Efficient Preparation of High-Purity Fucoxanthinol by SpyTag-Tailored Active Cholesterol Esterase Aggregates
Abstract
:1. Introduction
2. Results and Discussion
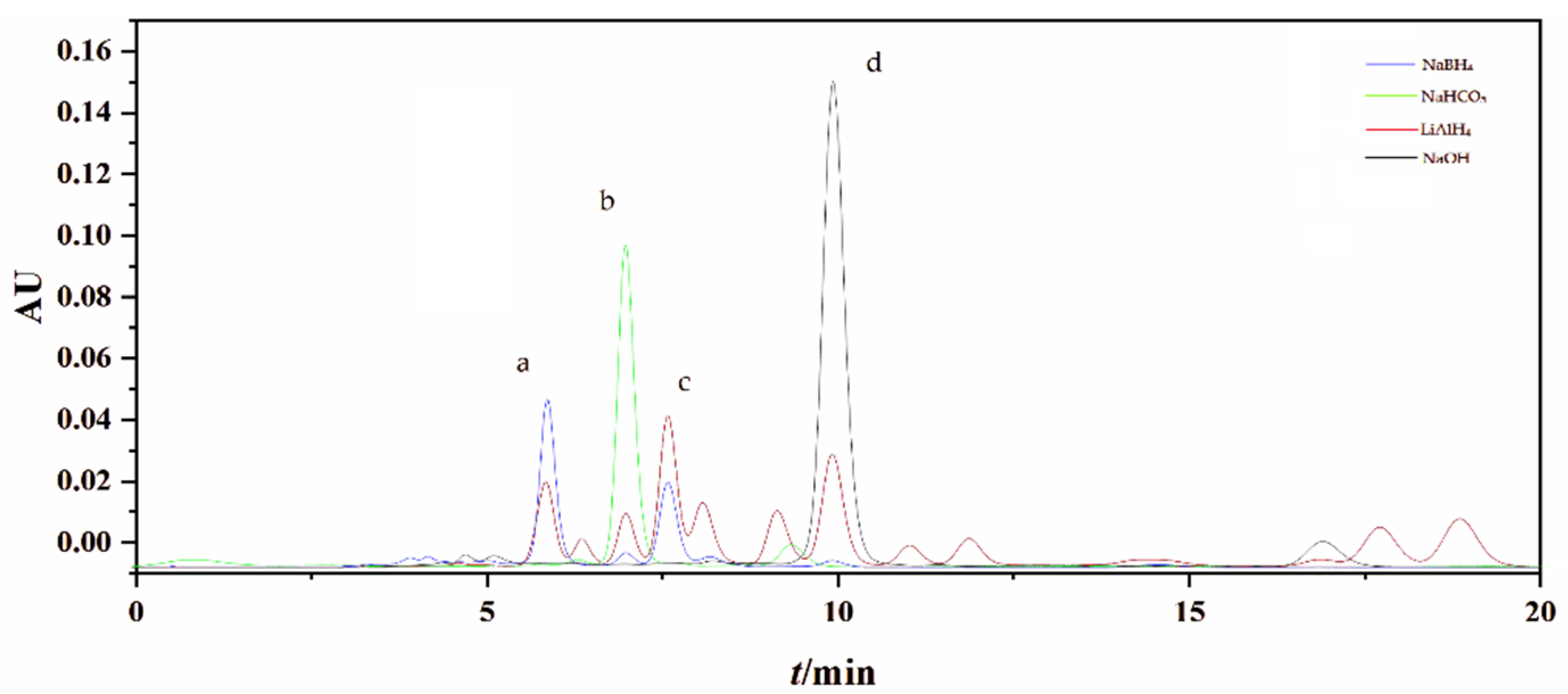
2.1. Preparation of FXOH by Chemical Reduction
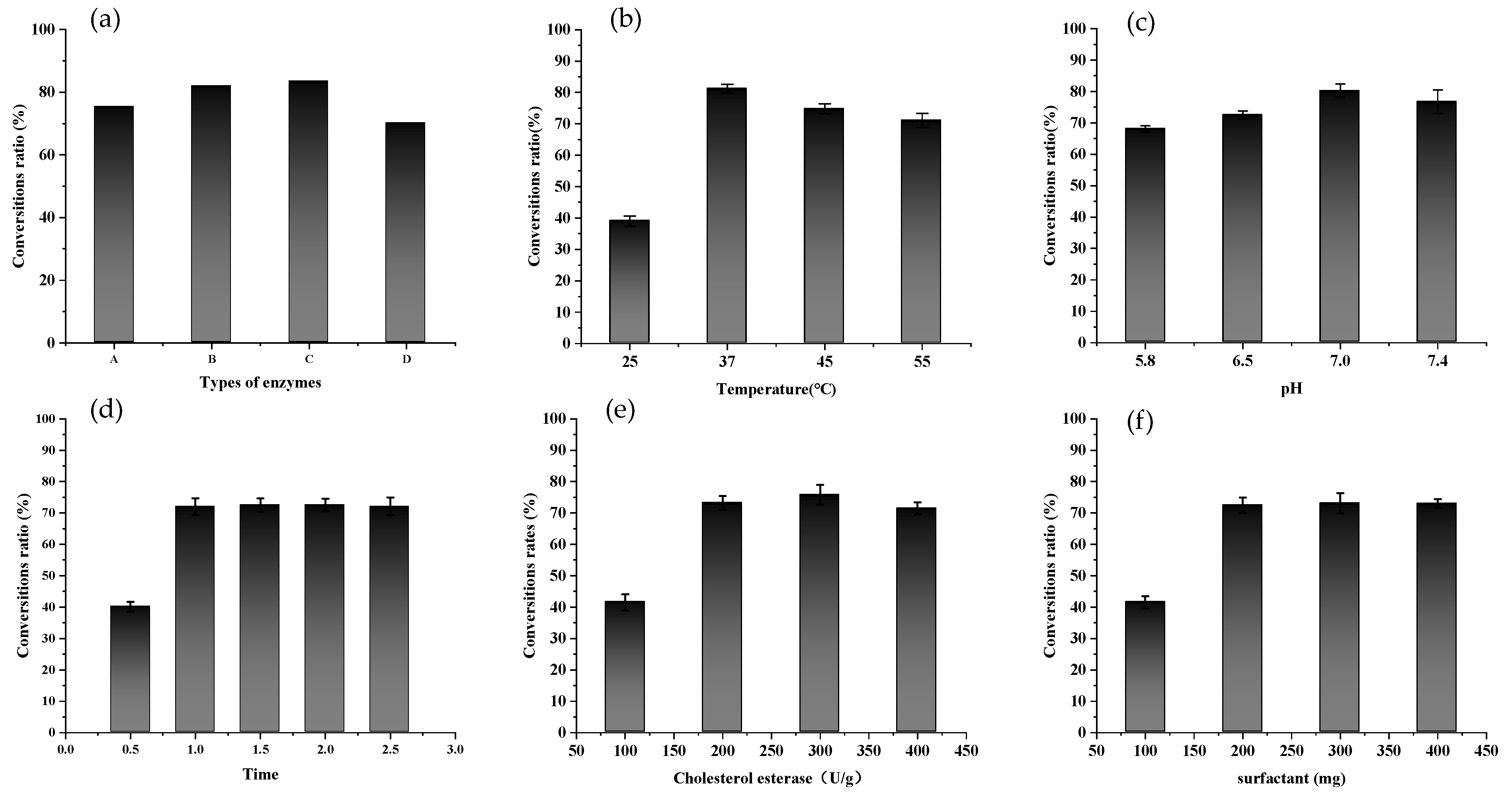
2.2. Enzymatic Preparation of FXOH
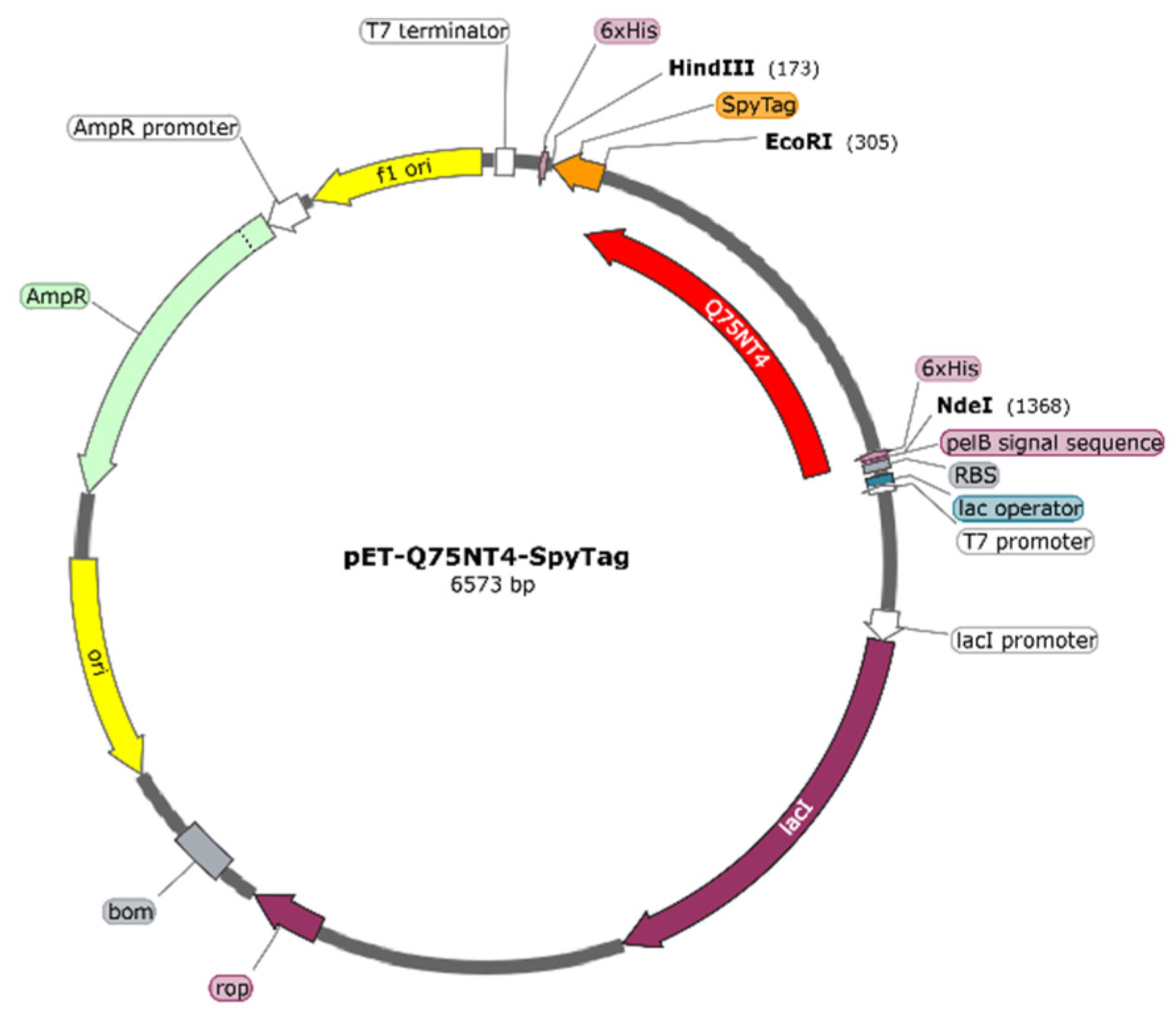
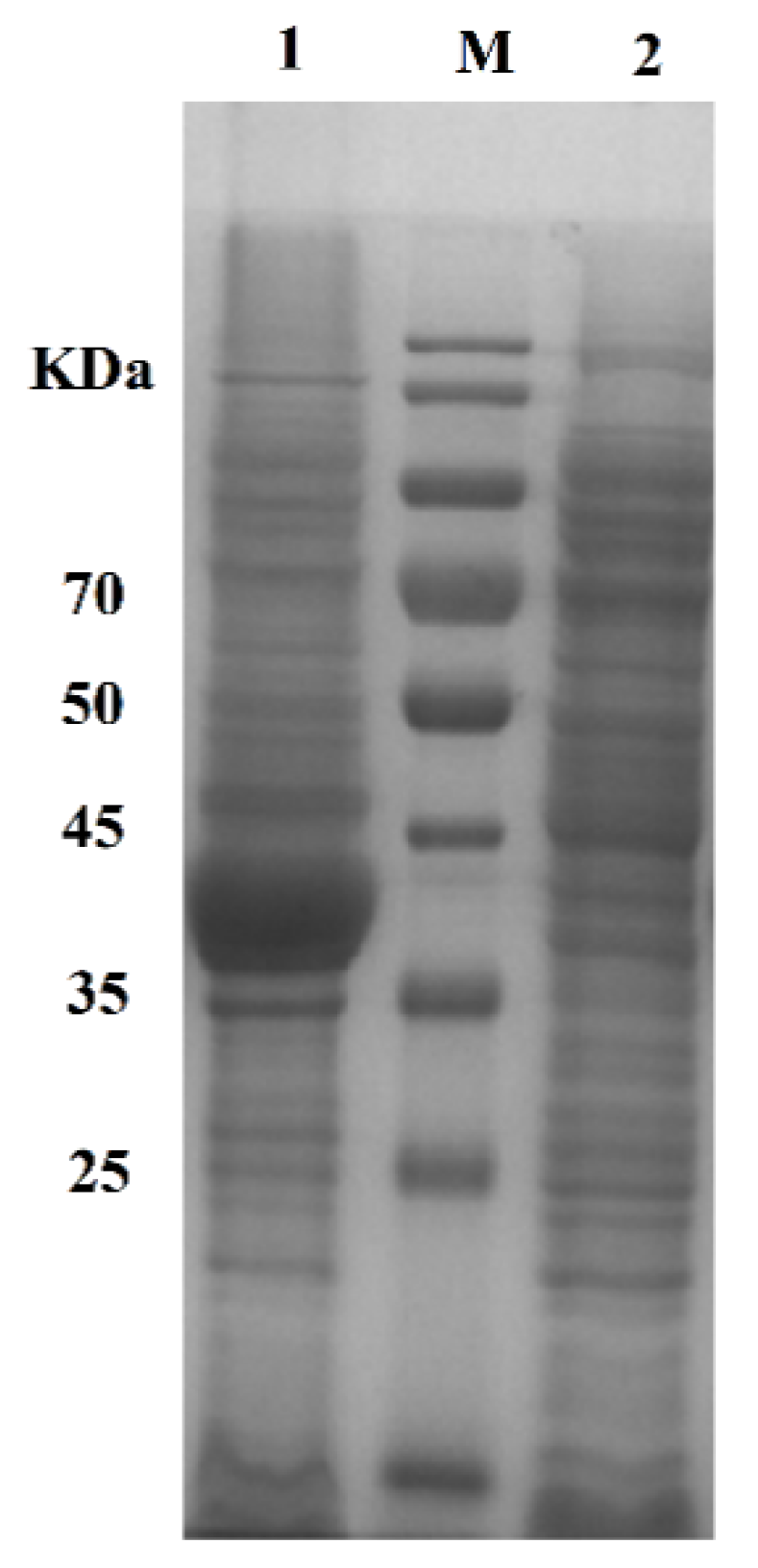
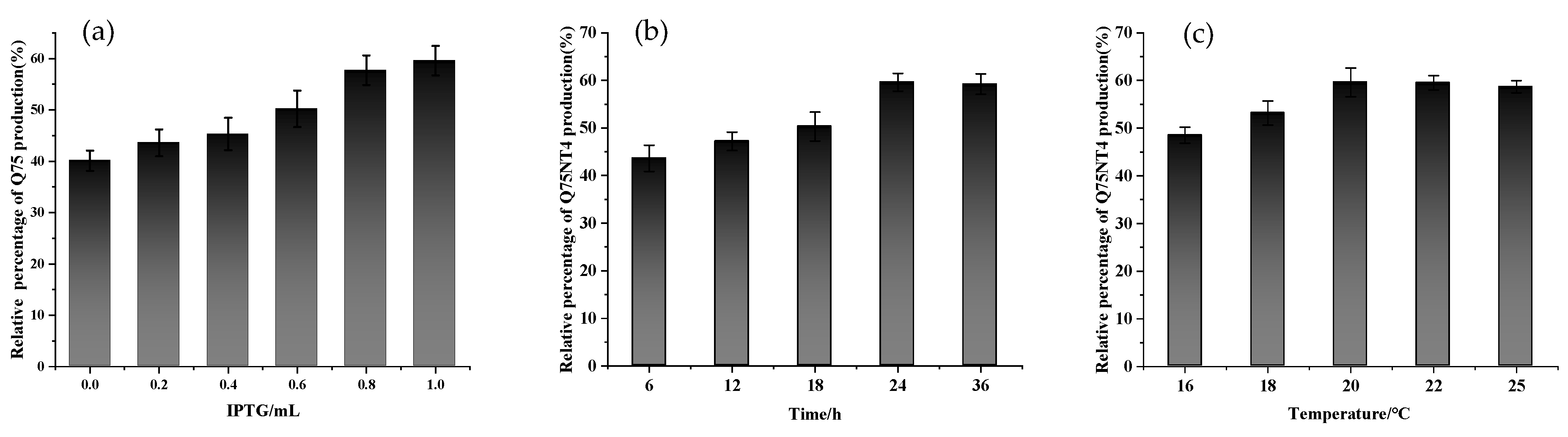
2.3. Preparation of Active Cholesterol Esterase Aggregates by SpyTag Tailoring
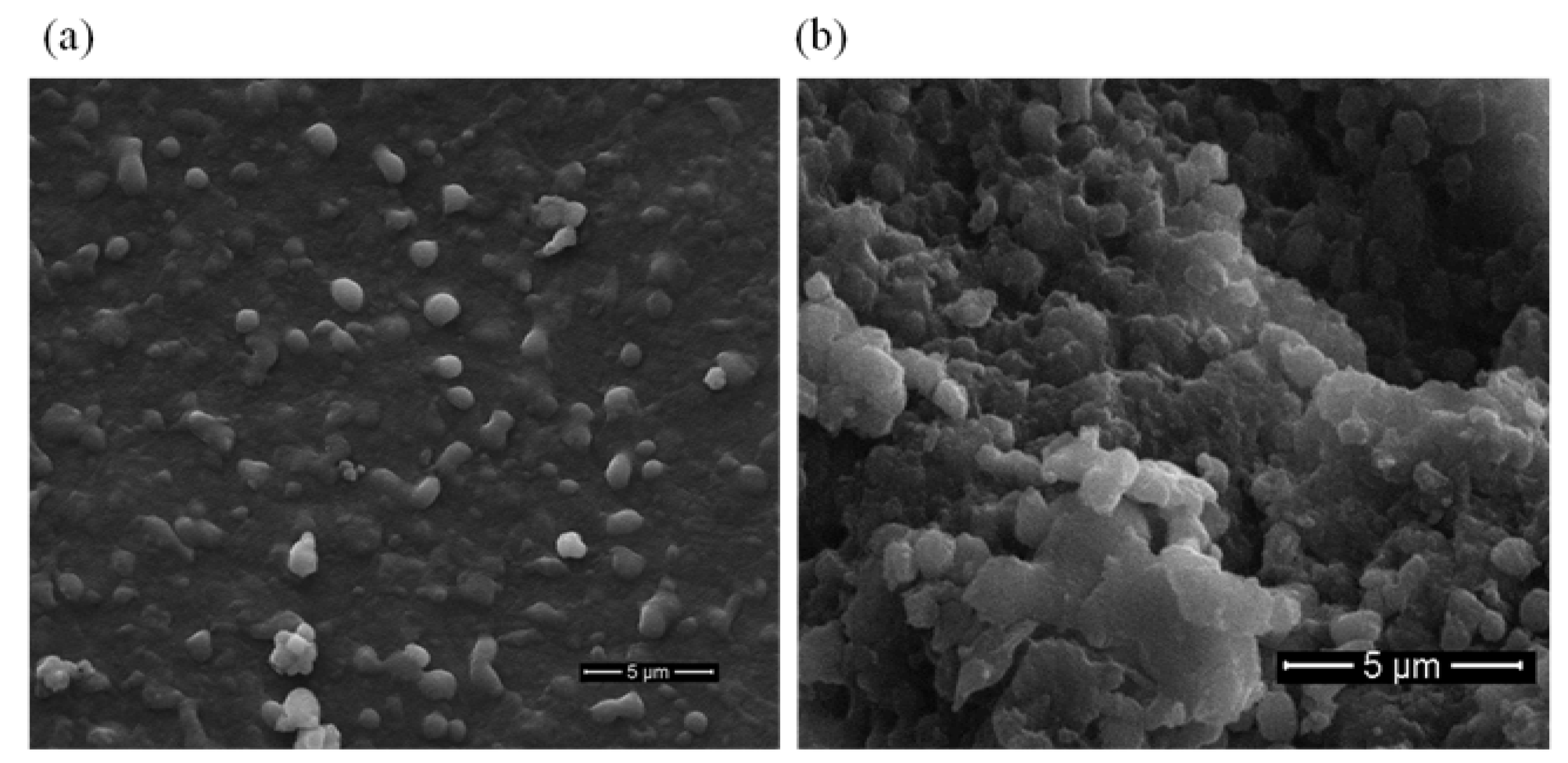
2.4. Morphological Analysis of Active Cholesterol Esterase Aggregates
2.5. Kinetic Parameters of Active Cholesterol Esterase Aggregates
2.6. Reusability of Active Cholesterol Esterase Aggregates
2.7. Preparation and Purification of FXOH by Using Different Methods
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of FXOH by Chemical Reduction
3.3. Preparation of FXOH by Enzymatic Hydrolysis
3.4. Protein Design and Gene Construction
3.5. Protein Expression and Purification
3.6. Enzyme Activity Determination
3.7. Morphological Analysis of Active Cholesterol Esterase Aggregates
3.8. Determination of Kinetic Parameters for Cholesterol Esterase
3.9. Reusability of Active Cholesterol Esterase Aggregates
3.10. Analysis of FXOH by Liquid Chromatography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Nascimiento, L.D.S.; Tredici, M.; Luceri, C. A Comparative In Vitro Evaluation of the Anti-Inflammatory Effects of a Tisochrysis lutea Extract and Fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef]
- Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V.; Harish. Bioprospecting of fucoxanthin from diatoms—Challenges and perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Wu, S.-J.; Liou, C.-J.; Chen, Y.-L.; Cheng, S.-C.; Huang, W.-C. Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Li, G.; Chang, X.; Luo, X.; Zhao, Y.; Wang, W.; Kang, X. Fucoxanthin induces prostate cancer PC-3 cell apoptosis by causing mitochondria dysfunction and oxidative stress. J. South. Med. Univ. 2021, 41, 953–959. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Yan, G.; Jin, W.; Chen, W.; Sun, J.; Yang, L.; Huang, M.; Hong, Z. Determination of fucoxanthinol in rat plasma by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Yang, L.; Yi, Z.; Fang, H.; Chen, W.; Hong, Z.; Zhang, Y.; Zhang, G.; Li, L. Anti-Inflammatory Effects of Fucoxanthinol in LPS-Induced RAW264.7 Cells through the NAAA-PEA Pathway. Mar. Drugs 2020, 18, 222. [Google Scholar] [CrossRef]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, R.; Kojima, H.; Takai, R.; Ohta, T.; Maeda, H.; Miyashita, K.; Mutoh, M.; Terasaki, M. Effects of CLIC4 on Fucoxanthinol-Induced Apoptosis in Human Colorectal Cancer Cells. Nutr. Cancer 2020, 73, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Hosokawa, M.; Sashima, T.; Sasak, T. Process for Production of Fucoxanthinol (Japan). Patent number: WO2007060811A1, 31 May 2007. [Google Scholar]
- Helfried, N. Neoxanthin and fucoxanthinol in Fucus Vesiculosus. Biochim. Biophys. Acta 1974, 338, 572–576. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.-C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2018, 277, 566–572. [Google Scholar] [CrossRef]
- Lai, O.-M.; Lee, Y.-Y.; Phuah, E.-T.; Akoh, C.C. Lipase/Esterase: Properties and Industrial Applications. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–11. [Google Scholar] [CrossRef]
- Ben Ali, Y.; Verger, R.; Abousalham, A. Lipases or Esterases: Does It Really Matter? Toward a New Bio-Physico-Chemical Classification. Methods Mol. Biol. 2012, 861, 31–51. [Google Scholar] [CrossRef]
- Erdem, E.; Woodley, J.M. Industrially useful enzymology: Translating biocatalysis from laboratory to process. Chem Catal. 2022, 2, 2499–2505. [Google Scholar] [CrossRef]
- Liu, T.; Pei, B.; Lin, J.; Zhang, G. Immobilization of β-1,3-xylanase on pitch-based hyper-crosslinked polymers loaded with Ni2+ for algal biomass manipulation. Enzym. Microb. Technol. 2020, 142, 109674. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, W.; Wang, J.; Cai, Z.; Wu, S.; Zhang, G. A novel method for simultaneous purification and immobilization of a xylanase-lichenase chimera via SpyTag/SpyCatcher spontaneous reaction. Enzym. Microb. Technol. 2018, 115, 29–36. [Google Scholar] [CrossRef]
- Bagde, P.; Vigneshwaran, N. Improving the stability of bacteriocin extracted from Enterococcus faecium by immobilization onto cellulose nanocrystals. Carbohydr. Polym. 2019, 209, 172–180. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, Ł.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Ehrlich, H.; Maciejewski, H.; Stelling, A.L.; Jesionowski, T. Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization. Mar. Drugs 2015, 13, 2424–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, S.; Princy; Arya, S.K.; Kaur, A. Enzyme immobilization: Implementation of nanoparticles and an insight into polystyrene as the contemporary immobilization matrix. Process Biochem. 2022, 120, 22–34. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Shen, T.; Wang, J.; Zhang, G. Investigation on the underlying mechanism: How fusion xylanase-ELPs self-assembles into insoluble active aggregates. J. Mol. Catal. B Enzym. 2016, 134, 247–252. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, B.; Wu, W.; Xing, L.; Zhao, Q. Self-assembling amphipathic alpha-helical peptides induce the formation of active protein aggregates in vivo. Faraday Discuss. 2013, 166, 243–256. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, W.; Cai, L.; Liu, X.; Qiu, Y.; Zhang, G. Green preparation of covalently co-immobilized multienzymes on silica nanoparticles for clean production of reducing sugar from lignocellulosic biomass. J. Clean. Prod. 2021, 314, 127994. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, W.; Qiu, Y.; Zhang, G. Programmable stimuli-responsive polypeptides for biomimetic synthesis of silica nanocomposites and enzyme self-immobilization. Int. J. Biol. Macromol. 2019, 134, 1156–1169. [Google Scholar] [CrossRef]
- Dong, W.; Sun, H.; Chen, Q.; Hou, L.; Chang, Y.; Luo, H. SpyTag/Catcher chemistry induces the formation of active inclusion bodies in E. coli. Int. J. Biol. Macromol. 2022, 199, 358–371. [Google Scholar] [CrossRef]
- Chen, Y.; Ming, D.; Zhu, L.; Huang, H.; Jiang, L. Tailoring the Tag/Catcher System by Integrating Covalent Bonds and Noncovalent Interactions for Highly Efficient Protein Self-Assembly. Biomacromolecules 2022, 23, 3936–3947. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, G. The fusions of elastin-like polypeptides and xylanase self-assembled into insoluble active xylanase particles. J. Biotechnol. 2014, 177, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xing, L.; Zhou, B.; Lin, Z. Active protein aggregates induced by terminally attached self-assembling peptide ELK16 in Escherichia coli. Microb. Cell Factories 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Fruitós, E.; González-Montalbán, N.; Morell, M.; Vera, A.; Ferraz, R.M.; Arís, A.; Ventura, S.; Villaverde, A. Aggregation as bacterial inclusion bodies does not imply inactivation of enzymes and fluorescent proteins. Microb. Cell Factories 2005, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Rinas, U.; Garcia-Fruitós, E.; Corchero, J.L.; Vázquez, E.; Seras-Franzoso, J.; Villaverde, A. Bacterial Inclusion Bodies: Discovering Their Better Half. Trends Biochem. Sci. 2017, 42, 726–737. [Google Scholar] [CrossRef]
- Hemamalini, R.; Khare, S.K. Halophilic lipase does forms catalytically active aggregates: Evidence from Marinobacter sp. EMB5 lipase (LipEMB5). Int. J. Biol. Macromol. 2018, 119, 172–179. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, Y.; Zhang, G. Unique silica biomimetic mineralization of acidic elastin-like polypeptides without hydroxyl and charged residues. Int. J. Biol. Macromol. 2020, 153, 224–231. [Google Scholar] [CrossRef]
- Jäger, V.D.; Lamm, R.; Küsters, K.; Ölçücü, G.; Oldiges, M.; Jaeger, K.-E.; Büchs, J.; Krauss, U. Catalytically-active inclusion bodies for biotechnology—General concepts, optimization, and application. Appl. Microbiol. Biotechnol. 2020, 104, 7313–7329. [Google Scholar] [CrossRef]
- Krauss, U.; Jäger, V.D.; Diener, M.; Pohl, M.; Jaeger, K.-E. Catalytically-active inclusion bodies—Carrier-free protein immobilizates for application in biotechnology and biomedicine. J. Biotechnol. 2017, 258, 136–147. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.-S.; Moon, I.S.; Hong, Y.-K. Neuroprotective effects of fucoxanthin and its derivative fucoxanthinol from the phaeophyte Undaria pinnatifida attenuate oxidative stress in hippocampal neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Zhuang, G.; Ye, Y.; Zhao, J.; Zhou, C.; Zhu, J.; Li, Y.; Zhang, J.; Yan, X. High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography. Mar. Drugs 2022, 20, 510. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.-W.; Chen, F. Fucoxanthinol from the Diatom Nitzschia Laevis Ameliorates Neuroinflammatory Responses in Lipopolysaccharide-Stimulated BV-2 Microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Ndengue, P.P.A.; Jing, Y.; Zhao, L.; Yang, X. Facile expression and purification of active human growth hormone in E. coli by a cleavable self-aggregating tag scheme. Protein Expr. Purif. 2021, 188, 105974. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wang, J.; Zheng, H.; Xiao, Q.; Wang, A.; Su, W. Catalytically active inclusion bodies (CatIBs) induced by terminally attached self-assembling coiled-coil domains: To enhance the stability of (R)-hydroxynitrile lyase. Enzym. Microb. Technol. 2021, 153, 109915. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.-Y.; Varjani, S.; Chang, J.-S. Producing fucoxanthin from algae—Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2021, 344, 126170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative Evaluation of the Radical-Scavenging Activities of Fucoxanthin and Its Stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2019, 90, 66–80. [Google Scholar] [CrossRef]
- Sigma Quality Control Test Procedure. Enzymatic Assay of CHOLESTEROL ESTERASE (EC 3.1.1.13). Available online: https://www.sigmaaldrich.cn/deepweb/assets/sigmaaldrich/product/documents/150/447/c1403enz.pdf (accessed on 16 March 1998).

| Enzyme | Concentration of Bacteria a/(g/L) | Concentration of Precipitates a/(g/L) | Mass of Active Aggregates b/(g/g) | Average Yield (%) |
|---|---|---|---|---|
| Q75NT4 | 14.10 ± 0.92 | 7.99 ± 0.63 | 0.57 ± 0.01 | 60 ± 3 |
| 15.81 ± 1.26 | 9.62 ± 0.72 | 0.61 ± 0.02 | ||
| 15.29 ± 1.19 | 9.45 ± 0.69 | 0.62 ± 0.02 |
| Enzyme | Km (mmol/L) | kcat/(s−1) | kcat/Km (mmol/L/s) |
|---|---|---|---|
| Free Cholesterol Esterase | 4.88 × 10−2 ± 0.01 | 1.36 ± 0.29 | 27.87 ± 2.37 |
| Active Cholesterol Esterase Aggregates | 6.25 × 10−2 ± 0.02 | 1.12 ± 0.18 | 17.92 ± 2.06 |
| Preparation Method | Product Type | Conversion Ratio (%) | Cost (USD/mg) |
|---|---|---|---|
| Chemical reduction | FXOH, multiple by-products | 55.86 | 2500.00 |
| Free enzyme | FXOH | 84.51 | 2000.00 |
| Immobilized enzyme | FXOH | 95.02 | 300.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Yang, T.; Chen, H.; Fang, H.; Chen, W.; Xie, Q.; Liu, Q.; Zhang, Y.; Hong, Z.; Zhang, G. Efficient Preparation of High-Purity Fucoxanthinol by SpyTag-Tailored Active Cholesterol Esterase Aggregates. Mar. Drugs 2022, 20, 709. https://doi.org/10.3390/md20110709
Jin W, Yang T, Chen H, Fang H, Chen W, Xie Q, Liu Q, Zhang Y, Hong Z, Zhang G. Efficient Preparation of High-Purity Fucoxanthinol by SpyTag-Tailored Active Cholesterol Esterase Aggregates. Marine Drugs. 2022; 20(11):709. https://doi.org/10.3390/md20110709
Chicago/Turabian StyleJin, Wenhui, Ting Yang, Hui Chen, Hua Fang, Weizhu Chen, Quanling Xie, Qian Liu, Yiping Zhang, Zhuan Hong, and Guangya Zhang. 2022. "Efficient Preparation of High-Purity Fucoxanthinol by SpyTag-Tailored Active Cholesterol Esterase Aggregates" Marine Drugs 20, no. 11: 709. https://doi.org/10.3390/md20110709
APA StyleJin, W., Yang, T., Chen, H., Fang, H., Chen, W., Xie, Q., Liu, Q., Zhang, Y., Hong, Z., & Zhang, G. (2022). Efficient Preparation of High-Purity Fucoxanthinol by SpyTag-Tailored Active Cholesterol Esterase Aggregates. Marine Drugs, 20(11), 709. https://doi.org/10.3390/md20110709








