Neoagaro-Oligosaccharides Ameliorate Chronic Restraint Stress-Induced Depression by Increasing 5-HT and BDNF in the Brain and Remodeling the Gut Microbiota of Mice
Abstract
:1. Introduction
2. Results
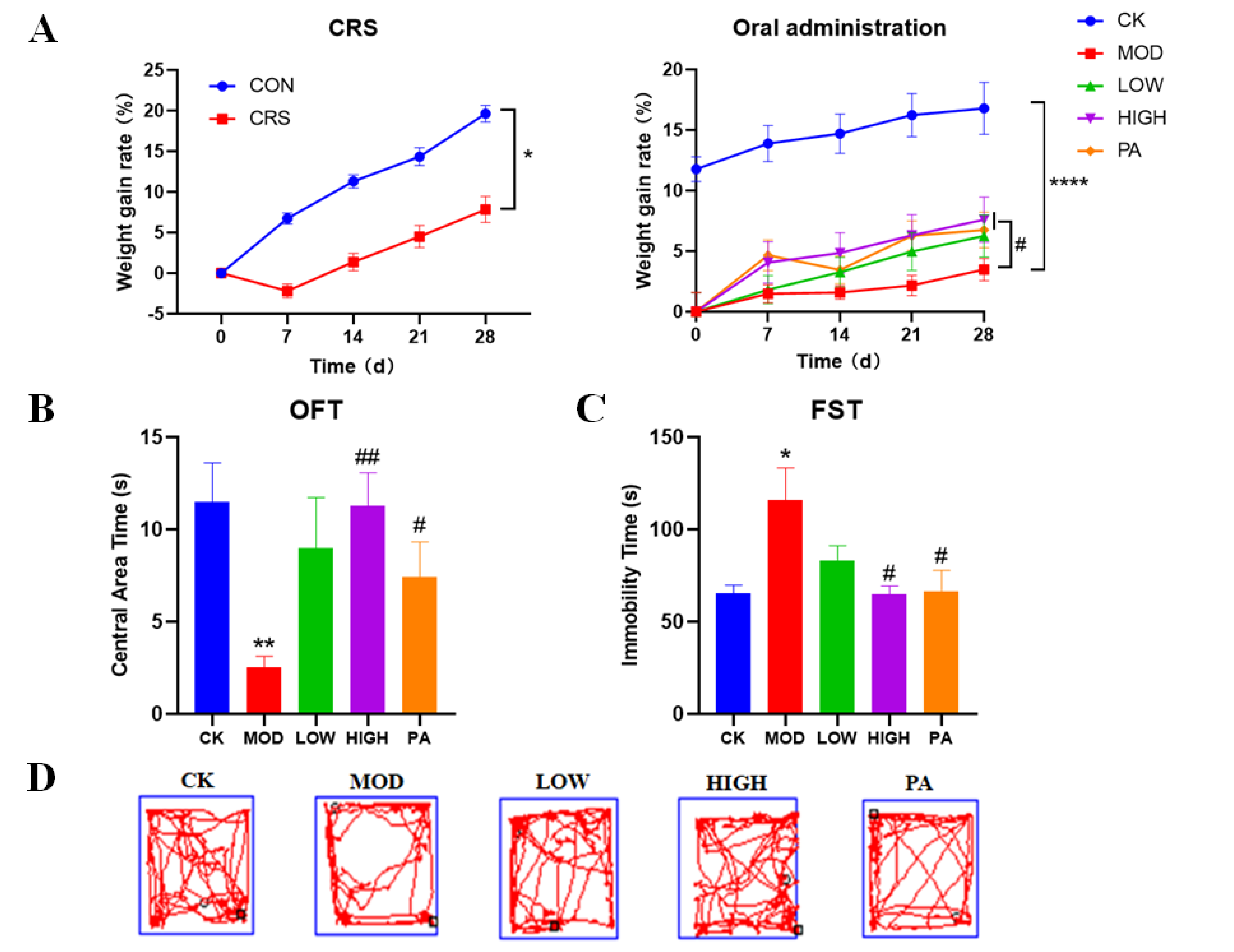
2.1. Effects of NAOs on Body Weight in CRS-Induced Mice
2.2. Effects of NAOs on CRS-Induced Mice in the Open Field Test (OFT)
2.3. Effects of NAOs on CRS-Induced Mice in the Forced Swimming Test (FST)
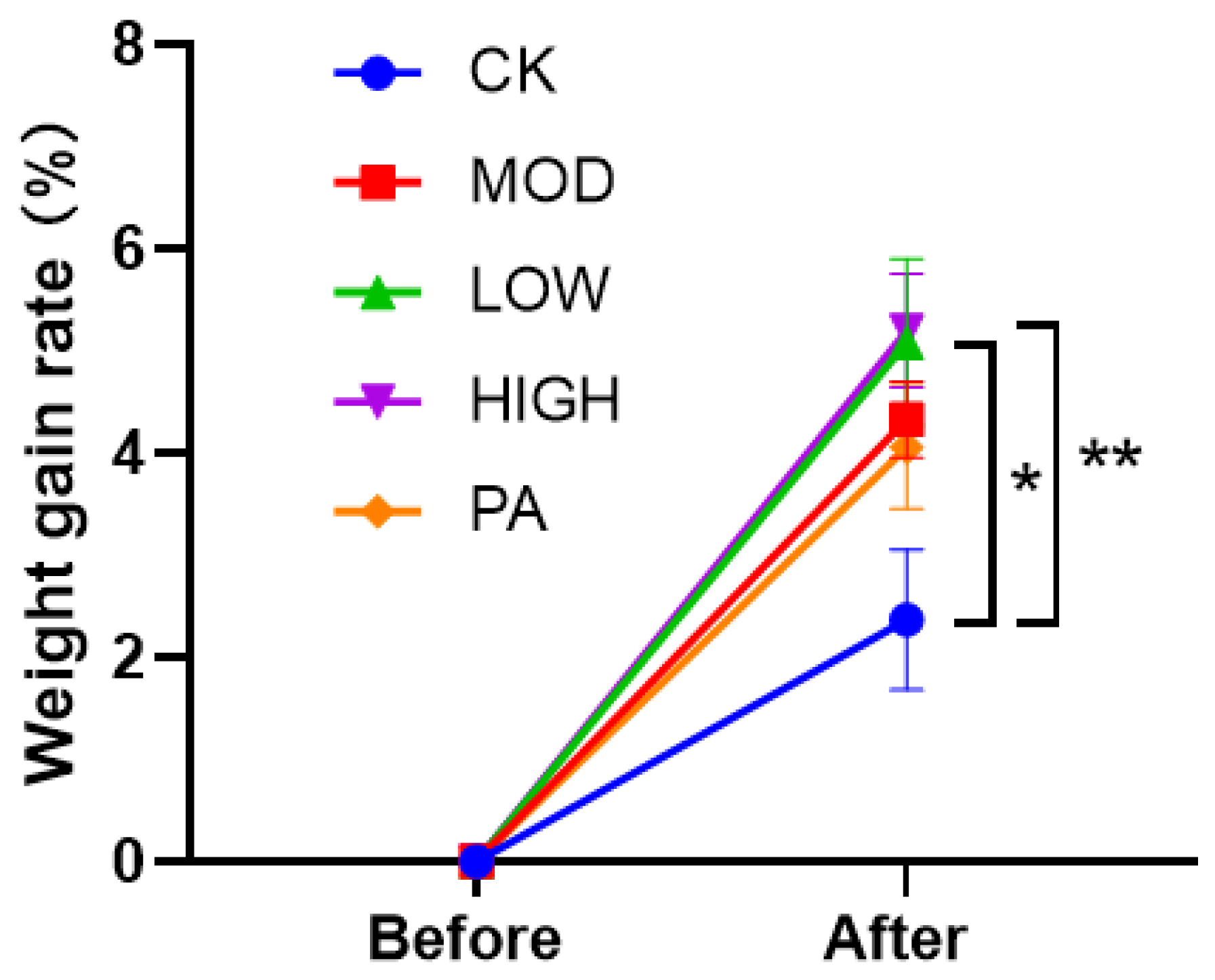
2.4. Effect on the Weight Gain Rate after Terminating the Gavage of NAOs
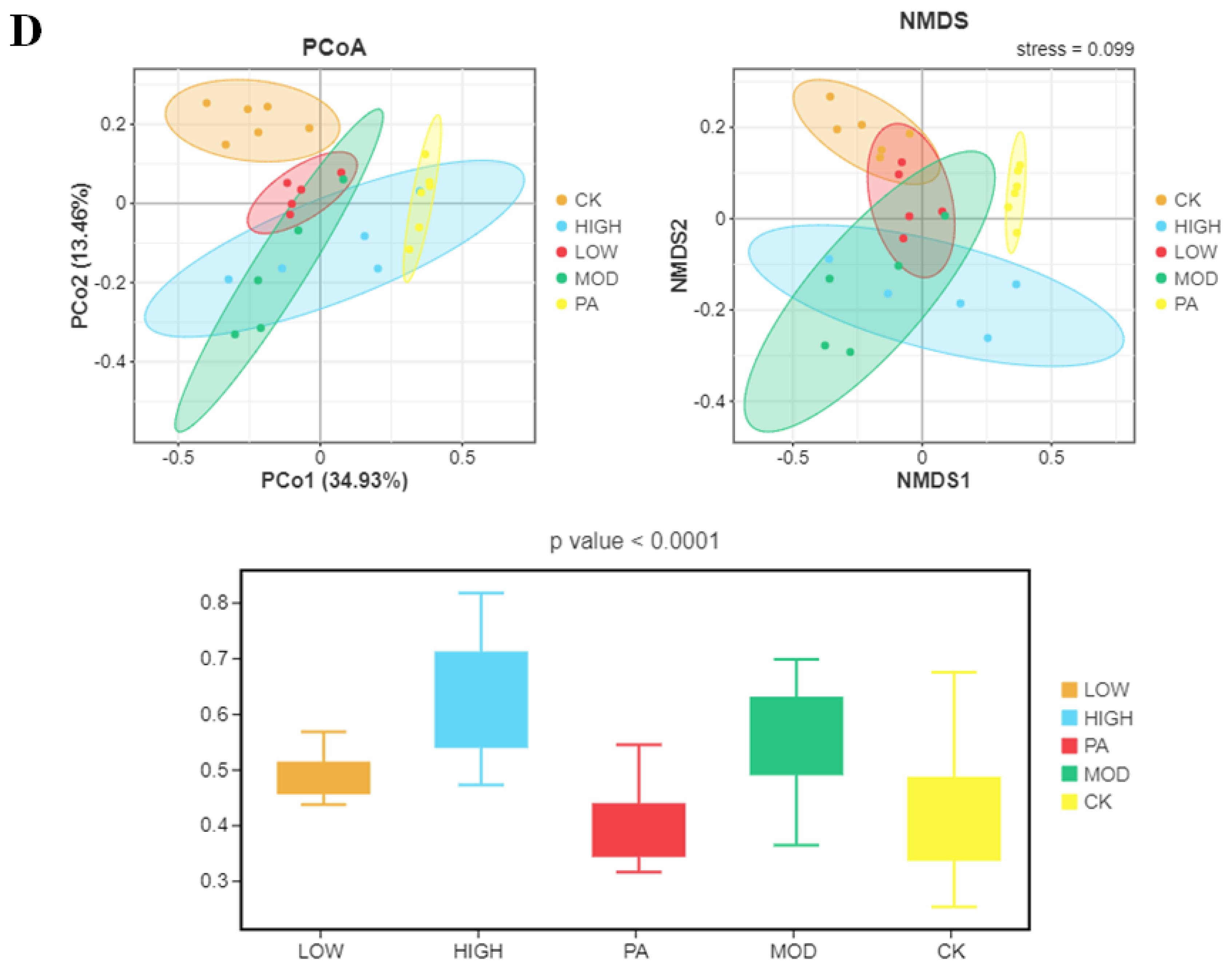
2.5. Oral Administration of NAOs Modulated the Gut Microbiota Composition of Depressed Mice
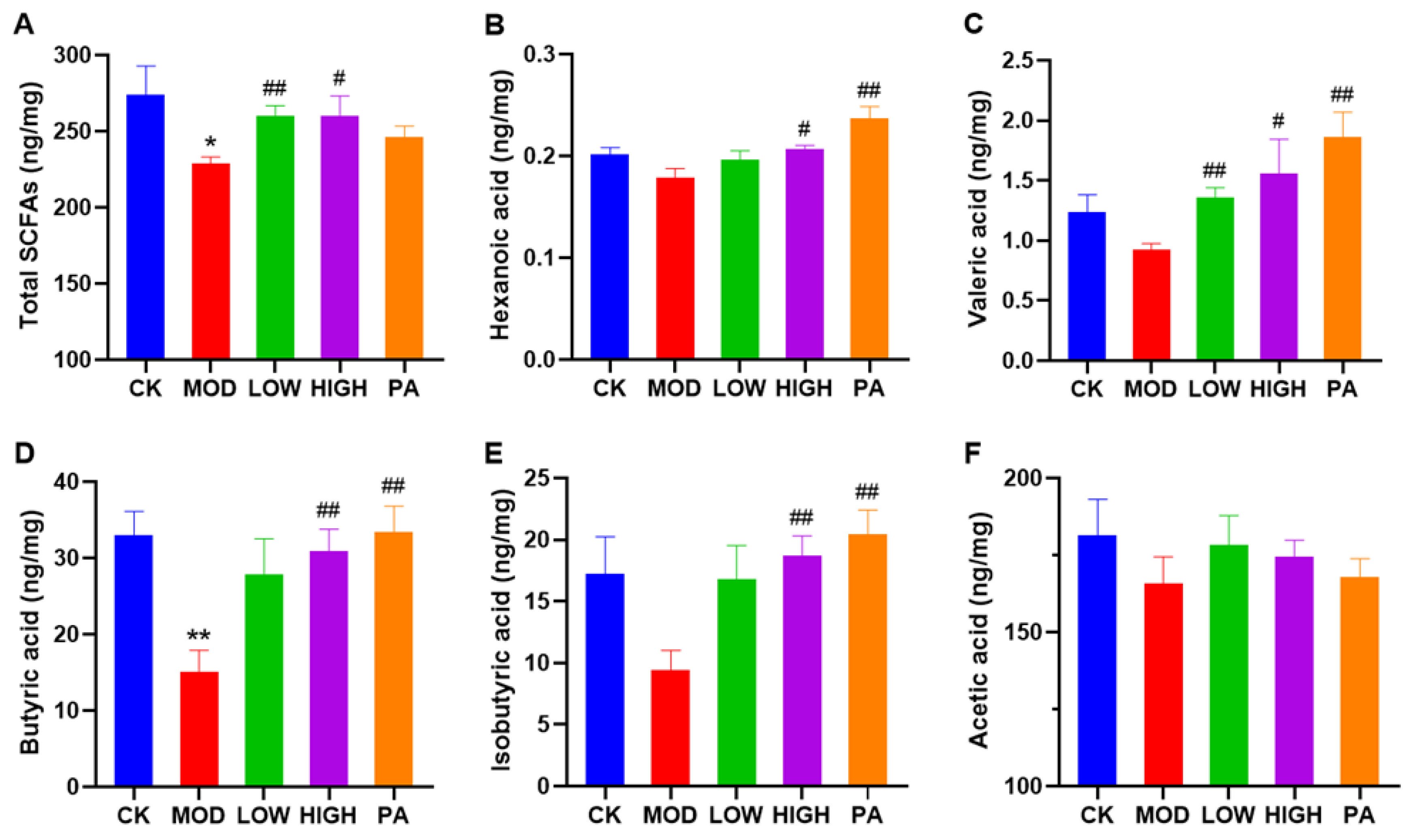
2.6. Oral Administration of NAOs Increased the Levels of Short-Chain Fatty Acids (SCFAs) in the Feces of Depressed Mice
2.7. Effect of NAOs on the Expression of IL-18 and 5-HT in the Serum of Mice
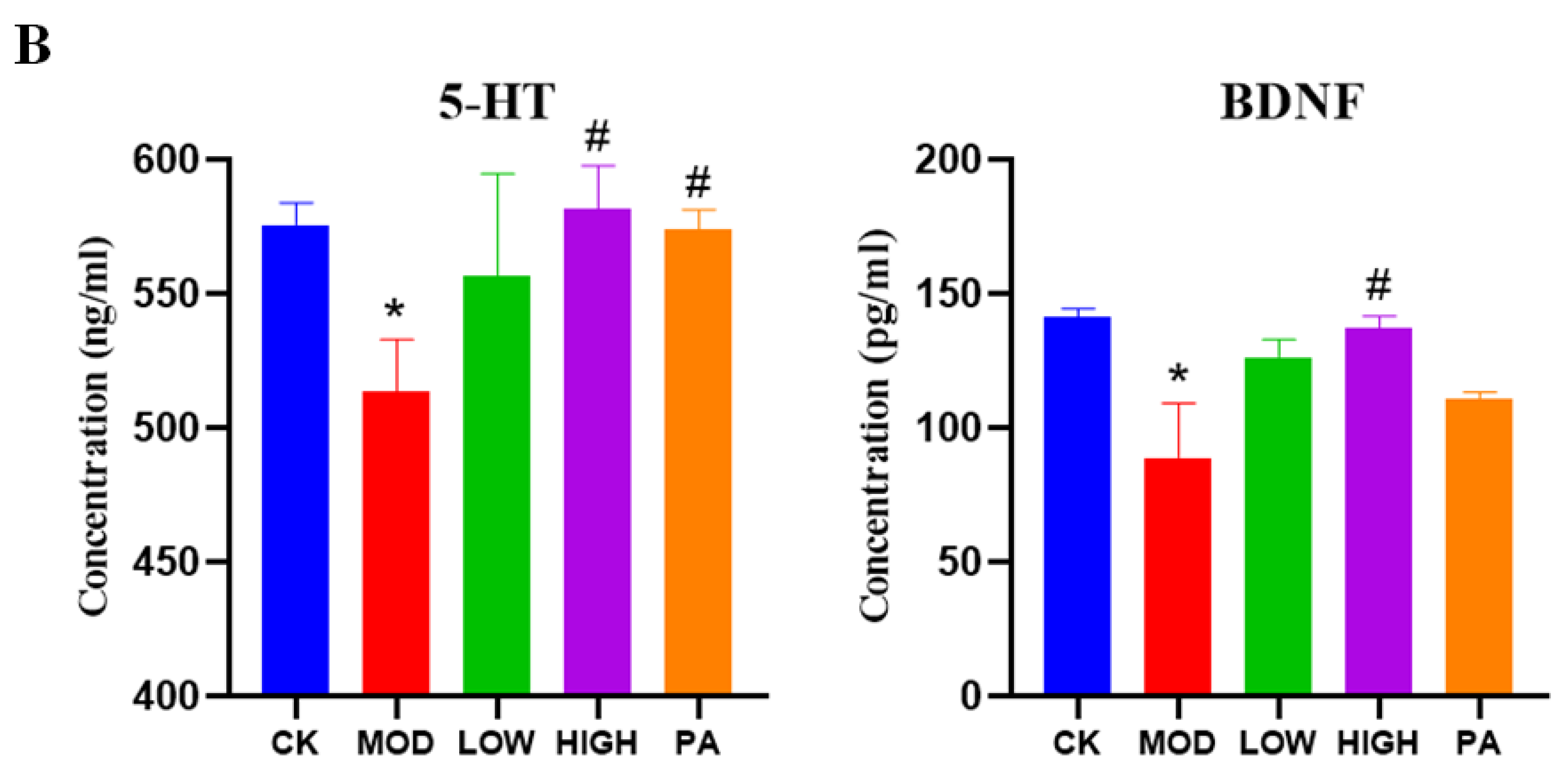
2.8. Effect of NAOs on the Expression of 5-HT and BDNF in the Whole Brain of Mice
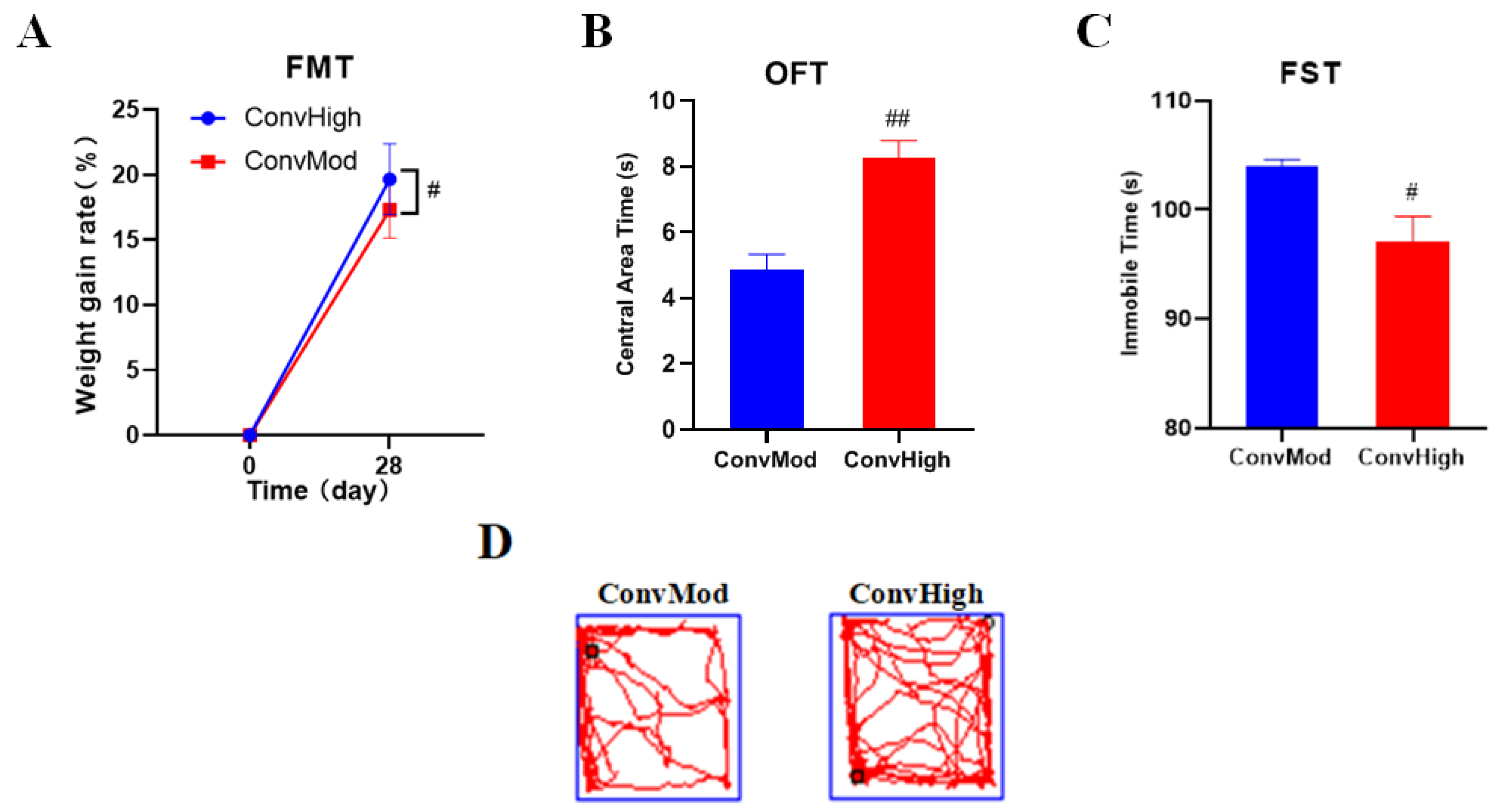
2.9. FMT Confirmed the Involvement of Gut Bacteria in the NAO Modulation of Weight Changes, as Well as Anxiety- and Depressive-Like Behavior
3. Discussion
4. Materials and Methods
4.1. Preparation and Determination of NAOs
4.2. Animals
4.3. CRS Procedure
4.4. Experimental Groups and Drug Administration
4.5. OFT
4.6. FST
4.7. Gut Microbiota Analysis
4.8. Biochemical Assays
4.9. FMT
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK group | Control group |
| NAOs | Neoagaro-oligosaccharides |
| OFT | Open field test |
| FST | Forced swimming test |
| SCFAs | Short-chain fatty acids |
| FMT | Fecal microbiota transplantation |
| BDNF | Brain-derived neurotrophic factor |
| MOD group | Model group |
| LOW group | The low-concentration NAOs group |
| HIGH group | The high-concentration NAOs group |
| PA group | Paroxetine group |
| ConvHigh | Microbiota depleted mice that were conventionalized with cecal samples from HIGH group mice |
| ConvMod | Microbiota depleted mice that were conventionalized with cecal samples from MOD group mice |
References
- Torre, J.A.I.; Vilagut, G.; Ronaldson, A.; Serrano-Blanco, A.; Martín, V.; Peters, M.; Valderas, J.M.; Dregan, A.; Alonso, J. Prevalence and variability of current depressive disorder in 27 European countries: A population-based study. Lancet Public Health 2021, 6, e729–e738. [Google Scholar] [CrossRef]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munshi, J.D.; Chang, C.K.; Dregan, A.; Hatch, S.L.; Morgan, C.; Thornicroft, G.; Stewart, R.; Hotopf, M. How do ethnicity and deprivation impact on life expectancy at birth in people with serious mental illness Observational study in the UK. Psychol. Med. 2021, 51, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N. Global, regional, and national incidence, prevalence, and years livedwith disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qi, H.; Liu, R.; Feng, Y.; Li, W.; Xiang, M.; Cheung, T.; Jackson, T.; Wang, G.; Xiang, Y. Depression, anxiety and associated factors among Chinese adolescents during the COVID-19 outbreak: A comparison of two cross-sectional studies. Transl. Psychiatry 2021, 11, 148. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef]
- Ivan, S.; Tomislav, K.; Mirko, D. Neurochemical and Behavioural Changes in Rat Models of Depression. Croat. Chem. Acta 2011, 84, 287–299. [Google Scholar]
- Yang, L.L.; Zhang, Z.J.; Sun, D.M.; Xu, Z.; Zhang, X.R.; Li, L.J. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurol. Res. 2010, 32, 340–346. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Lai, J.; Du, Y.; Xu, Y.; Ruan, L.; Hu, S. Current understanding of gut microbiota in mood disorders: An update of humanstudies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Akkasheh, E.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Cryan, J.F. Gut-brain axis in 2016: Brain-gut-microbiota axis-mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef]
- Cryan, J.F.; Riorddan, K.J.O.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Yan, R.; Zeng, R.; Zuo, Y.; Wang, D.; Qu, W. Expression and characterization of a novel cold-adapted and stableβ-agarase gene agaW1540 from the deep-sea bacterium shewanella sp. WPAGA9. Mar. Drugs 2021, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Chang, Y.K.; Park, J.S.; Hong, S.K. In vitro and in vivo investigation for biological activities of neoagarooligosaccharides prepared by hydrolyzing agar with β-agarase. Biotechnol. Bioproc. E 2017, 22, 489–496. [Google Scholar] [CrossRef]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocolloid. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Enoki, T.; Tanabe, M.; Shimomura, M.; Ohnogi, H. Induction mechanism of heme oxygenase-1 and anti-inflammatory activity by agaro-oligosaccharides. Arch. Biochem. Biophys. 2014, 57, 157–162. [Google Scholar]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Mizushima, K.; Hirai, Y.; Harusato, A.; Ohnogi, H.; Yamaji, R.; Inui, H.; Nakano, Y.; et al. Oligosaccharides from agar inhibit murine intestinal inflammation through the induction of heme oxygenase-1 expression. J. Gastroenterol. 2013, 48, 897–909. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Tanimura, Y.; Mizushima, K.; Harusato, A.; Fukui, A.; Yoriki, H.; Handa, O.; Ohnogi, H.; et al. Preventive effect of agaro-oligosaccharides on non-steroidalanti-inflammatory drug-induced small intestinal injury in mice. J. Gastroen. Hepatol. 2014, 29, 310–317. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Hao, C.; Wu, L.; Wan, W.; Mao, X. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappaB pathways. Sci. Rep. UK 2017, 7, 44252. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Fu, X.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. J. App. Phycol. 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Ushiroda, C.; Ohnogi, H.; Kudo, Y.; Yasui, M.; Inui, S.; et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am. J. Physiol. Gastrl. 2016, 310, G367–G375. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.; Park, J.; Hong, S. Anti-obesity and anti-diabetic effect of neoagarooligosaccharides on high-fat diet-induced obesity in mice. Mar. Drugs 2017, 15, 90. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Mace, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Morais, L.H.; IV, H.L.S.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Wu, W.L.; Adame, M.D.; Liou, C.W.; Barlow, J.T.; Lai, T.T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.Y.; et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature 2021, 595, 409–414. [Google Scholar] [CrossRef]
- Abed, S.; Ali, A.H.; Noman, A.; Niazi, S.; Ammar, A.F.; Bakry, A.M. Inulin as prebiotics and its applications in food industry and human health: A review. Int. J. Agric. Innov. Res. 2016, 5, 88–97. [Google Scholar]
- Zhang, N.; Mao, X.; Li, R.; Hou, E.; Wang, Y.; Xue, C.; Tang, Q. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol. Nutr. Food. Res. 2017, 61, 8. [Google Scholar] [CrossRef]
- Ma, C.; Yang, K.; Wang, Y.; Dai, X. Anti-aging effect of agar oligosaccharide on male Drosophila melanogaster and its preliminary mechanism. Mar. Drugs 2019, 17, 632. [Google Scholar] [CrossRef] [Green Version]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted Unraveling the role of the microbiome in major depressive disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Hul, M.V.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Thiele, I. Modeling metabolism of the human gut microbiome. Curr. Opin. Biotechnol. 2018, 51, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health. Perspe. 2014, 122, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, W.; Ding, S.; Wang, Y.; Ye, L.; Chen, X.; Ma, H. Exploring the potential antidepressant mechanisms of puerarin: Anti-inflammatory response via the gut-brain axis. J. Affect. Disord. 2022, 310, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Qu, Y.G.; Chang, L.J.; Pu, Y.Y.; Zhang, K.; Hashimoto, K.J. Antibiotic-induced microbiome depletion is associated with resilience in mice after chronic social defeat stress. J. Affect. Disord. 2020, 260, 448–457. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, A.Q.; Yang, Q.F.; Li, J.Y.; Wang, L.H.; Liu, X.X.; Huang, Y.X.; Liu, L. Beneficial Effect of Alkaloids From Sophora alopecuroides L. on CUMS-Induced Depression Model Mice via Modulating Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 665159. [Google Scholar] [CrossRef]
- Qu, W.; Liu, S.; Zhang, W.; Zhu, H.; Tao, Q.; Wang, H.; Yan, H. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: Intestinal microbiota and gut microbiome function. Food Funct. 2019, 10, 5886–5897. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Wei, C.L.; Wang, S.; Yen, J.T.; Cheng, Y.F.; Liao, C.L.; Hsu, C.C.; Wu, C.C.; Tsai, Y.C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.X.; Kang, Z.Y.; Bai, Z.H.; Li, K.H.; He, X.T.; Liu, Y.; Chen, X.Q.; Cheong, K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohyd. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Bendtsen, K.M.B.; Krych, L.; Sorensen, D.B.; Pang, W.Y.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sorensen, S.J.; Hansen, A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS ONE 2012, 7, E46231. [Google Scholar]
- Duncan, S.H.; Louis, P.; Flint, H.J. Cultivable bacterial diversity from the human colon. Lett. Appl. Microbiol. 2007, 44, 343–350. [Google Scholar] [CrossRef]
- Wong, J.M.W.; Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatr. 2016, 21, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Erny, D.; Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Fu, R.B.; Li, Z.P.; Zhao, R.; Li, C.Y.; Shao, S.; Li, J. The mechanism of intestinal flora dysregulation mediated by intestinal bacterial biofilm to induce constipation. Bioengineered 2021, 12, 6484–6498. [Google Scholar] [CrossRef]
- Yang, L.-M.; Yu, L.; Jin, H.-J.; Zhao, H. Substance P receptor antagonist in lateral habenula improves rat depression-like behavior. Brain Res. Bull. 2014, 100, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.F.; Shu, L.N.; Wu, C.L.; Tong, Y.P.; Xiong, Z.; Zhou, J.F.; Yu, C.N.; Xie, X.X.; Fu, Z.W. Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect. Disord. 2022, 276, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dar, D.; Glenda, M.M.Q.; Wang, J.; Young, L.T. Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Qin, T.; Li, H.; Ma, Z.; Ma, S. Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-kappaB regulating NLRP3 signal pathway. Int. Immunopharmacol. 2017, 53, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kirkland, R.A.; Lee, S.H.; Cawthon, C.R.; Rzepka, K.W.; Minaya, D.M.; de Lartigue, G.; Czaja, K.; de la Serre, C.B. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Behav. Brain Res. 2020, 225, 113082. [Google Scholar] [CrossRef]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression—Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Jin, M.; Zeng, R.; Lin, M. Characterization of a novel alkaline beta-agarase and its hydrolysates of agar. Food Chem. 2019, 295, 311–319. [Google Scholar] [CrossRef]
- Mason, S.S.; Baker, K.B.; Davis, K.W.; Pogorelov, V.M.; Malbari, M.M.; Ritter, R.; Wray, S.P.; Gerhardt, B.; Lanthorn, T.H.; Savelieva, K.V. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur. J. Pharmacol. 2009, 602, 306–315. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, K.Z.; Zhang, Y.W.; Huang, H.; Lv, J.; Wang, Q.; Wang, H.; Xia, T.; Liu, X. Protective effect of ginsenoside Rb1 against chronic restraint stress (CRS)-induced memory impairments in rats. Behav. Brain Res. 2021, 405, 113146. [Google Scholar] [CrossRef]
- Hei, M.; Chen, P.; Wang, S.; Li, X.; Xu, M.; Zhu, X.; Wang, Y.; Duan, J.; Huang, Y.; Zhao, S. Effects of chronic mild stress induced depression on synaptic in mouse hippocampus. Behav. Brain Res. 2019, 365, 26–35. [Google Scholar] [CrossRef]
- Ostadhadi, S.; Akbarian, R.; Norouzi-Javidan, A.; Nikoui, V.; Zolfaghari, S.; Chamanara, M.; Dehpour, A.R. Possible involvement of ATP-sensitive potassium channels in the antidepressant-like effects of gabapentin in mouse forced swimming test. Can. J. Physiol. Pharm. 2017, 95, 795–802. [Google Scholar] [CrossRef]
- Nazari, S.K.; Nikoui, V.; Ostadhadi, S.; Chegini, Z.H.; Oryan, S.; Bakhtiarian, A. Possible involvement of ATP-sensitive potassium channels in the antidepressant-like effect of baclofen in mouse forced swimming test. Pharmacol. Rep. 2016, 68, 1214–1220. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.; Rodrigues, S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: Melon dehydration. LWT Food Sci. Technol. 2008, 41, 604–610. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Zeng, R.; Liu, X.; Yang, L.; Chan, Z. Neoagaro-Oligosaccharides Ameliorate Chronic Restraint Stress-Induced Depression by Increasing 5-HT and BDNF in the Brain and Remodeling the Gut Microbiota of Mice. Mar. Drugs 2022, 20, 725. https://doi.org/10.3390/md20110725
Zhuang Y, Zeng R, Liu X, Yang L, Chan Z. Neoagaro-Oligosaccharides Ameliorate Chronic Restraint Stress-Induced Depression by Increasing 5-HT and BDNF in the Brain and Remodeling the Gut Microbiota of Mice. Marine Drugs. 2022; 20(11):725. https://doi.org/10.3390/md20110725
Chicago/Turabian StyleZhuang, Yan, Runying Zeng, Xiao Liu, Longhe Yang, and Zhuhua Chan. 2022. "Neoagaro-Oligosaccharides Ameliorate Chronic Restraint Stress-Induced Depression by Increasing 5-HT and BDNF in the Brain and Remodeling the Gut Microbiota of Mice" Marine Drugs 20, no. 11: 725. https://doi.org/10.3390/md20110725
APA StyleZhuang, Y., Zeng, R., Liu, X., Yang, L., & Chan, Z. (2022). Neoagaro-Oligosaccharides Ameliorate Chronic Restraint Stress-Induced Depression by Increasing 5-HT and BDNF in the Brain and Remodeling the Gut Microbiota of Mice. Marine Drugs, 20(11), 725. https://doi.org/10.3390/md20110725



