Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants
Abstract
:1. Introduction
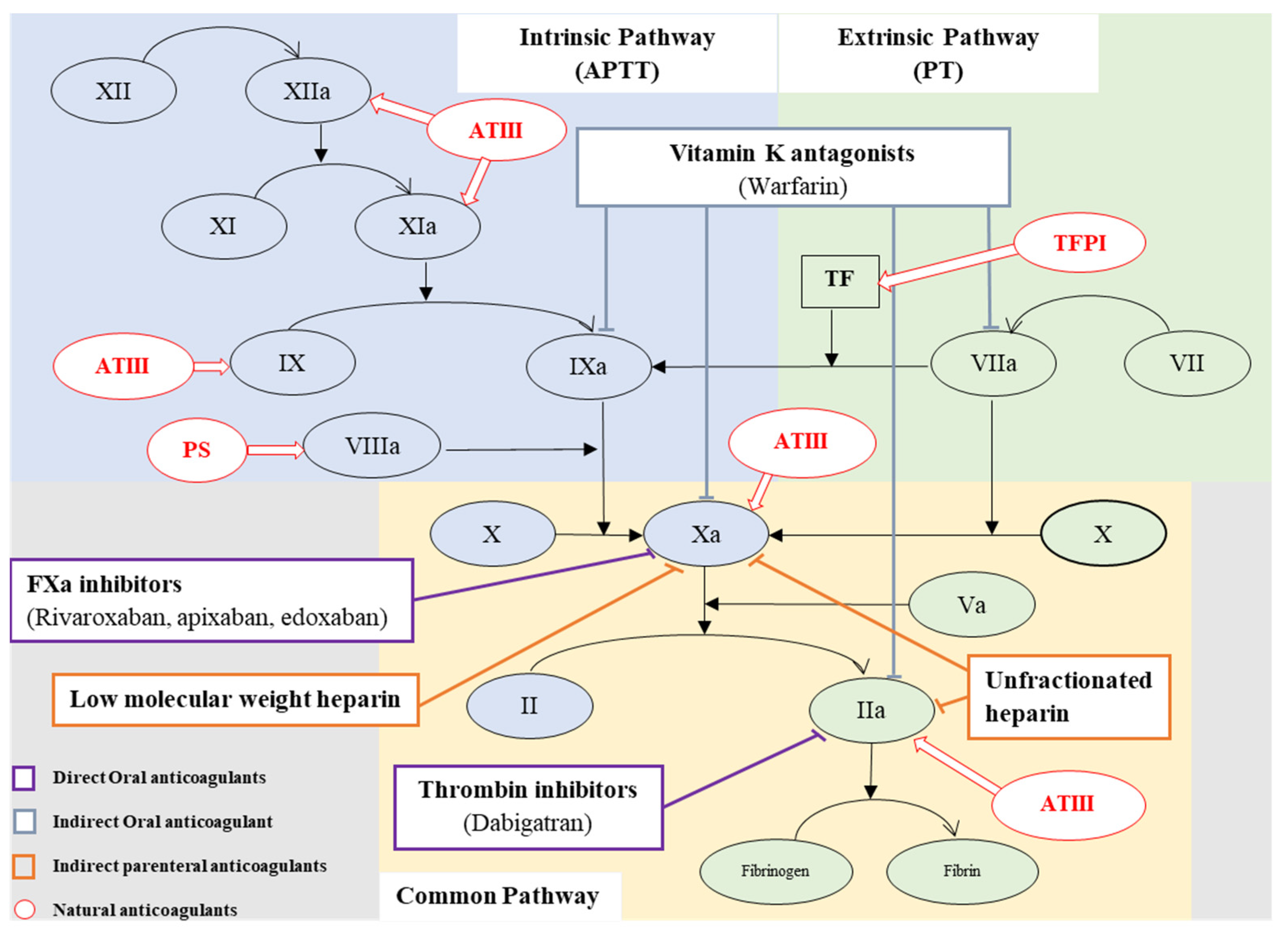
2. Coagulation
3. Natural Anticoagulants
4. Thrombosis
5. Anticoagulant Therapy
6. Issues Related with Current Anticoagulants
7. Marine-Derived Anticoagulant
8. Sulfated Polysaccharides
8.1. Glycosaminoglycans
8.1.1. Heparin and Heparin Sulfate
8.1.2. Chondroitin/Dermatan Sulfate
8.1.3. Fucosylated Chondroitin Sulfate
8.2. Glycosaminoglycans Mimicking
8.2.1. Ulvan
8.2.2. Carrageenan
8.2.3. Fucoidan or Fucan Sulfate
8.2.4. Rhaman Sulfate
8.3. Chemically Sulfated Polysaccharides and Oligosaccharides
8.3.1. Sulfonated and Sulfated Chitosan and Chitosan Derivatives
8.3.2. Sulfated Alginate
8.4. Proteins and Peptides
9. Clinical Use and Efficacy
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredenburgh, J.C.; Gross, P.L.; Weitz, J.I. Emerging anticoagulant strategies. Blood J. Am. Soc. Hematol. 2017, 129, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.-H.; Chen, Y.-Y.; Zhou, G.-S.; Liu, X.; Tang, Y.-P.; Liu, R.; Liu, P.; Li, N.; Yang, J.; Wang, J. A novel antithrombotic protease from marine worm Sipunculus nudus. Int. J. Mol. Sci. 2018, 19, 3023. [Google Scholar] [CrossRef] [Green Version]
- Schulman, S. Advantages and limitations of the new anticoagulants. J. Intern. Med. 2014, 275, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Ruppert, C. Anticoagulants; Elsevier: Amsterdam, The Netherlands, 2006; pp. 115–128. [Google Scholar]
- Dabbous, M.K.; Sakr, F.R.; Malaeb, D.N. Anticoagulant therapy in pediatrics. J. Basic Clin. Pharm. 2014, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef] [Green Version]
- Franchini, M.; Liumbruno, G.M.; Bonfanti, C.; Lippi, G. The evolution of anticoagulant therapy. Blood Transfus. 2016, 14, 175. [Google Scholar]
- Eikelboom, J.W.; Weitz, J.I. New anticoagulants. Circulation 2010, 121, 1523–1532. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct oral anticoagulant use: A practical guide to common clinical challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.-C.; Jung, W.-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 283–308. [Google Scholar]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S. Marine-derived polymeric materials and biomimetics: An overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhao, J.; Xing, M.; Xiao, H.; Zhang, Q.; Liang, H.; Ji, A.; Song, S. Current research landscape of marine-derived anti-atherosclerotic substances. Mar. Drugs 2020, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Perry, D. Laboratory monitoring of haemostasis. Anaesthesia 2015, 70, 68-e24. [Google Scholar] [CrossRef]
- Adams, R.L.; Bird, R.J. coagulation cascade and therapeutics update: Relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology 2009, 14, 462–470. [Google Scholar] [CrossRef]
- Sira, J.; Eyre, L. Physiology of haemostasis. Anaesth. Intensive Care Med. 2016, 17, 79–82. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515. [Google Scholar] [CrossRef]
- Austin, S.K. Haemostasis. Medicine 2017, 45, 204–208. [Google Scholar] [CrossRef]
- Sagripanti, A.; Carpi, A. Natural anticoagulants, aging, and thromboembolism. Exp. Gerontol. 1998, 33, 891–896. [Google Scholar] [CrossRef]
- Ezihe-Ejiofor, J.A.; Hutchinson, N. Anticlotting mechanisms 1: Physiology and pathology. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 87–92. [Google Scholar] [CrossRef]
- Opal, S.M.; Kessler, C.M.; Roemisch, J.; Knaub, S. Antithrombin, heparin, and heparan sulfate. Crit. Care Med. 2002, 30, S325–S331. [Google Scholar] [CrossRef] [PubMed]
- Rigby, A.C.; Grant, M.A. Protein S: A conduit between anticoagulation and inflammation. Crit. Care Med. 2004, 32, S336–S341. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, A.T.; Elliott, W.G.; Zia, A.A. Heparin-induced thrombocytopenia in the cardiovascular patient: Diagnostic and treatment guidelines. Eur. J. Cardio-Thorac. Surg. 2005, 27, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120. [Google Scholar] [PubMed]
- Eisert, W.G.; Hauel, N.; Stangier, J.; Wienen, W.; Clemens, A.; van Ryn, J. Dabigatran: An oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1885–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Armaganijan, L.V. Newer oral anticoagulants should be used as first-line agents to prevent thromboembolism in patients with atrial fibrillation and risk factors for stroke or thromboembolism. Circulation 2012, 125, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitz, J.I.; Gross, P.L. New oral anticoagulants: Which one should my patient use? Hematol. 2010 Am. Soc. Hematol. Educ. Program Book 2012, 2012, 536–540. [Google Scholar] [CrossRef]
- Bauer, K. Recent progress in anticoagulant therapy: Oral direct inhibitors of thrombin and factor Xa. J. Thromb. Haemost. 2011, 9, 12–19. [Google Scholar] [CrossRef]
- Triggers, M.N. Targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar]
- Tanaka, K.A.; Szlam, F.; Dickneite, G.; Levy, J.H. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb. Res. 2008, 122, 117–123. [Google Scholar] [CrossRef]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Am. Heart Assoc. 2001, 21, 1094–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harter, K.; Levine, M.; Henderson, S.O. Anticoagulation drug therapy: A review. West. J. Emerg. Med. 2015, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D. Limitations of traditional anticoagulants. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 62S–65S. [Google Scholar] [CrossRef]
- Lee, L.H. DOACs–advances and limitations in real world. Thromb. J. 2016, 14, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burn, J.; Pirmohamed, M. Direct oral anticoagulants versus warfarin: Is new always better than the old? Open Heart 2018, 5, e000712. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Comparative Biochemistry and Physiology Part C: Toxicology &. Pharmacology 2003, 135, 357. [Google Scholar]
- Vasconcelos, A.A.; Pomin, V.H. Marine carbohydrate-based compounds with medicinal properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G. Sulfated Polysaccharide Extracted from the Green Algae Codium bernabei: Physicochemical Characterization and Antioxidant, Anticoagulant and Antitumor Activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine polysaccharides for wound dressings application: An overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Wan, M.-C.; Qin, W.; Lei, C.; Li, Q.-H.; Meng, M.; Fang, M.; Song, W.; Chen, J.-H.; Tay, F.; Niu, L.-N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Benvenuti, M.; Casolaro, M.; Lamponi, S.; Magnani, A. Sulfated hyaluronic acid as heparin-like material: Physicochemical and biological characterization. J. Mater. Sci. Mater. Med. 1994, 5, 830–833. [Google Scholar] [CrossRef]
- Magnani, A.; Albanese, A.; Lamponi, S.; Barbucci, R. Blood-interaction performance of differently sulphated hyaluronic acids. Thromb. Res. 1996, 81, 383–395. [Google Scholar] [CrossRef]
- Chen, G.; Ito, Y.; Imanishi, Y.; Magnani, A.; Lamponi, S.; Barbucci, R. Photoimmobilization of sulfated hyaluronic acid for antithrombogenicity. Bioconjugate Chem. 1997, 8, 730–734. [Google Scholar] [CrossRef]
- Paoli, A.; Celussi, M.; Del Negro, P. Attività fosfatasica di Synechococcus, isolato nel Golfo di Trieste. Inf. Bot. Ital. 2005, 37, 590–591. [Google Scholar]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, present, and future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.; Hughes, A.J.; Andrade, G.P.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A non-hemorrhagic hybrid heparin/heparan sulfate with anticoagulant potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, G.F.; Mendes, A.; Castro, R.A.; Baú, E.C.; Nader, H.B.; Dietrich, C.P. Distribution of sulfated glycosaminoglycans in the animal kingdom: Widespread occurrence of heparin-like compounds in invertebrates. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2000, 1475, 287–294. [Google Scholar] [CrossRef]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavão, M.S. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Giji, S. Biological activities of heparan sulfate. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 125–135. [Google Scholar]
- Cesaretti, M.; Luppi, E.; Maccari, F.; Volpi, N. Isolation and characterization of a heparin with high anticoagulant activity from the clam Tapes phylippinarum: Evidence for the presence of a high content of antithrombin III binding site. Glycobiology 2004, 14, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavaki, C.; Mizumoto, S.; Karamanos, N.; Sugahara, K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, J.M.; Gallo, R.L. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002, 12, 117R–125R. [Google Scholar] [CrossRef] [PubMed]
- Dellias, J.M.; Onofre, G.R.; Werneck, C.C.; Landeira-Fernandez, A.M.; Melo, F.R.; Farias, W.R.; Silva, L.-C.F. Structural composition and differential anticoagulant activities of dermatan sulfates from the skin of four species of rays, Dasyatis americana, Dasyatis gutatta, Aetobatus narinari and Potamotrygon motoro. Biochimie 2004, 86, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, H.; Ghlissi, Z.; Kallel, R.; Amor, I.B.; Boudawara, T.; Gargouri, J.; Sahnoun, Z.; Volpi, N.; Sila, A.; Bougatef, A. Chondroitin/dermatan sulfate purified from corb (Sciaena umbra) skin and bone: In vivo assessment of anticoagulant activity. Int. J. Biol. Macromol. 2020, 164, 131–139. [Google Scholar] [CrossRef]
- Bougatef, H.; Krichen, F.; Capitani, F.; Amor, I.B.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; Bougatef, A.; Sila, A. Chondroitin sulfate/dermatan sulfate from corb (Sciaena umbra) skin: Purification, structural analysis and anticoagulant effect. Carbohydr. Polym. 2018, 196, 272–278. [Google Scholar] [CrossRef]
- Saravanan, R.; Shanmugam, A. Is isolation and characterization of heparan sulfate from marine scallop Amussium pleuronectus (Linne.) an alternative source of heparin? Carbohydr. Polym. 2011, 86, 1082–1084. [Google Scholar] [CrossRef]
- Andrade, G.P.; Lima, M.A.; de Souza, A.A., Jr.; Fareed, J.; Hoppensteadt, D.A.; Santos, E.A.; Chavante, S.F.; Oliveira, F.W.; Rocha, H.A.; Nader, H.B. A heparin-like compound isolated from a marine crab rich in glucuronic acid 2-O-sulfate presents low anticoagulant activity. Carbohydr. Polym. 2013, 94, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.S.; Cavalcante, R.S.; Cavalheiro, R.P.; Palhares, L.C.; Nobre, L.T.; Andrade, G.P.; Nader, H.B.; Lima, M.A.; Chavante, S.F. Anti-IIa activity and antitumor properties of a hybrid heparin/heparan sulfate-like compound from Litopenaeus vannamei shrimp. Int. J. Biol. Macromol. 2018, 118, 1470–1478. [Google Scholar] [CrossRef]
- Dietrich, C.P.; Paiva, J.F.; Castro, R.A.; Chavante, S.F.; Jeske, W.; Fareed, J.; Gorin, P.A.; Mendes, A.; Nader, H.B. Structural features and anticoagulant activities of a novel natural low molecular weight heparin from the shrimp Penaeus brasiliensis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1428, 273–283. [Google Scholar] [CrossRef]
- Krichen, F.; Bougatef, H.; Sayari, N.; Capitani, F.; Amor, I.B.; Koubaa, I.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N. Isolation, purification and structural characterestics of chondroitin sulfate from smooth hound cartilage: In vitro anticoagulant and antiproliferative properties. Carbohydr. Polym. 2018, 197, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nifantiev, N.E.; Usov, A.I. Oversulfated dermatan sulfate and heparinoid in the starfish Lysastrosoma anthosticta: Structures and anticoagulant activity. Carbohydr. Polym. 2021, 261, 117867. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xue, C.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3054–3066. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Yang, S.; Lv, Z. Separation, purification, structures and anticoagulant activities of fucosylated chondroitin sulfates from Holothuria scabra. Int. J. Biol. Macromol. 2018, 108, 710–718. [Google Scholar] [CrossRef]
- Mansour, M.B.; Balti, R.; Ollivier, V.; Jannet, H.B.; Chaubet, F.; Maaroufi, R.M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym. 2017, 174, 760–771. [Google Scholar] [CrossRef]
- Chahed, L.; Balti, R.; Elhiss, S.; Bouchemal, N.; Ajzenberg, N.; Ollivier, V.; Chaubet, F.; Maaroufi, R.M.; Mansour, M.B. Anticoagulant activity of fucosylated chondroitin sulfate isolated from Cucumaria syracusana. Process Biochem. 2020, 91, 149–157. [Google Scholar] [CrossRef]
- Gao, N.; Lu, F.; Xiao, C.; Yang, L.; Chen, J.; Zhou, K.; Wen, D.; Li, Z.; Wu, M.; Jiang, J. β-Eliminative depolymerization of the fucosylated chondroitin sulfate and anticoagulant activities of resulting fragments. Carbohydr. Polym. 2015, 127, 427–437. [Google Scholar]
- Li, J.-H.; Li, S.; Zhi, Z.-J.; Yan, L.-F.; Ye, X.-Q.; Ding, T.; Yan, L.; Linhardt, R.J.; Chen, S.-G. Depolymerization of fucosylated chondroitin sulfate with a modified fenton-system and anticoagulant activity of the resulting fragments. Mar. Drugs 2016, 14, 170. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A novel structural fucosylated chondroitin sulfate from Holothuria Mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym. 2018, 181, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.-C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm: Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, H.; Enjyoji, K.-I.; Minamiguchi, K.; Kitazato, K.T.; Kitazato, K.; Saito, H.; Kato, H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: Antithrombin III-and heparin cofactor II-independent inhibition of factor X activation by factor IXa-factor VIIIa complex and heparin cofactor II-dependent inhibition of thrombin. Blood 1995, 85, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, R.J.; Santos, G.R.; Mourão, P.A. Effects of polysaccharides enriched in 2,4-disulfated fucose units on coagulation, thrombosis and bleeding. Thromb. Haemost. 2009, 102, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, R.J.; Oliveira, S.-N.M.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef] [Green Version]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef]
- Reis, S.E.; Andrade, R.G.C.; Accardo, C.M.; Maia, L.F.; Oliveira, L.F.; Nader, H.B.; Aguiar, J.A.; Medeiros, V.P. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Res. 2020, 45, 101750. [Google Scholar] [CrossRef]
- Li, P.; Wen, S.; Sun, K.; Zhao, Y.; Chen, Y. Structure and bioactivity screening of a low molecular weight ulvan from the green alga ulothrix flacca. Mar. Drugs 2018, 16, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Liu, X.; Wang, K.; Liu, D.; Huang, L.; Liu, S.; Zhang, Q. Subchronic toxicity study of ulvan from Ulva pertusa (Chlorophyta) in Wistar rats. Food Chem. Toxicol. 2013, 62, 573–578. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.M.; Noseda, M.D.; Dallagnol, J.C.; Ferreira, L.G.; Ducatti, D.R.; Gonçalves, A.G.; de Freitas, R.A.; Duarte, M.E.R. Conformational analysis of ulvans from Ulva fasciata and their anticoagulant polycarboxylic derivatives. Int. J. Biol. Macromol. 2020, 162, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Saluri, K.; Tuvikene, R. Anticoagulant and antioxidant activity of lambda-and theta-carrageenans of different molecular weights. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100243. [Google Scholar] [CrossRef]
- Yermak, I.M.; Barabanova, A.O.; Aminin, D.L.; Davydova, V.N.; Sokolova, E.V.; Solov’Eva, T.F.; Kim, Y.H.; Shin, K.S. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydr. Polym. 2012, 87, 713–720. [Google Scholar] [CrossRef]
- Silva, F.; Dore, C.; Marques, C.; Nascimento, M.; Benevides, N.; Rocha, H.; Chavante, S.; Leite, E. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- de Araújo, C.A.; Noseda, M.D.; Cipriani, T.R.; Gonçalves, A.G.; Duarte, M.E.R.; Ducatti, D.R. Selective sulfation of carrageenans and the influence of sulfate regiochemistry on anticoagulant properties. Carbohydr. Polym. 2013, 91, 483–491. [Google Scholar] [CrossRef] [Green Version]
- dos Santos-Fidencio, G.C.; Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydr. Polym. 2019, 214, 286–293. [Google Scholar] [CrossRef]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.-M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan oligosaccharides of distinct anti-heparanase and anticoagulant activities inhibit MDA-MB-231 breast cancer cell migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Shi, Z.; Wang, Y.; Song, X.; Wang, L.; Han, M.; Du, H.; He, C.; Zhao, W. Anticoagulant chitosan-kappa-carrageenan composite hydrogel sorbent for simultaneous endotoxin and bacteria cleansing in septic blood. Carbohydr. Polym. 2020, 243, 116470. [Google Scholar] [CrossRef]
- Song, X.; Wang, K.; Tang, C.-Q.; Yang, W.-W.; Zhao, W.-F.; Zhao, C.-S. Design of carrageenan-based heparin-mimetic gel beads as self-anticoagulant hemoperfusion adsorbents. Biomacromolecules 2018, 19, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Gerbst, A.G.; Ushakova, N.A.; Tsvetkova, E.A.; Dmitrenok, A.S.; Usov, A.I.; Nifantiev, N.E. Anticoagulant and antithrombotic activities of modified xylofucan sulfate from the brown alga Punctaria plantaginea. Carbohydr. Polym. 2016, 136, 826–833. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Shang, F.; Mou, R.; Zhang, Z.; Gao, N.; Lin, L.; Li, Z.; Wu, M.; Zhao, J. Structural analysis and anticoagulant activities of three highly regular fucan sulfates as novel intrinsic factor Xase inhibitors. Carbohydr. Polym. 2018, 195, 257–266. [Google Scholar] [CrossRef]
- He, W.; Sun, H.; Su, L.; Zhou, D.; Zhang, X.; Shanggui, D.; Chen, Y. Structure and anticoagulant activity of a sulfated fucan from the sea cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020, 164, 87–94. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, W.; Yin, R.; Zhou, L.; Li, Z.; Wu, M.; Zhao, J. An anticoagulant fucan sulfate with hexasaccharide repeating units from the sea cucumber Holothuria albiventer. Carbohydr. Res. 2018, 464, 12–18. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, N.; Zuo, Z.; Li, S.; Zheng, W.; Shi, X.; Liu, Q.; Ma, T.; Yin, R.; Li, X. Five distinct fucan sulfates from sea cucumber Pattalus mollis: Purification, structural characterization and anticoagulant activities. Int. J. Biol. Macromol. 2021, 186, 535–543. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Xian, H.; Qin, L.; Mao, W. Structure and anticoagulant property of a sulfated polysaccharide isolated from the green seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, X.; Cao, S.; Qin, L.; He, M.; He, X.; Mao, W. Anticoagulant properties of a green algal rhamnan-type sulfated polysaccharide and its low-molecular-weight fragments prepared by mild acid degradation. Mar. Drugs 2018, 16, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulfated rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, T.; Akita, N.; Terasawa, M.; Hayashi, T.; Suzuki, K. Rhamnan sulfate extracted from Monostroma nitidum attenuates blood coagulation and inflammation of vascular endothelial cells. J. Nat. Med. 2019, 73, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Sinurat, E.; Marraskuranto, E. Fucoidan from brown seaweed and its bioactivity. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2012, 7, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Lahrsen, E.; Schoenfeld, A.-K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Obluchinsksya, E.; Makarova, M.; Pozharitskaya, O.; Shikov, A. Effects of ultrasound treatment on the chemical composition and anticoagulant properties of dry fucus extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Song, Y.; He, P.; Rodrigues, A.L.; Datta, P.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Chen, C.; Dordick, J.; Zhang, F. Anti-SARS-CoV-2 activity of rhamnan sulfate from Monostroma nitidum. Mar. Drugs 2021, 19, 685. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cai, J.; Hu, Y.; Li, D.; Du, Y. Preparation, characterization and antimicrobial activity of 6-amino-6-deoxychitosan. Carbohydr. Polym. 2012, 87, 202–209. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Wahid, F.; Wang, H.-S.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Preparation, characterization and antibacterial applications of carboxymethyl chitosan/CuO nanocomposite hydrogels. Int. J. Biol. Macromol. 2017, 101, 690–695. [Google Scholar] [CrossRef]
- Shao, K.; Han, B.; Gao, J.; Song, F.; Yang, Y.; Liu, W. Synthesis and characterization of a hydroxyethyl derivative of chitosan and evaluation of its biosafety. J. Ocean. Univ. China 2015, 14, 703–709. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Thinesh, T.; Selvin, J.; Selvan, K.M.; Shanmugam, V.; Shanmugam, A. Characterization of bioactive chitosan and sulfated chitosan from Doryteuthis singhalensis (Ortmann, 1891). Int. J. Biol. Macromol. 2017, 99, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Moovendhan, M.; Vairamani, S.; Shanmugam, A. Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int. J. Biol. Macromol. 2017, 99, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Heo, S.-Y.; Oh, G.-W.; Choi, I.-W.; Park, W.S.; Jung, W.-K. Antithrombin III-mediated blood coagulation inhibitory activity of chitosan sulfate derivatized with different functional groups. Int. J. Biol. Macromol. 2020, 161, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Suwan, J.; Zhang, Z.; Li, B.; Vongchan, P.; Meepowpan, P.; Zhang, F.; Mousa, S.A.; Mousa, S.; Premanode, B.; Kongtawelert, P. Sulfonation of papain-treated chitosan and its mechanism for anticoagulant activity. Carbohydr. Res. 2009, 344, 1190–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronghua, H.; Yumin, D.; Jianhong, Y. Preparation and anticoagulant activity of carboxybutyrylated hydroxyethyl chitosan sulfates. Carbohydr. Polym. 2003, 51, 431–438. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Xie, W.; Chen, L.; Zheng, H.; Fan, L. Preparation and anticoagulant activity of N-succinyl chitosan sulfates. Int. J. Biol. Macromol. 2012, 51, 808–814. [Google Scholar] [CrossRef]
- Huang, R.; Du, Y.; Yang, J.; Fan, L. Influence of functional groups on the in vitro anticoagulant activity of chitosan sulfate. Carbohydr. Res. 2003, 338, 483–489. [Google Scholar] [CrossRef]
- Song, W.; Zeng, Q.; Yin, X.; Zhu, L.; Gong, T.; Pan, C. Preparation and anticoagulant properties of heparin-like electrospun membranes from carboxymethyl chitosan and bacterial cellulose sulfate. Int. J. Biol. Macromol. 2018, 120, 1396–1405. [Google Scholar] [CrossRef]
- Fan, L.; Wu, P.; Zhang, J.; Gao, S.; Wang, L.; Li, M.; Sha, M.; Xie, W.; Nie, M. Synthesis and anticoagulant activity of the quaternary ammonium chitosan sulfates. Int. J. Biol. Macromol. 2012, 50, 31–37. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Dimassi, S.; Tabary, N.; Leclercq, L.; Degoutin, S.; Chai, F.; Pierlot, C.; Cazaux, F.; Ung, A.; Staelens, J.-N. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohydr. Polym. 2018, 196, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, K.; Li, D.; Yu, S.; Cai, J.; Chen, L.; Du, Y. Preparation, characterization and in vitro anticoagulant activity of highly sulfated chitosan. Int. J. Biol. Macromol. 2013, 52, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vikhoreva, G.; Bannikova, G.; Stolbushkina, P.; Panov, A.; Drozd, N.; Makarov, V.; Varlamov, V.; Gal’Braikh, L. Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydr. Polym. 2005, 62, 327–332. [Google Scholar] [CrossRef]
- Vongchan, P.; Sajomsang, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activity of a sulfated chitosan. Carbohydr. Res. 2002, 337, 1239–1242. [Google Scholar] [CrossRef]
- Karthik, R.; Manigandan, V.; Saravanan, R.; Rajesh, R.P.; Chandrika, B. Structural characterization and in vitro biomedical activities of sulfated chitosan from Sepia pharaonis. Int. J. Biol. Macromol. 2016, 84, 319–328. [Google Scholar] [CrossRef]
- Park, P.-J.; Je, J.-Y.; Jung, W.-K.; Ahn, C.-B.; Kim, S.-K. Anticoagulant activity of heterochitosans and their oligosaccharide sulfates. Eur. Food Res. Technol. 2004, 219, 529–533. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Power, K.; Kadir, O.; Dékány, I.; Yannopoulos, S.N.; Bouropoulos, N.; Bakandritsos, A.; Antonijevic, M.D.; Zouganelis, G.D.; Roldo, M. Stabilisation of SWNTs by alkyl-sulfate chitosan derivatives of different molecular weight: Towards the preparation of hybrids with anticoagulant properties. Nanoscale 2011, 3, 1218–1224. [Google Scholar] [CrossRef]
- Vongchan, P.; Sajomsang, W.; Kasinrerk, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activities of the chitosan polysulfate synthesized from marine crab shell by semi-heterogeneous conditions. Sci. Asia 2003, 29, 115–120. [Google Scholar] [CrossRef]
- Fan, L.; Jiang, L.; Xu, Y.; Zhou, Y.; Shen, Y.; Xie, W.; Long, Z.; Zhou, J. Synthesis and anticoagulant activity of sodium alginate sulfates. Carbohydr. Polym. 2011, 83, 1797–1803. [Google Scholar] [CrossRef]
- Salimi, E.; Ghaee, A.; Ismail, A.F.; Karimi, M. Anti-thrombogenicity and permeability of polyethersulfone hollow fiber membrane with sulfonated alginate toward blood purification. Int. J. Biol. Macromol. 2018, 116, 364–377. [Google Scholar] [CrossRef]
- Ronghua, H.; Yumin, D.; Jianhong, Y. Preparation and in vitro anticoagulant activities of alginate sulfate and its quaterized derivatives. Carbohydr. Polym. 2003, 52, 19–24. [Google Scholar] [CrossRef]
- Xue, Y.-T.; Li, S.; Liu, W.-J.; Xin, M.; Li, H.-H.; Yu, G.-L.; Guan, H.-S.; He, X.-X.; Li, C.-X. The mechanisms of sulfated polysaccharide drug of propylene glycol alginate sodium sulfate (PSS) on bleeding side effect. Carbohydr. Polym. 2018, 194, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-T.; Ren, L.; Li, S.; Wang, L.-L.; He, X.-X.; Zhao, X.; Yu, G.-L.; Guan, H.-S.; Li, C.-X. Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS). Carbohydr. Polym. 2016, 144, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Zhang, Y.; Zeng, Y.; Zhang, L.; Zhao, X. Anticoagulant and FGF/FGFR signal activating activities of the heparinoid propylene glycol alginate sodium sulfate and its oligosaccharides. Carbohydr. Polym. 2016, 136, 641–648. [Google Scholar] [CrossRef]
- Xin, M.; Ren, L.; Sun, Y.; Li, H.-H.; Guan, H.-S.; He, X.-X.; Li, C.-X. Anticoagulant and antithrombotic activities of low-molecular-weight propylene glycol alginate sodium sulfate (PSS). Eur. J. Med. Chem. 2016, 114, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiu, P.; Xin, M.; Xu, X.; Wang, Z.; Xu, H.; Yu, R.; Xu, X.; Zhao, C.; Wang, X. Structure-activity relationship of propylene glycol alginate sodium sulfate derivatives for blockade of selectins binding to tumor cells. Carbohydr. Polym. 2019, 210, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cheng, C.; Nie, C.; He, C.; Deng, J.; Wang, L.; Xia, Y.; Zhao, C. Anticoagulant sodium alginate sulfates and their mussel-inspired heparin-mimetic coatings. J. Mater. Chem. B 2016, 4, 3203–3215. [Google Scholar] [CrossRef]
- Pulsawat, W.; Tongmalee, S. Synthesis and Anticoagulant activity of Sulfated alginate. Asia-Pac. J. Sci. Technol. 2014, 19, 60–66. [Google Scholar]
- Chandika, P.; Ko, S.-C.; Oh, G.-W.; Heo, S.-Y.; Nguyen, V.-T.; Jeon, Y.-J.; Lee, B.; Jang, C.H.; Kim, G.; Park, W.S. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int. J. Biol. Macromol. 2015, 81, 504–513. [Google Scholar] [CrossRef]
- Li, Q.-H.; Zou, H. Study on perparation of heparin-immobilized poly (vinyl-alcohol)-alginate and its blood compatibility. J.-Jinan Univ. Nat. Sci. Med. Ed. 1999, 20, 73–77. [Google Scholar]
- Kim, S.-K.; Wijesekara, I. Marine-Derived Peptides: Development and Health Prospects. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S.-K., Ed.; Wiley: New York, NY, USA, 2013; pp. 1–3. [Google Scholar]
- Jung, W.-K.; Je, J.-Y.; Kim, H.-J.; Kim, S.-K. A novel anticoagulant protein from Scapharca broughtonii. BMB Rep. 2002, 35, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-K.; Jo, H.-Y.; Qian, Z.-J.; Jeong, Y.-J.; Park, S.-G.; Choi, I.-W.; Kim, S.-K. A novel anticoagulant protein with high affinity to blood coagulation factor Va from Tegillarca granosa. BMB Rep. 2007, 40, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Jung, W.-K.; Mendis, E.; Moon, S.-H.; Kim, S.-K. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005, 76, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and bioactive properties of peptides derived from marine side streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the Nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Jo, H.-Y.; Jung, W.-K.; Kim, S.-K. Purification and characterization of a novel anticoagulant peptide from marine echiuroid worm, Urechis unicinctus. Process Biochem. 2008, 43, 179–184. [Google Scholar] [CrossRef]
- Nasri, R.; Amor, I.B.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Gargouri, J.; Châabouni, M.K.; Nasri, M. Anticoagulant activities of goby muscle protein hydrolysates. Food Chem. 2012, 133, 835–841. [Google Scholar] [CrossRef]
- Qiao, M.; Tu, M.; Chen, H.; Mao, F.; Yu, C.; Du, M. Identification and in silico prediction of anticoagulant peptides from the enzymatic hydrolysates of Mytilus edulis proteins. Int. J. Mol. Sci. 2018, 19, 2100. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-K.; Kim, S.-K. Isolation and characterisation of an anticoagulant oligopeptide from blue mussel, Mytilus edulis. Food Chem. 2009, 117, 687–692. [Google Scholar] [CrossRef]
- Cheng, S.; Tu, M.; Chen, H.; Xu, Z.; Wang, Z.; Liu, H.; Zhao, G.; Zhu, B.; Du, M. Identification and inhibitory activity against α-thrombin of a novel anticoagulant peptide derived from oyster (Crassostrea gigas) protein. Food Funct. 2018, 9, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Feng, N.; Zhang, L. Efficacy of heparinoid PSS in treating cardiovascular diseases and beyond—A review of 31 years clinical experiences in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 75–93. [Google Scholar] [PubMed]
- Zeng, Y.; Yang, D.; Qiu, P.; Han, Z.; Zeng, P.; He, Y.; Guo, Z.; Xu, L.; Cui, Y.; Zhou, Z. Efficacy of Heparinoid PSS in treating cardiovascular diseases and beyond—A review of 27 years clinical experiences in China. Clin. Appl. Thromb./Hemost. 2016, 22, 222–229. [Google Scholar] [CrossRef] [PubMed]




| Compound | Source | Species | MW | Concentration (μg/mL) | Anticoagulant Activity | Anti-Factor | Additional Findings | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| APTT | PT | TT | ||||||||
| Heparan sulfate | Mollusks | Nodipecten nodosus | - | 0.001–1 | ~40–~120 s | - | - | FXa-IC50 0.835 μg/mL fiia- IC50 9.3 μg/mL | In vivo assays demonstrated that at a dose of 1 mg/kg, it inhibited thrombus growth in photochemically injured arteries. | [51] |
| Heparin/heparan sulfate | Shrimp | Litopenaeus vannamei | - | 0–15 | ~40–~250 s | - | - | Inhibit FXa | Anti-Xa activity coupled with low bleeding effects. | [49] |
| Heparan sulfate | Scallop | Amussium pleuronectus | 15 kDa | - | 135 IU/mg | 100 IU/mg | - | - | APTT and PT were lower than standard bovine heparin sulfate. | [59] |
| Heparan like | Crab | Goniopsis cruentata | 19 kDa | 25–100 | ~100–~300 | - | ~175–~300 | Inhibit FXa and FIIa | No effect in the extrinsic pathway. | [60] |
| Heparin/heparan sulfate | Shrimp | Litopenaeus vannamei | 15 kDa | 0.5 μg/mL | - | - | - | Inhibit FIIa | Greater inhibitory effect; 90.7% than heparin. | [61] |
| LMWH | Shrimp | Penaeus brasiliensis | 8.5 kDa | 5–100 | ~50->300 s | ~15 s | ~20–~>300 s | Inhibit FXa and FIIa | Inhibits FXa, HCII. | [62] |
| Chondroitin sulfate | Smooth hound | - | 68.78 kDa | 25–500 | ~35–~65 s | ~14–~18 s | ~20–~60 s | - | Prolong the clotting time APTT, PT, and TT. | [63] |
| Dermatan sulfate | Pacific starfish | Lysastrosoma anthosticta | - | 2–10 | ~30–~100 s | - | - | Inhibit FXa | Prolongs the clotting time. | [64] |
| Chondroitin sulfate/dermatan sulfate | Corb skin | Sciaena umbra | 15.46 kDa | 25–1000 | ~30–70 s | ~13.5–~19 s | ~18–~50 | - | Remarkably high anticoagulant, Prolongs the clotting time APTT, PT, and TT. | [58] |
| Chondroitin sulfate/dermatan sulfate | Corb skin and bone | Sciaena umbra | - | 25–75 | ~22–~26 s ~20–~24 s | - | ~40–~50 s ~39–~41 s | - | Prolongs the clotting time APTT and TT. | [57] |
| Fucosylated chondroitin sulfates | Sea cucumbers | Pearsonothuria graeffei Stichopus tremulus Holothuria vagabunda Isostichopus badionotus | 73–320 kDa 81–340 kDa 100–380 kDa 109–460 kDa | 5–65 | ~30–~50 s ~35–~55 s ~40–~70 s ~45–~75 s | ~18–~35 s ~18–~45 s ~25–~50 s ~24–~55 s | - | Prolongs the clotting time APTT and TT., are related to the sulfation pattern. | [65] | |
| Fucosylated chondroitin sulfates | Sea cucumbers | Stichopus monotuberculatus Holothuria scabra Apostichopus japonicas Holothuria nobilis Thelenata ananas | 50–70 kDa 10–15 kDa | - | 2.5–7.0 μg/mL | 4–23 μg/mL | Inhibit FXa | Stronger AT-dependent anti-FIIa activities and potent HCII-dependent anti-FIIa activities. | [66] | |
| Fucosylated Chondroitin sulfates | Sea cucumbers | Sostichopus badionotus Pearsonothuria graeffei | - | - | 35, 183 U/mg | 78,157 μg/mL | Inhibit FXa and FIIa | Prolongs APTT and PT, inhibits FXa, and activates FXII. EC50 (Anti-FIIa/HCII); 0.86, 0.05 μg/mL, and EC50 Anti-FIIa/ATIII; 12.5, 0.56 μg/mL. | [67] | |
| Fucosylated chondroitin sulfates | Sea cucumbers | Holothuria scabra | 69 kDa kDa | 20–60 | ~50–~100 s | - | ~20–~25 s | - | Prolongs the coagulation and was evaluated by APTT and TT. | [68] |
| Fucosylated chondroitin sulfates | Sea cucumbers | Holothuria polii | 45 kDa kDa | 5–25 | >110s | - | >100 | Inhibit FXa and FIIa | High anticoagulant activity mediated by HCII, and to a lesser extent by ATIII. | [69] |
| Fucosylated chondroitin sulfates | Sea cucumbers | Cucumaria syracusana | - | 5–25 | >100 | - | >100 | Inhibit FXa and FIIa | High anticoagulant activity mediated by HCII and to a lesser extent by ATIII with IC50 of 0.05 μg/mL and 0.09 μg/mL. | [70] |
| Fucosylated chondroitin sulfates | Sea cucumbers | - | 3.2–8.8 kDa | - | 1.62–8.25 μg/mL | - | - | Inhibit FXa and FIIa | Anticoagulant activities through inhibition of intrinsic tenase, and of FXII. | [71] |
| Fucosylated chondroitin sulfates | Sea cucumbers | Isostichopus badionotus | 4.3–109 | - | - | - | - | Inhibit FXa and FIIa | High anticoagulant activity mediated by HCII and to a lesser extent by ATIII, results in a significant increase of the anti-FXa /anti-FIIa activity ratio. | [72] |
| Fucosylated chondroitin sulfates | Sea cucumbers | Holothuria Mexicana | - | 50–500 | ~75–>300 s | ~20–>800 s | Inhibit FXa and FIIa | Anticoagulant activity is similar to LMWH while inhibiting FIIa and FXa mediated by ATIII. | [73] | |
| Compound | Source | Species | MW | Concentration (μg/mL) | Anticoagulant Activity | Anti-Factor | Additional Findings | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| APTT | PT | TT | ||||||||
| Ulvan | Green algae | Ulva lactuca | - | 0.78–12.50 | ~29–~74 s | ~12–~18 s | ~23–>60 s | - | The highest APTT and TT clotting time with high concentrations of acid extracts. | [81] |
| Ulvan | Green algae | Ulva lactuca | 185.28 kDa 163. | - | - | - | - | Inhibit FXa and FIIa | Antithrombin-mediated inhibition of FXa and FIIa. The inhibition of venous thrombus formation of rats | [82] |
| Ulvan | Green macroalga | Ulva rigida | - | - | 2.5 μg/mL | 45 μg/mL | 2.62 μg/mL | Inhibit FXa and FIIa | Low ATIII-mediated inhibition activity | [80] |
| Ulvan | Green algae | Ulva pertusa | - | 0.6 μg/g 1.2 μg/g 3 μg/g | ~62 ~81 ~80 | ~19 ~25 ~20 | - | - | Prolongs the clotting in the male and female Wistar rats | [84] |
| Low molecular–weight ulvan | Green algae | Ulothrix flacca | 5 kDa | 2.5–50 | ~48–>200 s | ~15–~19 | ~24–>120 s | - | Mild anticoagulant activities similar to those of LMWH | [83] |
| Ulvans and their polycarboxyl derivatives | Green algae | Ulva fasciata | - | 10–150 | ~28–~220 s | - | - | - | Exhibited a dose-dependent prolongation of APTT | [85] |
| λ-carrageenan ι-carrageenan | Red algae | Gigartina skottsbergii | 4.7–3100 kDa 1.1–63 kDa | - | - | - | - | - | High molecular weight λ-carrageenan was comparable to the anticoagulant activity of heparin | [86] |
| k, k/β, k/ι, λ, iks- carrageenan | Red algae | Chondrus armatus, C. yendoi, C. pinnulatus and Tichocarpus crinitus | - | ~187, 580, 81, 343, >600, and 59 s | - | - | -- | Anticoagulant activity depends on the monosaccharide composition, number, position, and distribution of sulfate | [87] | |
| λ-carrageenan ι-carrageenan | Red algae | Sigma Chemical Co. (St. Louis, MO, EUA) | - | - | 240 s 132 s | - | - | - | No anticoagulant action in the PT test | [88] |
| λ-carrageenan ι-carrageenan θ-carrageenan | Red algae | Kappaphycus alvarezii Eucheuma denticulatum Gigartina skottsbergii | - | 10–150 | ~34–>300 s | - | - | - | Anticoagulant activity depends on molecular weight and/or differences in the sulfation degree or sulfation pattern | [89] |
| k- carrageenan λ-carrageenan ι-carrageenan ι/υ-carrageenan θ-carrageenan | Red algae | Kappaphycus alvarezzi Gigartina skottsbergii | 36,000 57,800 84,000 70,000 23,700 (g/mol) | - | - | - | - | - | Prolonged coagulation with the carrageenan and oxidized carrageenan | [90] |
| λ-carrageenan oligosaccharides | Red algae | FMC Biopolymer (Villefranche-Sur-Saône, France) | 5.9 kDa | - | - | - | - | Inhibit FXa and FIIa | The anticoagulant activitydepended on the degree of sulfation | [91] |
| Chitosan-kappa-carrageenan composite hydrogels | Red algae | Aladdin Reagent Co., Ltd. | - | - | 110.5 s | - | 37.4 s | Attenuate FVIII, IX FIX, XI FXI and FXII | Composite hydrogels had better anticoagulant properties than raw chitosan hydrogels | [92] |
| Carrageenan-based gel beads | Red algae | Aladdin Reagent Co., Ltd. | 100–300 kDa | >600 s | >250 s | ~73 s | - | The self-anticoagulant and biocompatible beads prolong the coagulation time significantly | [93] | |
| Fucoidans | Brown algae | Saccharina japonica | 8.4–50.1 kDa | 3.6–14.4 | ~28–95 s | ~8.5–10.5 s | ~24–~53 s | - | Prolonged the coagulation dose-dependent manner in APTT ant TT assays | [94] |
| Heterofucans | Brown algae | Dictyopteris delicatula | - | - | - | - | - | No inhibition was in PT and prolonged the APTT | [95] | |
| Xylofucan | Brown algae | Punctaria plantaginea | 1–5 | 05–1.4 | ~4–>100 s | - | - | Inhibit FXa and FIIa | ATIII-medicated anticoagulant activity | [96] |
| Low molecular fucoidans | Brown algae | Laminaria japonica | - | 0.7–28 | ~85–~240 s | ~88–~170 s | ~53–~160 | - | Prolonged the coagulation evaluated by APTT, PT, TT | [97] |
| Fucan sulfates | Sea cucumber | Holothuria fuscopunctata Thelenota ananas Stichopus horrens | 36.8 kDa 61.2 kDa 487.9 kDa | - | 11.3 s 10.4 s 19.6 s | - | - | Inhibit FXa and FIIa | Strong inhibition of the intrinsic coagulation pathway through the intrinsic FXase | [98] |
| Fucan sulfates | Sea cucumber | Acaudina leucoprocta | - | 2.5–20 | ~43–~72 s | ~9.6–~11.6 s | ~13–~13.5s | - | Anticoagulant activity through ATIII activity through HCII. | [99] |
| Fucan sulfates | Sea cucumber | Holothuria albiventer | - | - | ~26 μg/mL | - | ~116 μg/mL | Inhibit FXa | Prolongation of APTT and TT and intrinsic FXase inhibitory activity | [100] |
| Fucan sulfates | Sea cucumber | Pattalus mollis | 6.12–238.3 kDa | - | ~20–~23 s | >128 | ~40–>128s | - | Strong prolongation of coagulation evaluated by APTT and PT | [101] |
| Rhamnan sulfates | Green algae | Monostroma angicava | 88.1 kDa | 10–100 | ~33–>200S | ~13–~16S | ~18–>120 | - | Anticoagulant activity mediated by potentiation thrombin by HCII | [102] |
| Low molecular Rhamnan sulfates | Green algae | Monostroma angicava | 24–240 kDa | 10–100 | ~40–~200s | ~15–~35 | ~10–~100 | - | Prolongs the clotting time | [103] |
| Rhamnan sulfates | Green algae | Monostroma angicava | - | 5–100 | ~40–~200 s | ~15–~30 s | ~10–~120 | - | Prolongs the clotting time | [104] |
| Rhamnan sulfates | Green algae | Monostroma nitidum | - | - | - | - | - | Inhibit FXa and FIIa | Anticoagulant activity through inhibition of FXa and FII, inhibits tissue factor expression and von Willebrand factor release | [105] |
| Low molecular rhamnan sulfates | Green algae | Monostroma latissimum. | 33.6 kDa | 2–50 | ~30–~200 s | - | - | Inhibit FIIa | Anticoagulant activity mediated by potentiation thrombin by HCII | [106] |
| Chemically Sulfated Polysaccharide | Sulfation Technique | MW | Degree of Sulfation/Substitution | Concentration (μg/mL) | In Vitro Anticoagulant Assay | Anti-Factor | Additional Findings | In Vivo or In Silico | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| APTT | PT | TT | |||||||||
| Quaternary ammonium chitosan sulfate | - Quaternary ammonium chitosan by N-(3-chloro-2-hydroxypropyl) trimethyl ammonium chloride - Quaternary ammonium chitosan sulfates by N(SO3Na)3 | - | 0.52–1.55 1.55 | 75 25–75 | ~90 –~160 s ~90 –~160 s | ~10 s ~10–~30 s | ~25–~45 s ~40–~50 s | Inhibits FIIa and FXa | Prolonged coagulation Best anticoagulant activity at MW-2.27 × 104. | - | [133] |
| polyampholytic aryl-sulfonated chitosans | formyl benzene sulfonic acid | <0.803 | 31–>250 s | 11.2–21.8 s | - | Inhibits FXa 0.09 UI/mL | Very low activity on the extrinsic pathway | - | [134] | ||
| Silylated chitosan sulfate | sulfur trioxide–pyridine complex in DMSO | 18.1–54.5 kDa | 1.65–2.46 | 20–80 s | ~-35–~85 s | ~13 s | ~20–~35 s | Requires a high degree of sulfation (DS > 2.1) | - | [135] | |
| N-propanoyl-, N-hexanoyl- and N,O-quaternary substituted chitosan sulfate | chlorosulfonic acid (ClSO3H I) in formamide | 0.18–0.81 | 16.67–66.7s | 60.78–138.99 s | 0.90–1.11 (INR) | 9.60–18.08 s | - | Prolonged coagulation | - | [131] | |
| Carboxybutyrylated hydroxyethyl chitosan sulfate derivative | ClSO3H I in N,N-dimethylformamide | 0.18–0.77 | 360 s at 40 μg/mL | - | TT: 20 s at 10 μg/mL | - | Prolonged APTT and TT. Best result when the DS of the carboxyl groups is 0.4/unit | - | [129] | ||
| N-succinyl chitosan sulfate | N-succinyl chitosan N(SO3Na)3 | 4.5 kDa 13.7 kDa 45.4 kDa 119.6 kDa | 1.97 | 2–75 | ~86 s ~80 s ~82 s | ~25 s ~24 s ~23 s | ~20 s ~18 s ~17 s | Depended on DS, MW, and concentration of N-succinyl chitosan sulfate. | - | [130] | |
| Low-molecular-weight chitosan | Oleum to N,N-dimethylformamide | 10–50 kDa | 1.10–1.63 | - | - | - | - | Anti-Xa and anti-IIa activity | Regular increase of anti-Xa activity like heparins | - | [136] |
| Sulfated chitosan derivative | ClSO3H IiH2NCHO | - | - | 5–100 | ~25–~175 s | ~15–~60 s | ~15 s | Inhibit FIIa and FXa | Prolonged coagulation mediated by AT III | - | [127] |
| Low-molecular-weight chitosan polysulfate | ClSO3H I in N,N-dimethylformamide | 5.1–26.2 kDa | - | - | 40.3–51.7 s | 0.88–0.86 (INR) | 19.7–12.6 s | Inhibit FIIa and FXa | Prolonged coagulation mediated by AT III and HC II | - | [128] |
| -N-alkyl derivatives of chitosan sulfate -Quaternary derivatives/chitosan sulfate | ClSO3H I in N,N-dimethylformamide | - | - | - | - | - | - | N-alkyl derivatives of chitosan sulfate are more highly potent than Quaternary derivatives/chitosan sulfate | The tail bleeding method in Wistar rats | [126] | |
| Chitosan sulfate (chitosan from Doryteuthis singhalensis) | ClSO3H I in N,N-dimethylformamide | 83.76% | - | 6.91 IU/mg | 1.85 IU/mg | - | Inhibit FXa | Prolonged coagulation Inhibits FXa through ATIII and thrombin | - | [124] | |
| Chitosan sulfate (chitosan from (Somanniathelphusa dugasti) | ClSO3H I in N,N-dimethylformamide (obtained 3 fractions of chitosan) | 0.21 | - | - | 21.6–23.2 s | - | Inhibit FXa | Prolonged coagulation Inhibits FXa through ATIII and thrombin | - | [137] | |
| Low molecular weight chitosan sulfate (chitosan from Sepia pharaonic) | ClSO3H I in N,N-dimethylformamide | 1277 Da | - | - | ~ 67 s | ~ 95 s | - | - | Prolonged the coagulation | - | [138] |
| Heterochitosans and heterochitooligosacharides | trimethylamine-sulfur trioxide | 10–5 kDa 5–1 kDa >1 kDa | - | 5–100 5–100 5–100 | ~37–~44 s ~37–~43 s ~37–~42 s | ~15–~25 s ~15–~25 s ~15–~20 s | - | - | Prolonged the coagulation Highest anticoagulant activity: 90% deacetylated chitosan sulfates | - | [139] |
| Chitosan sulfate (chitosan from Sepia prashadi) | ClSO3H I in N,N-dimethylformamide | - | - | - | 6.90 IU/mg | 1.2 IU/mg | Anticoagulant activity depends on sulfate content and the position of sulfate groups. | - | [125] | ||
| N-octyl-O-sulfate chitosan and derivatives | ClSO3H I in N,N-dimethylformamide | 150 kDa 400 kDa 600 kDa | - | 0–5 0–5 0–5 | - | - | - | FXa~100–~10% FXa~100–~6% FXa~100–~6% | Percentage residual activity of factor Xa after inhibition | - | [140] |
| Chitosan polysulfate (crab shell chitosan from Sigma-Aldrich) | ClSO3H I in N,N-dimethylformamide | 66 kDa 35 kDa 18 kDa | 0.89 | 27.8 s 22.2 s 22.4 s | Prolonged coagulation Inhibits FXa through ATIII and thrombin | - | [141] | ||||
| Sulfated alginate | N (SO3Na)3 | - | 0.58 0.95 1.25 | - | ~120–~165 s | ~20 s | ~38–~42 s | High Degrees of sulfation and concentration inhibit FIIa and FXa Low molecular weight results in higher anti-Xa activity | - | [142] | |
| Sulfonated alginate (Immobilized in membrane_ | ClSO3H I in N,N-dimethylformamide | - | - | - | >35 s | >14s | >10s | - | Platelet adhesion resistance | - | [143] |
| Alginate sulfate and quaterized derivatives (QAS-1, QAS-2, and QAS-3) | ClSO3H I in N,N-dimethylformamide | - | - | 33 | ~225 s ~200 s ~125 s | ~18.5 ~15.5 ~15.5 | ~22 s ~19 s ~15 s | - | The very high anticoagulant activity of alginate sulfate was reduced by quaternization | - | [144] |
| Propylene glycol sodium alginate sulfate with low mannuronic acid (M)/guluronic acid (G) ratio | ClSO3H I in N,N-dimethylformamide | 8403 Da 9446 Da 19716 Da | 11.43 11.48 12.27 | 25 | - | ~15 s ~15 s ~15 s | ~15 s ~20 s ~50 s | Inhibit FIIa | Fractions with low (M)/(G) and high MW prolong APTT and TT, and over-inhibit the FIIa activity mediated by ATIII to induce bleeding risk. | - | [145] |
| Propylene glycol sodium alginate sulfate with low mannuronic acid (M)/guluronic acid (G) ratio | ClSO3H I in N,N-dimethylformamide | 8403 Da 9446 Da 19716 Da | 11.43 11.48 12.27 | 25 | ~50 s ~90 s ~170 s | - | - | - | Prolonged coagulation low M/G ratio or high MW | - | [146] |
| Propylene glycol sodium alginate and oligosaccharides | - | - | - | 5–50 | ~50–~40 s | ~13–~15 s | ~10–~40 s | - | Prolonged the APTT, TT, and PT with various fractions, Weaker than heparin | - | [147] |
| Low-molecular-weight propylene glycol sodium alginate | ClSO3H I in N,N-dimethylformamide | ~21 kDa ~9 kDa ~7 kDa ~4 kDa ~3 kDa | 1.15 1.05 1.01 1.07 1.06 | 0.78–50 | ~40–~120 s ~40–~60 s ~40–~80 s ~40–~80 s ~40–~85 s | - | - | Inhibit FIIa | Inhibit FIIa in the presence of ATIII and heparin cofactor II. | Decreased the wet weights and lengths of the thrombus in mice | [148] |
| Propylene glycol sodium alginate sulfate | ClSO3H I in N,N-dimethylformamide | - | - | 12.5–200 | 2.7–>240 s | - | - | - | Prolonged coagulation yet mild anticoagulant | - | [149] |
| Alginate sulfate (heparin mimetic coating) | H2SO4 in N,N-dimethylformamide | - | - | 1–25 | ~50–>600 s | - | ~0–>24 s | - | Prolonged coagulation and non-coagulation | - | [150] |
| Alginate sulfate and fragments | ClSO3H I in formamide | - | 1.75–1.35 | 75 | 288 and 102 s | - | - | - | Prolonged coagulation but no increase in PT | - | [151] |
| Disease | Types | Cases | Effective Rate (%) |
|---|---|---|---|
| Hyperviscosity and hyperlipidemia | Hyperviscosity | 1518 | 80.00–96.67 |
| Hyperlipidemia | 3581 | 75.50–95.08 | |
| Others | 81 | ||
| Cerebrovascular disease | Ischemic cerebrovascular disease | 2666 | 86.80–98.30 |
| Cerebral infarction | 2689 | 84.20–95.12 | |
| Stroke prevention and treatment | 487 | 90.00 | |
| Cerebral thrombosis | 1294 | 81.60–96.00 | |
| Others | 690 | 87.04–98.33 | |
| Cardiovascular disease | Coronary heart disease | 1216 | 90.00–92.00 |
| Ischemic heart disease | 554 | 91.30 | |
| Angina | 966 | 77.00–98.08 | |
| Pulmonary heart disease | 2156 | 81.80–97.50 | |
| Others | 609 | 66.70–77.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandika, P.; Tennakoon, P.; Kim, T.-H.; Kim, S.-C.; Je, J.-Y.; Kim, J.-I.; Lee, B.; Ryu, B.; Kang, H.W.; Kim, H.-W.; et al. Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants. Mar. Drugs 2022, 20, 654. https://doi.org/10.3390/md20100654
Chandika P, Tennakoon P, Kim T-H, Kim S-C, Je J-Y, Kim J-I, Lee B, Ryu B, Kang HW, Kim H-W, et al. Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants. Marine Drugs. 2022; 20(10):654. https://doi.org/10.3390/md20100654
Chicago/Turabian StyleChandika, Pathum, Pipuni Tennakoon, Tae-Hee Kim, Se-Chang Kim, Jae-Young Je, Jae-Il Kim, Bonggi Lee, BoMi Ryu, Hyun Wook Kang, Hyun-Woo Kim, and et al. 2022. "Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants" Marine Drugs 20, no. 10: 654. https://doi.org/10.3390/md20100654
APA StyleChandika, P., Tennakoon, P., Kim, T. -H., Kim, S. -C., Je, J. -Y., Kim, J. -I., Lee, B., Ryu, B., Kang, H. W., Kim, H. -W., Kim, Y. -M., Kim, C. S., Choi, I. -W., Park, W. S., Yi, M., & Jung, W. -K. (2022). Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants. Marine Drugs, 20(10), 654. https://doi.org/10.3390/md20100654









