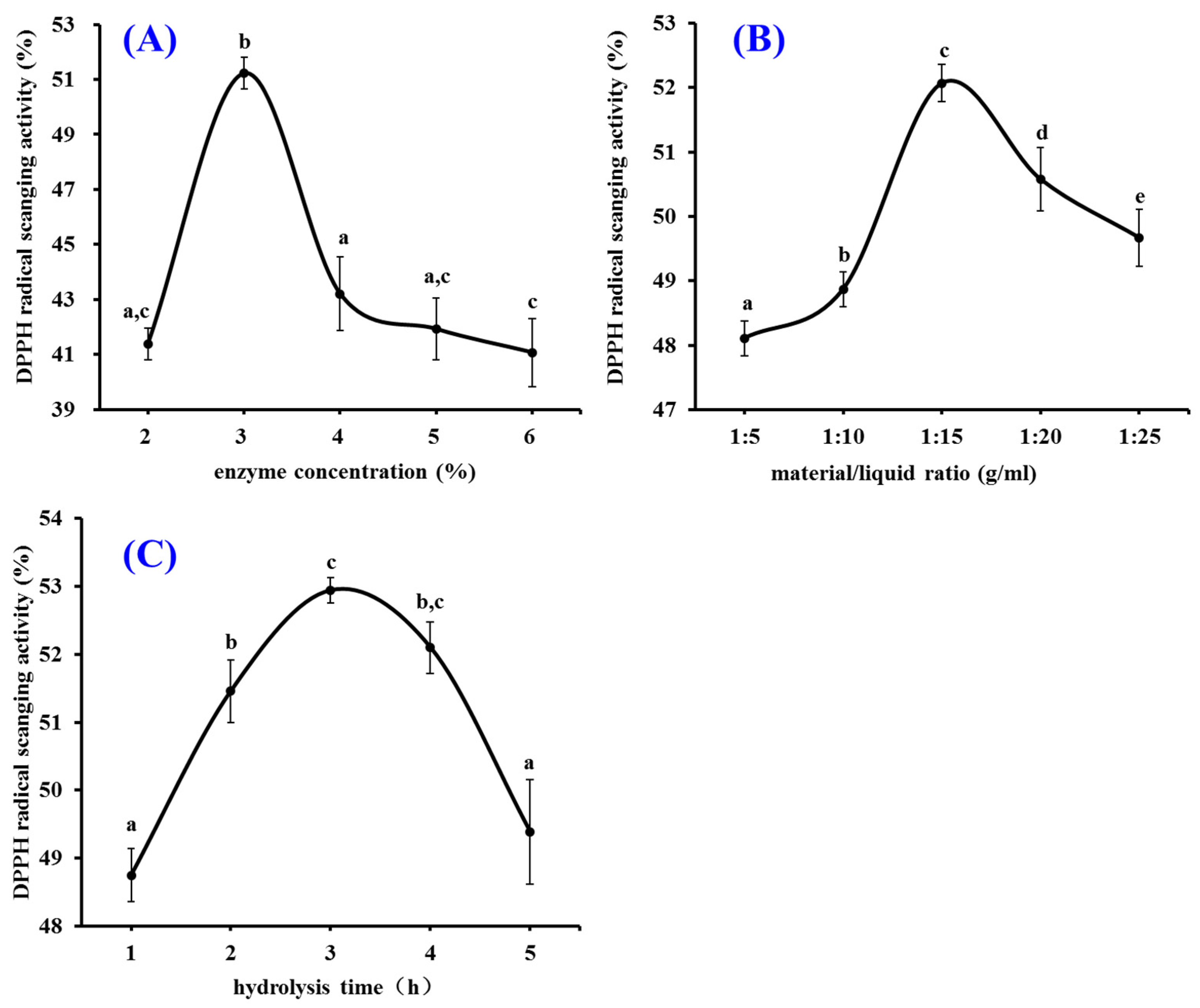

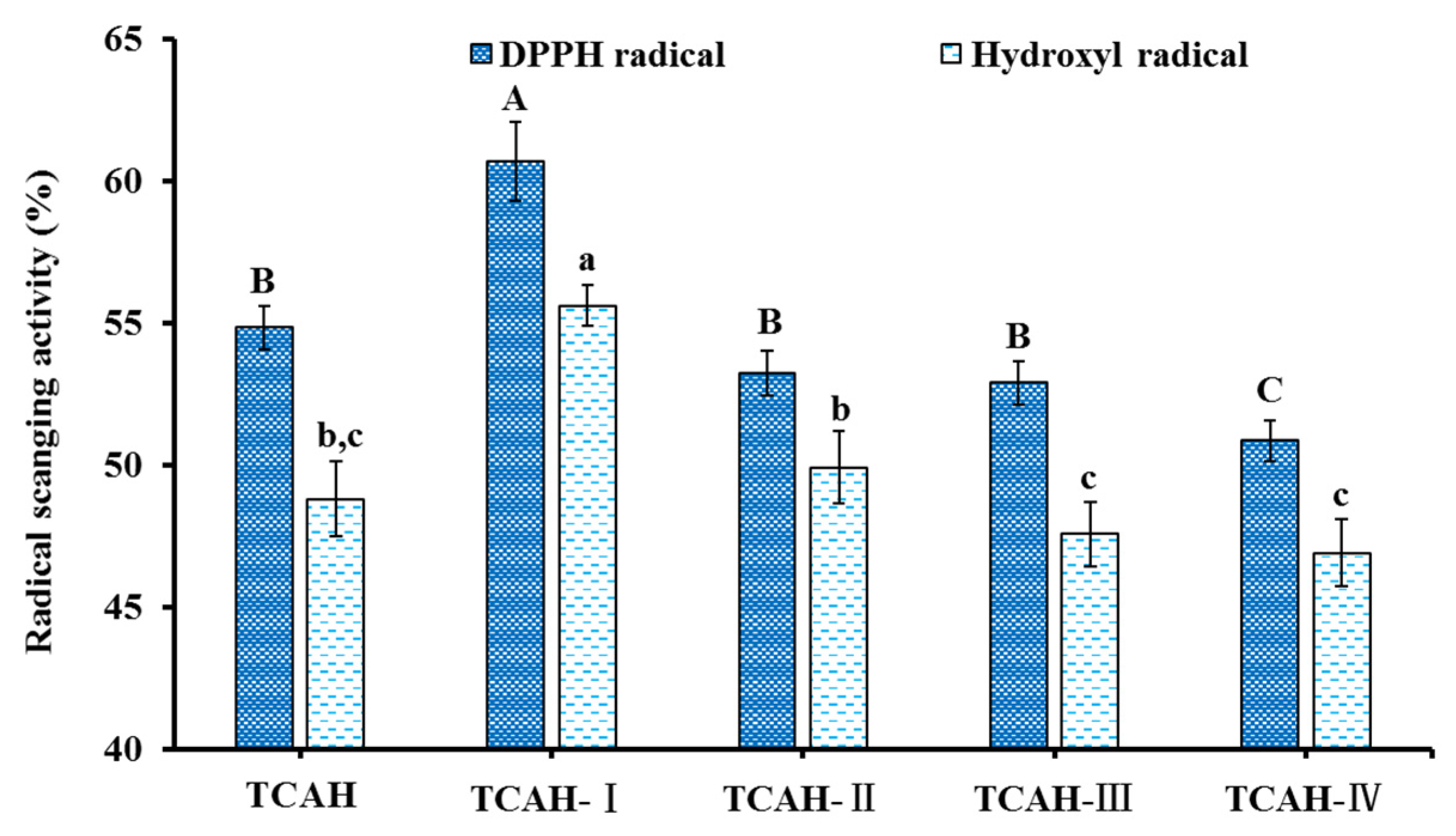


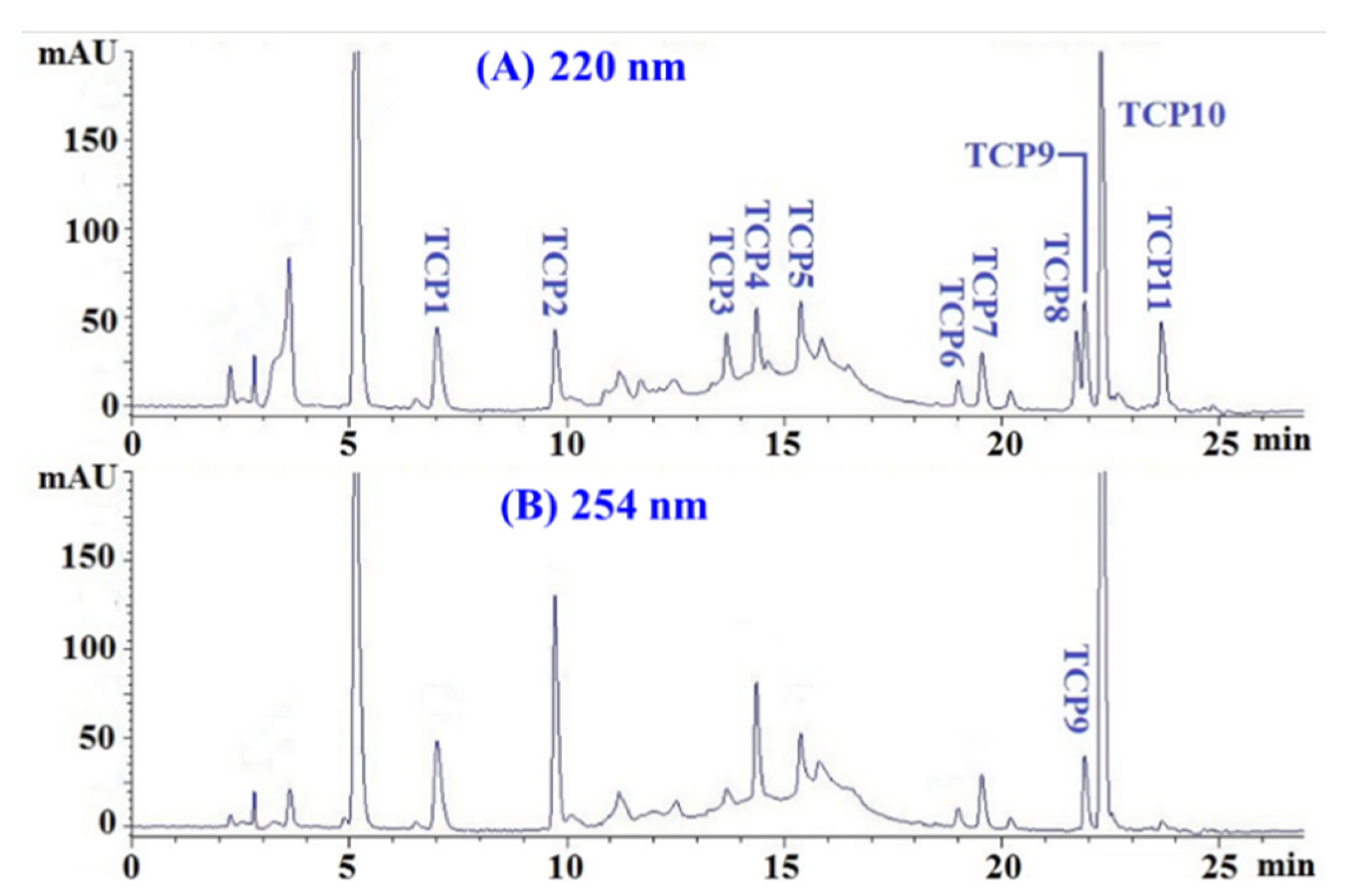

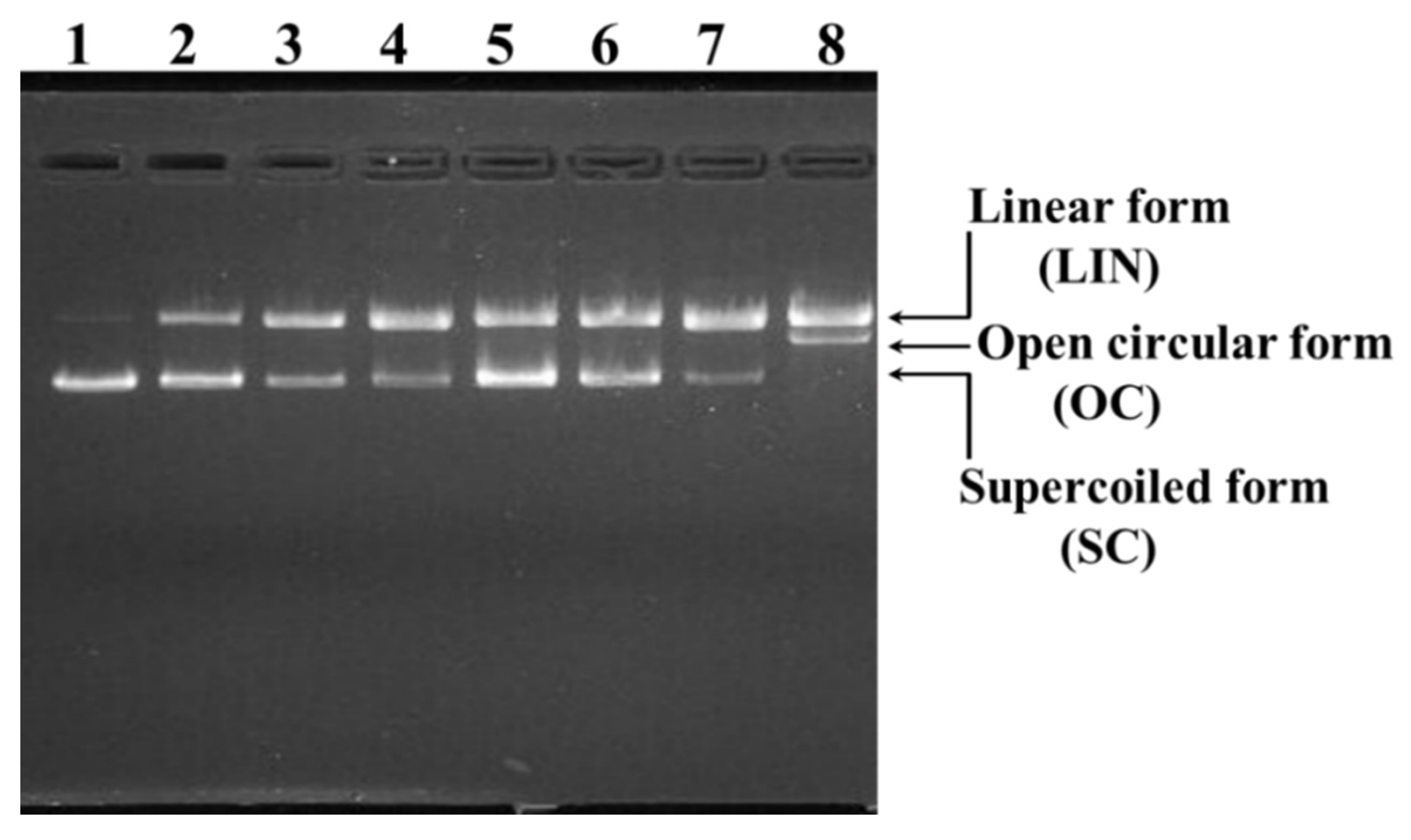



| Run | Independent Variables | Dependent Variables | ||
|---|---|---|---|---|
| X1 (Hydrolysis Time/h) | X2 (Material/Liquid Ratio/mg/mL) | X3 (Enzyme Concentration/%) | Y (DPPH· Scavenging Activity, %) | |
| 1 | 2 | 1:10 | 3 | 48.11 |
| 2 | 4 | 1:10 | 3 | 48.57 |
| 3 | 2 | 1:20 | 3 | 49.73 |
| 4 | 4 | 1:20 | 3 | 51.35 |
| 5 | 2 | 1:15 | 2 | 47.97 |
| 6 | 4 | 1:15 | 2 | 50.84 |
| 7 | 2 | 1:15 | 4 | 43.39 |
| 8 | 4 | 1:15 | 4 | 48.39 |
| 9 | 3 | 1:10 | 2 | 45.94 |
| 10 | 3 | 1:20 | 2 | 53.67 |
| 11 | 3 | 1:10 | 4 | 48.53 |
| 12 | 3 | 1:20 | 4 | 50.89 |
| 13 | 3 | 1:15 | 3 | 52.42 |
| 14 | 3 | 1:15 | 3 | 53.53 |
| 15 | 3 | 1:15 | 3 | 52.28 |
| 16 | 3 | 1:15 | 3 | 53.34 |
| 17 | 3 | 1:15 | 3 | 54.92 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 130.89 | 9 | 14.54 | 5.47 | 0.0178 | significant |
| X1-hydrolysis time | 26.87 | 1 | 26.87 | 10.11 | 0.0155 | |
| X2-material-to-liquid ratio | 0.41 | 1 | 0.41 | 0.15 | 0.7068 | |
| X3-enzyme concentration | 1.23 | 1 | 1.23 | 0.46 | 0.5180 | |
| X1X2 | 0.33 | 1 | 0.33 | 0.13 | 0.7330 | |
| X1X3 | 1.13 | 1 | 1.13 | 0.43 | 0.5345 | |
| X2X3 | 7.21 | 1 | 7.21 | 2.71 | 0.1435 | |
| X12 | 37.40 | 1 | 37.40 | 14.07 | 0.0072 | |
| X22 | 3.22 | 1 | 3.22 | 1.21 | 0.3077 | |
| X32 | 29.97 | 1 | 29.97 | 11.28 | 0.0121 | |
| Residual | 18.60 | 7 | 2.66 | |||
| Lack of Fit | 14.11 | 3 | 4.70 | 4.19 | 0.1001 | not significant |
| Pure Error | 4.49 | 4 | 1.12 | |||
| Cor Total | 149.49 | 16 | 0.0178 | |||
| R2 = 0.8756 | R2Adj = 0.7156 | CV(%) = 3.25 | Adeq Precision = 7.073 | |||
| Retention Time (min) | Amino Acid Sequence | Observed MW/Theoretical MW (Da) | |
|---|---|---|---|
| TCP1 | 7.05 | Gln-Gly-Asp (QGD) | 318.3/318.3 |
| TCP2 | 9.78 | Gly-Glu-Gln-Ser-Asn (GEQSN) | 533.5/553.5 |
| TCP3 | 13.68 | Pro-Lys-Lys (PKK) | 371.4/371.5 |
| TCP4 | 14.35 | Gly-Pro-Gln (GPQ) | 300.3/300.3 |
| TCP5 | 15.38 | Gly-Glu-Glu-Gly-Asp (GEEGD) | 505.3/505.4 |
| TCP6 | 19.03 | Tyr-Glu-Gly-Gly-Asp (YEGGD) | 539.4/539.5 |
| TCP7 | 19.57 | Gly-Glu-Gly-Glu-Arg (GEGER) | 546.5/546.5 |
| TCP8 | 21.81 | Gly-Glu-Gly-Gln-Arg (GEGQR) | 545.5/545.6 |
| TCP9 | 21.95 | Gly-Pro-Gly-Leu-Met (GPGLM) | 473.6/473.6 |
| TCP10 | 22.21 | Gly-Leu-Asn (GLN) | 302.4/302.3 |
| TCP11 | 23.69 | Gly-Asp-Arg-Gly-Asp (GDRGD) | 518.4/518.5 |
| EC50 (mg/mL) | ||||
|---|---|---|---|---|
| DPPH· | HO· | ABTS+· | O2−· | |
| TCP1 | 1.867 ± 0.018 a | 1.931 ± 0.006 a | 0.273 ± 0.013 a | 1.558 ± 0.032 a |
| TCP2 | 2.054 ± 0.021 b | 2.123 ± 0.031 b | 0.529 ± 0.003 b | 1.857 ± 0.002 b |
| TCP3 | 0.978 ± 0.006 c | 1.158 ± 0.032 c | 0.188 ± 0.002 c | 0.924 ± 0.003 c |
| TCP4 | 1.186 ± 0.008 d, g | 1.307 ± 0.006 d | 0.269 ± 0.013 a | 1.063 ± 0.007 d |
| TCP5 | 2.296 ± 0.011 e | 2.744 ± 0.108 e | 1.447 ± 0.016 d | 2.980 ± 0.012 e |
| TCP6 | 1.062 ± 0.032 c, d | 1.243 ± 0.027 c, d | 0.200 ± 0.002 c, e | 0.933 ± 0.011 c |
| TCP7 | 1.964 ± 0.031 a, b | 2.049 ± 0.018 b | 0.407 ± 0.008 f | 1.640 ± 0.055 f |
| TCP8 | 2.257 ± 0.038 e | 2.505 ± 0.035 f | 1.218 ± 0.005 g | 2.143 ± 0.040 g |
| TCP9 | 1.149 ± 0.039 d, g | 1.285 ± 0.016 c,d | 0.216 ± 0.007 e, h | 0.969 ± 0.014 c |
| TCP10 | 3.204 ± 0.177 f | 3.556 ± 0.042 g | 1.641 ± 0.003 i | 3.022 ± 0.015 e |
| TCP11 | 1.214 ± 0.036 g | 1.739 ± 0.050 h | 0.226 ± 0.006 h | 0.951 ± 0.008 c |
| GSH | 0.085 ± 0.002 h | 0.504 ± 0.011 i | 0.097 ± 0.002 j | 0.278 ± 0.016 h |
| Peptides | Declined Percentage | Peptides | Declined Percentage |
|---|---|---|---|
| TCP1 | 5.69% | TCP7 | 6.08% |
| TCP2 | 4.49% | TCP8 | 4.21% |
| TCP3 | 3.25% | TCP9 | 4.30% |
| TCP4 | 3.22% | TCP10 | 3.15% |
| TCP5 | 4.25% | TCP11 | 2.65% |
| TCP6 | 3.87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. https://doi.org/10.3390/md20100626
Cai W-W, Hu X-M, Wang Y-M, Chi C-F, Wang B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Marine Drugs. 2022; 20(10):626. https://doi.org/10.3390/md20100626
Chicago/Turabian StyleCai, Wei-Wei, Xiao-Meng Hu, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2022. "Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments" Marine Drugs 20, no. 10: 626. https://doi.org/10.3390/md20100626
APA StyleCai, W.-W., Hu, X.-M., Wang, Y.-M., Chi, C.-F., & Wang, B. (2022). Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Marine Drugs, 20(10), 626. https://doi.org/10.3390/md20100626









