Potential Cosmetic Active Ingredients Derived from Marine By-Products
Abstract
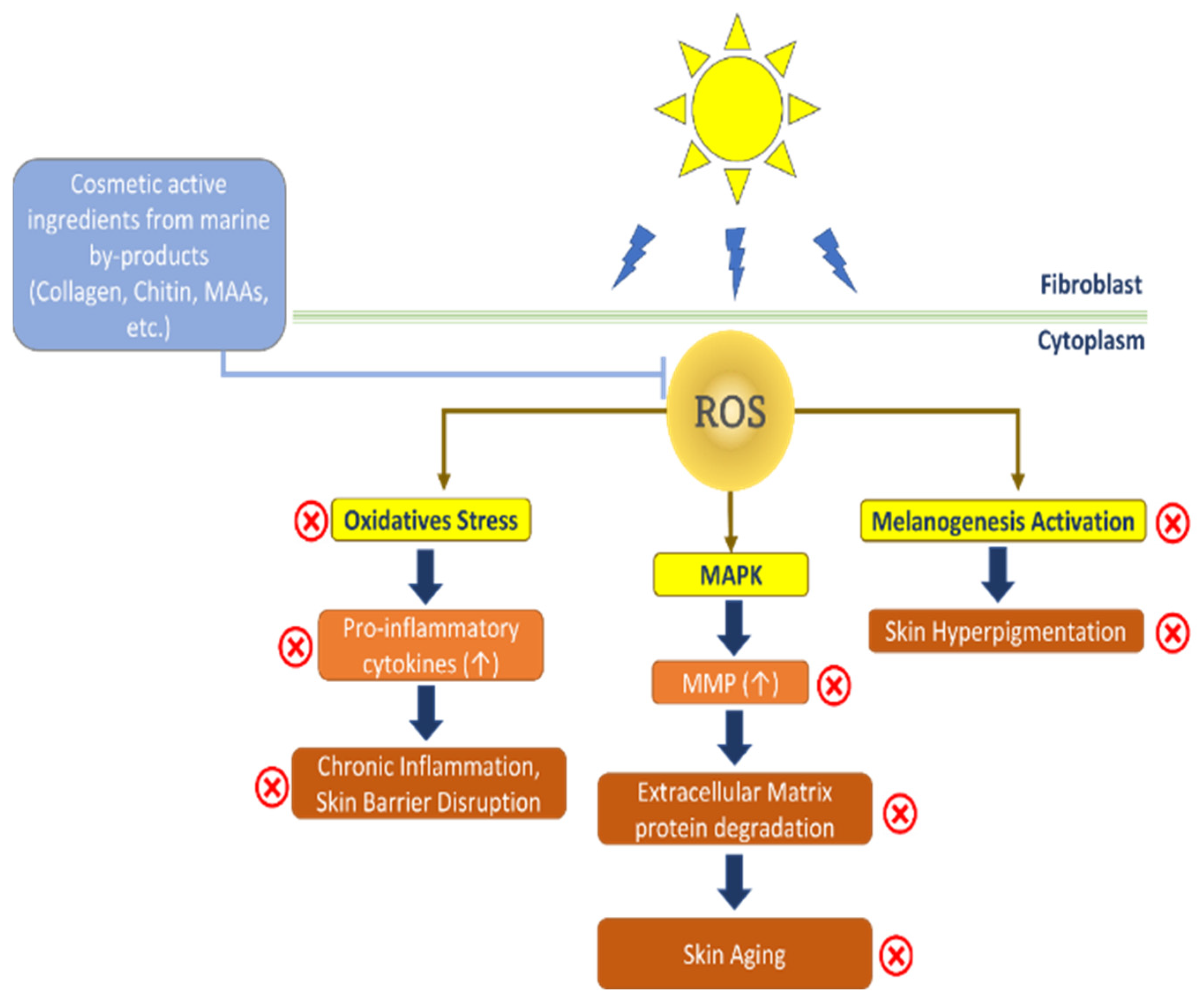
:1. Introduction
2. Potential Cosmeceutical Ingredient from Marine By-Products
2.1. Cosmetic Active Ingredients from Finfish By-Product
2.1.1. Collagen, Gelatin, and Collagen Derivatives as Cosmetic Ingredients
2.1.2. Active Ingredients of Fish Oil for Cosmetic
2.1.3. Natural Calcium Phosphates (CaPs) as Cosmetic Ingredients
2.2. Cosmetic Active Ingredients from Crustacean By-Products
2.2.1. Chitin and Its Derivatives as Cosmetic Ingredients
2.2.2. Astaxanthin as Cosmetic Ingredients
2.3. Cosmetic Active Ingredients from Molluscan By-Products
2.4. Cosmetic Active Ingredients from Seaweed Biomass Waste
3. Cosmeceutical Properties of Compounds from Marine By-Products on Skin Health
3.1. Skin-Whitening Properties
3.2. Antiaging and Skin Rejuvenation Properties
| Source | Enzymatic Hydrolysis | Organ | Sequence | Assay | Activity | Size | Ref. |
|---|---|---|---|---|---|---|---|
| Alaska pollack (Gadus chalcogrammus) | Alcalase, Pronase E, and collagenase | Skin | - | TBA, in vitro | - | - | [155] |
| Hoki (Johnius belengerii) | Trypsin | Skin | His-Gly-Pro-Leu-Gly-Pro-Leu | DPPH, carbon-centered, superoxide radicals, linoleic acid peroxide | - | 797.00 Da | [148] |
| Croceine croaker (Pseudosciaena crocea) | Pepsin and Trypsin | Skin | Gly-Phe-Arg-Gly-Thr-Ile-Gly-Leu-Val-Gly | DPPH | IC50: 1.271 mg/mL | 976.55 Da | [149] |
| Superoxide radical | IC50: 0.463 mg/mL | ||||||
| ABTS radical | IC50: 0.421 mg/mL | ||||||
| Gly-Pro-Ala-Gly-Pro-Ala-Gly | DPPH | IC50: 0.675 mg/mL | 526.24 Da | [149] | |||
| Superoxide radical | IC50: 0.099 mg/mL | ||||||
| ABTS radical | IC50: 0.309 mg/mL | ||||||
| Gly-Phe-Pro-Ser-Gly | DPPH | IC50: 0.283 mg/mL | 463.41 Da | [149] | |||
| Superoxide radical | 0.151 mg/mL | ||||||
| ABTS | IC50: 0.210 mg/mL | ||||||
| Pepsin | Frame | Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-His-Pro-Ala-Cys-Pro-Asp-Phe-Asn | DPPH | IC50: 41.37 µM | 1801.00 Da | [156] | |
| Hydroxyl radical | IC50: 17.77 µM | ||||||
| Peroxyl radical | IC50: 18.99 µM | ||||||
| Superoxide radical | IC50: 172.10 µM | ||||||
| Japanese flounder (Palatichtys olivaceus) | Pepsin | Skin | Gly-Gly-Phe-Asp-Met-Gly | In vitro, macromolecules damage | - | 582.00 Da | [147] |
| Speckled shrimp (Metapenaeus monoceros) | Crude protease from Bacillus cereus | Shells | Protein hydrolysates | DPPH, reducing power, β-carotene | - | - | [157] |
| Spotless smoothhound (Mustelus griseus) | Trypsin | Cartilage | Gly-Ala-Glu-Arg-Pro | DPPH | EC50: 3.73, mg/mL | 528.57 Da | [158] |
| Hydroxyl radical | EC50: 0.25 mg/mL | ||||||
| ABTS | EC50: 0.10 mg/mL | ||||||
| Superoxide radical | EC50: 0.09 mg/mL | ||||||
| Gly-GluArg-Glu-Ala-Asn-Val-Met | DPPH | EC50: 1.87 mg/mL | 905.00 Da | [158] | |||
| Hydroxyl radical | EC50: 0.34 mg/mL | ||||||
| ABTS | EC50: 0.05 mg/mL | ||||||
| Superoxide radical | EC50: 0.33 mg/mL | ||||||
| Ala-Glu-Val-Gly | DPPH | EC50: 2.30 mg/mL | 374.40 Da | [158] | |||
| Hydroxyl radical | EC50: 0.06 mg/mL | ||||||
| ABTS | EC50: 0.07 mg/mL | ||||||
| Superoxide radical | EC50: 0.18 mg/mL | ||||||
| Horse mackerel (Magalaspis cordyla) | Combination (pepsin, trypsin and α-chymotrypsin) | Viscera | Ala–Cys–Phe–Leu | DPPH | 89.2% (treatment at 0.2 mg/mL) | 518.50 Da | [159] |
| - | Hydroxyl radical | 59.1% (treatment at 0.2 mg/mL) | - | ||||
| Bigeye snapper (Priacanthus macracanthus) | Alcalase, neutrase, pyloric caeca extract | Skin | - | DPPH, ABTS, FRAP | - | - | [160] |
| Brownstripe red snapper (Lutjanus vitta) | Pyloric caeca extract | Skin | - | DPPH, ABTS, FRAP | - | - | [161] |
| Yellowfin sole (Limanda aspera) | Pepsin | Frame | Arg-Pro-Asp-Phe-Asp-Leu-Glu-Pro-Pro-Tyr | Linoleic acid model | - | 13.00 kDa | [162] |
| Tuna | Pepsin | Backbone | Val-Lys-Ala-Gly-Phe-Ala-Trp-Thr-Ala-Asn-Gln-Gln-Leu-Ser | DPPH, hydroxyl and superoxide | - | 1519.00 Da | [163] |
| Yellowtail fish (Seriola lalandi) | Protease | Scales and bone | Hydrolysates | DPPH, ABTS, reducing power, and Cu2+ and Fe2+ chelating activity | - | - | [164] |
| Horned turban sea snail (Turbo cornutus) | Protamex | Viscera | Thr-Asp-Ala | H2O2 radical, MPO inhibition | IC50: 646.0 ± 45.0 µM | - | [165] |
| Phe-Ala-Pro-Gln-Tyr | H2O2 radical | IC50: 57.1 ± 17.7 µM | - | [165] | |||
| Mackerel | Alcalase | Waste | - | - | - | - | [166] |
| Atlantic horse mackerel (Trachurus trachurus) | Alcalase | Head, Skin, and Bone, Waste meat | - | DPPH, reducing power and Cu2+ chelating activity | - | - | [167] |
3.3. Skin Moisturizing Effect
4. Future Prospects and Challenges of Marine By-Products in the Cosmetic Industry
| By-Product Source | Functional Product | Processing Method | Cosmeceutical Function | Ref. |
|---|---|---|---|---|
| Salmon and Codfish skins | Collagen | Acid-soluble collagen (ASC) extraction | Good moisture absorption, prevents skin dehydration without irritation | [14,177] |
| Milkfish scale | Hydrolyzed collagen | Pepsin hydrolysis | Moisturizers, antiaging agents, and skin-whitening agents | [145] |
| Salmon skin | Collagen peptides | Water, protease | Wound healing | [31] |
| Salmon skin | Hydrolysates gelatin | Hot water, alkaline protease | Antiaging against the UV-induced photo-aging | [178] |
| Fish scale | Collagen peptide | Hot water, enzymatic | Improving skin elasticity | [27] |
| Olive flounder and Alaska pollock skins | Fish skin hydrolysates | Enzymatic hydrolysis (pepsin, alcalase, protemax) | Minimize ROS levels, enhanced the viability of UV-B irradiated HaCat cells and human dermal fibroblast | [179] |
| Pacific whiting skin | Hydrolysates gelatin | Hot water | Anti-photoaging, delayed skin wrinkling | [180] |
| Manhaden fish oil | Rich in omega-3 | N.a | Reduce the irradiation effect | [51,52] |
| Marbled rock cod by-product | Fish oil in capsule | Solvent extraction (hexane) | Suppressed MMP-1 | [55] |
| Shark liver | Squalen (Semosqualene®) | N.a | Preventing and repairing cutaneous photoaging | [181] |
| Codfish bone | Hydroxyapatite-Fe2O3 | Calcination 700 °C | Active sunscreen filter | [66,67] |
| Pacific cod skin | Hydrolysates gelatin | Alkaline protease | Anti-photoaging, delaying skin wrinkling | [182] |
| Fringescale sardinella bone | Hydroxyapatite, hydroxyapatite- Mn, hydroxyapatite- Fe | Calcination 900 °C | Active sunscreen filter | [68] |
| Salmon skin | Collagen peptide | Water, protease | Antioxidant and anti-inflammatory | [183] |
| Tuna skin | Hydrolyzed collagen | Static hydrothermal hydrolysis | Antiaging (inhibiting tyroanase and gelatinase) and antioxidant | [25] |
| Codfish skin | Collagen polypeptides | Water, pepsin and alkaline protease | Moisturizer, antioxidant | [184] |
| Pacific cod skin | Gelatin and polypeptides | Hot water extraction, pepsin, and alkaline protease hydrolysis | Melanogenesis inhibition | [185] |
| Salmon skin | Gelatin hydrolysates | Enzymatic hydrolysis | Prevent collagen loss in photoaging skin caused by UV irradiation | [178] |
| Shrimp shell | Chitosan oligosaccharide | Enzymatic hydrolysis | Exhibit antiaging activity | [186] |
| Crab shell | Chitin nanofibrils, Oligochitosan-tetracycline and erythromycin | Acid hydrolysis | Prevents skin dryness, Anti-inflammatory and antioxidant (delivery system), Antibacterial (P.acne) | [187,188,189] |
| Oyster shell | Powdered oyster shell, organic shell extract | Fine grinding, Acid for decalcination, water extract | Utilize as emulsion stabilizer for cosmetic, Improving collagen content | [116,190] |
| Mussel and oyster shell | Shell extract | Acid aqueous extraction | Induced the synthesis of type i and iii collagens and sulfated gags | [115,191] |
| Pearl oyster shell | Water-soluble matric and fraction (SE4) of nacre, Nacre extract (pearl), | Water | Increase proliferation and collagen, Promoted the differentiation, Enhanced collagen synthesis in a rat skin | [109,110,111] |
| Outer and inner squid skins | Collagen hydrolysates | Enzymatic hydrolysis (alcalase) | Demonstrate great water-holding capacity | [192] |
| Squid pens | N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (HTCC) | Glycidyl trimethyl ammonium chloride (GTMAC) synthesis | Indicate good moisture absorption and retention capacity | [193] |
| Squid ink | Squid ink polysaccharides | Enzymatic hydrolysis (papain) | Prevent oxidative stress in human dermal fibroblast | [194] |
| Company | Country | By-Product Resource | Bioactive Compounds | Cosmeceutical’s Function | Ref. |
|---|---|---|---|---|---|
| Finn Canada | Canada | Salmon skin | Collagen | Improve skin condition. Treat various skin problems such as wrinkles, spots, dryness, dullness, and acne | [195] |
| Kenney and Ross Limited | Canada | Fish skin | Collagen | Stimulates healthy skin, nails, and hair | [196] |
| Copalis | France | Fish skin and bone | Collagen type I-III, elastin | Skin moisturization, anti-wrinkle, skin regeneration, enhance skin elasticity, | |
| Revolution fibres Ltd. | New Zealand | Fish skin | Collagen | Reduce the appearance of wrinkles and sunspots | [197] |
| Rousselot | France | Fisk skin and bone | Collagen peptides | Skin moisturization, enhance skin collagen density | [29] |
| Celergen Inc | Switzerland | Fish skin | Collagen hydrolysate | Enhance skill elasticity | [27] |
| Abyss | France | Fish skin | Collagen hydrolysate | Reduce the appearance of wrinkles | [30] |
| Nuwen | France | Fish skin | Collagen hydrolysate | Skin moisturization | [198] |
| One Ocean | United States | Fish skin | Collagen | Skin moisturization, anti-wrinkle | [199] |
| Osteralia | France | Nacre | Oyster shell | Antiaging, skin nourishment | [200] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022: Toward Blue Transfromation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Mozumder, M.M.H.; Uddin, M.M.; Schneider, P.; Raiyan, M.H.I.; Trisha, M.G.A.; Tahsin, T.H.; Newase, S. Sustainable utilization of fishery waste in Bangladesh—A qualitative study for a circular bioeconomy initiative. Fishes 2022, 7, 84. [Google Scholar] [CrossRef]
- Rustad, T. Utilisation of marine by-products. J. Environ. Agric. Food Chem. 2003, 2, 458–463. [Google Scholar]
- Gilman, E.; Perez Roda, A.; Huntington, T.; Kennelly, S.; Suuronen, P.; Chaloupka, M.; Medley, P. Benchmarking global fisheries discards. Sci. Rep. 2020, 10, 14017. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Beheshti Foroutani, M.; Parrish, C.C.; Wells, J.; Taylor, R.G.; Rise, M.L.; Shahidi, F. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): Effects on growth performance and muscle lipid and fatty acid composition. PLoS ONE 2018, 13, e0198538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.-K. Fish and fish waste-based fertilizers in organic farming–with status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- IFFO. By-Product. Available online: www.iffo.com/product (accessed on 13 September 2022).
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Al Khawli, F.; Ferrer, E.; Berrada, H.; Barba, F.J.; Pateiro, M.; Dominguez, R.; Lorenzo, J.M.; Gullon, P.; Kousoulaki, K. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues-A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musial, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Sugibayashi, K.; Yusuf, E.; Todo, H.; Dahlizar, S.; Sakdiset, P.; Arce, F., Jr.; See, G.L. Halal cosmetics: A review on ingredients, production, and testing methods. Cosmetics 2019, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Van Der Rest, M.; Garrone, R. Collagen family of proteins. FASEB J. 1991, 5, 2814–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Rodriguez, F.; Moran, L.; Gonzalez, G.; Troncoso, E.; Zuniga, R.N. Collagen extraction from mussel byssus: A new marine collagen source with physicochemical properties of industrial interest. J. Food Sci. Technol. 2017, 54, 1228–1238. [Google Scholar] [CrossRef] [Green Version]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Zamorano-Apodaca, J.C.; García-Sifuentes, C.O.; Carvajal-Millán, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sánchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, X.; Wu, X.; Lin, S.; Wang, S. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake. J. Funct. Foods 2020, 65, 103717. [Google Scholar] [CrossRef]
- Blanco, M.; Vazquez, J.A.; Perez-Martin, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Jo, Y.-J. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process Biochem. 2019, 83, 176–182. [Google Scholar] [CrossRef]
- Li, G.Y.; Fukunaga, S.; Takenouchi, K.; Nakamura, F. Comparative study of the physiological properties of collagen, gelatin and collagen hydrolysate as cosmetic materials. Int. J. Cosmet. Sci. 2005, 27, 101–106. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxid. Med. Cell Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Maia Campos, P.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral supplementation with hydrolyzed fish cartilage improves the morphological and structural characteristics of the skin: A double-blind, placebo-controlled clinical study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Smith, T.K. A brief journey into the history of and future sources and uses of fatty acids. Front. Nutr. 2021, 8, 570401. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, J.; Faridayanti, S.; Mohamed, E.S.M.; Hamzah, M.S.; Torla, H.H.; Che Man, Y.B. Extraction and characterization of gelatin from different marine fish species in Malaysia. Int. Food Res. J. 2009, 16, 381–389. [Google Scholar]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol., Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Zhang, Z.-J.; Li, G.; Shi, B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J. Soc. Leather Technol. Chem. 2006, 90, 23–28. [Google Scholar]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Kwak, K.S.; Park, D.C.; Gu, Y.S.; Ji, C.I.; Jang, D.H.; Lee, Y.B.; Kim, S.B. Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocoll. 2004, 18, 573–579. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S. Effect of ethanolic extract of coconut husk on gel properties of gelatin from swim bladder of yellowfin tuna. LWT—Food Sci. Technol. 2015, 62, 955–961. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hanifah, H.; Euis, K.; Eni, K.; Yoshihiro, O. Antioxidant activity of collagen, gelatin and the derived peptides from yellowfin tuna, skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeya Shakila, R.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Functional characterization of gelatin extracted from bones of red snapper and grouper in comparison with mammalian gelatin. LWT—Food Sci. Technol. 2012, 48, 30–36. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Thitipramote, N.; Benjakul, S. Preparation and functional characterisation of fish skin gelatin and comparison with commercial gelatin. Int. J. Food Sci. Technol. 2013, 48, 1093–1102. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Antioxidant activity of several marine skin gelatins. LWT—Food Sci. Technol. 2011, 44, 407–413. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ryu, B.; Kim, S.-K. Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Krishnaswamy, K.; Khalesi, M. Optimization of gelatin production from Barred mackerel by-products: Characterization and hydrolysis using native and commercial proteases. Food Hydrocoll. 2020, 108, 105970. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Mirghani, M.E.S.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ. 2013, 11, 73–79. [Google Scholar]
- Liaset, B.; Julshamn, K.; Espe, M. Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymic hydrolysis of salmon frames with ProtamexTM. Process Biochemistry 2003, 38, 1747–1759. [Google Scholar] [CrossRef]
- Sayana, K.S.; Sirajudheen, T.K. By-products from tuna processing wastes-an economic approach to coastal waste management. In Proceedings of the International Seminar on Coastal Biodiversity Assessment, Kottarakkara, India, 5–7 January 2017; pp. 411–420. [Google Scholar]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef] [Green Version]
- Orengo, I.F.; Black, H.S.; Wolf, J.E. Influence of fish oil supplementation on the minimal erythema dose in humans. Arch. Dermatol. Res. 1992, 284, 219–221. [Google Scholar] [CrossRef]
- Orengo, I.F.; Black, H.S.; Kettler, A.H.; Wolf Jr, J.E. Influence of dietary menhaden oil upon carcinogenesis and various cutaneous responses to ultraviolet radiation. Photochem. Photobiol. 1989, 49, 71–77. [Google Scholar] [CrossRef]
- Khayef, G.; Young, J.; Burns-Whitmore, B.; Spalding, T. Effects of fish oil supplementation on inflammatory acne. Lipids Health Dis. 2012, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, L.; Azurdia, R.; Dean, M.; Moison, R.; Steenwinkel, N.; Beijersbergen van Henegouwen, G.; Vink, A. Systemic eicosapentaenoic acid reduces UVB-induced erythema and p53 induction in skin, while increasing oxidative stress, in a double-blind randomised study. Br. J. Dermatol. 2000, 142, 601–602. [Google Scholar]
- Lee, S.; Koo, M.H.; Han, D.W.; Kim, I.C.; Lee, J.H.; Kim, J.H.; Sultana, R.; Kim, S.Y.; Youn, U.J.; Kim, J.H. Comparison of fatty acid contents and MMP-1 inhibitory effects of the two antarctic fish, Notothenia rossii and Champsocephalus gunnari. Molecules 2022, 27, 4554. [Google Scholar] [CrossRef] [PubMed]
- Bittiner, S.B.; Cartwright, I.; Tucker, W.F.G.; Bleehen, S.S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet 1988, 331, 378–380. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.K. Hydroxyapatite synthesis methodologies: An overview. Int. J. ChemTech Res. 2010, 2, 903–907. [Google Scholar]
- Duta, L.; Dorcioman, G.; Grumezescu, V. A review on biphasic calcium phosphate materials derived from fish discards. Nanomaterials 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dou, W.; Zhu, Q.; Jiang, D.; Xia, J.; Wang, X.; Tang, W.; Wang, S. The extraction and characterization of porous HA/β-TCP biphasic calcium phosphate from sole fish bones at different temperatures. Mater. Res. Express 2019, 6, 125412. [Google Scholar] [CrossRef]
- Chai, Y.; Tagaya, M. Simple preparation of hydroxyapatite nanostructures derived from fish scales. Mater. Lett. 2018, 222, 156–159. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Costa, E.; Santos-Silva, A.; Pintado, M.M.; Castro, P.M. Hydroxyapatite-based materials of marine origin: A bioactivity and sintering study. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, M.; Nagai, T.; Kai, N.; Tanoue, Y.; Mae, H.; Hashimoto, M.; Miyoshi, K.; Kumagai, H.; Saeki, K. Inorganic constituents of bone of fish. Fish. Sci. 1995, 61, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and biocompatibility properties of calcium phosphate bioceramics derived from salmon fish bone wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Degli Esposti, L.; Adamiano, A.; Iafisco, M. The use of calcium phosphates in cosmetics, state of the art and future perspectives. Materials 2021, 14, 6398. [Google Scholar] [CrossRef]
- Piccirillo, C.; Rocha, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. A hydroxyapatite-Fe2O3 based material of natural origin as an active sunscreen filter. J. Mater. Chem. B 2014, 2, 5999–6009. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.M.A.; Piccirillo, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. Effect of preparation and processing conditions on UV absorbing properties of hydroxyapatite-Fe2O3 sunscreen. Mater. Sci. Eng. C 2017, 71, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rozaini, M.Z.H.; Hamzah, H.; Chia, P.; Razali, M.; Osman, M.U.; Anuar, S.; Che Soh, S.; Ghazali, S.; Nor Hayati, I.; Fei, l.; et al. Calcium hydroxyapatite-based marine origin: Novel sunscreen materials for cosmeceutical treatments. Orient. J. Chem. 2018, 34, 2770–2776. [Google Scholar] [CrossRef] [Green Version]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable valorization of seafood processing by-product/discard. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 111–139. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, K.; Singh, A. Chitin, chitosan and their pharmacological activities: A review. IJPSR 2018, 9, 2626–2635. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.-d.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chapter 5—Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–164. [Google Scholar] [CrossRef]
- Feng, M.; Lu, X.; Hou, D.; Zhang, S. Chapter 4—Solubility, chain characterization, and derivatives of chitin. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–129. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Ahmed, S.; Ali, M.A. Chitooligosaccharides and their structural-functional effect on hydrogels: A review. Carbohydr. Polym. 2021, 261, 117882. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and UF-purified annatto and saffron for the preparation of UV protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, Y.A.; El-Khordagui, L.K.; Boraei, N.A.; Darwish, I.A. Chitosan microparticles incorporating a hydrophilic sunscreen agent. Carbohydr. Polym. 2010, 81, 234–242. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.; Morganti, G.; Cardillo, M.; Chianese, A. Green nanotechnology serving the bioeconomy: Natural beauty masks to save the environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Eom, S.-H.; Yu, D.; Lee, M.-S.; Kim, Y.-M. Oligochitosan as a potential anti-acne vulgaris agent: Combined antibacterial effects against Propionibacterium acnes. Food Sci. Biotechnol. 2017, 26, 1029–1036. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Simat, V.; Rathod, N.B.; Cagalj, M.; Hamed, I.; Generalic Mekinic, I. Astaxanthin from crustaceans and their by-products: A bioactive metabolite candidate for therapeutic application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in aquatic ecosystems and aquaculture: A colorful business with implications for human health. Front. Mar. Sci. 2017, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae derived astaxanthin: Research and consumer trends and industrial use as food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods–a review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Cheema, S.K.; Hawboldt, K. Extraction of astaxanthin from atlantic shrimp by-products using fish oil: Process optimization and operational parameter effects. J. Clean. Prod. 2022, 371, 133609. [Google Scholar] [CrossRef]
- Hamdi, S.A.H.; Ghonaim, G.M.; El Sayed, R.R.; Rodríguez-Couto, S.; Abd El-Ghany, M.N. Bioprocess of astaxanthin extraction from shrimp waste via the common microorganisms Saccharomyces cerevisiae and Lactobacillus acidophilus in comparison to the chemical method. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin extraction from marine crustacean waste streams: An integrate approach between microwaves and supercritical fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Niu, T.; Xuan, R.; Jiang, L.; Wu, W.; Zhen, Z.; Song, Y.; Hong, L.; Zheng, K.; Zhang, J.; Xu, Q.; et al. Astaxanthin induces the Nrf2/HO-1 antioxidant pathway in human umbilical vein endothelial cells by generating trace amounts of ROS. J. Agric. Food Chem. 2018, 66, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.Y.; Lee, C.; Pan, J.L.; Wen, Z.H.; Huang, S.H.; Lan, C.W.; Liu, W.T.; Hour, T.C.; Hseu, Y.C.; Hwang, B.H.; et al. Enriched astaxanthin extract from Haematococcus pluvialis augments growth factor secretions to increase cell proliferation and induces MMP1 degradation to enhance collagen production in human dermal fibroblasts. Int. J. Mol. Sci. 2016, 17, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Hama, S.; Uenishi, S.; Yamada, A.; Ohgita, T.; Tsuchiya, H.; Yamashita, E.; Kogure, K. Scavenging of hydroxyl radicals in aqueous solution by astaxanthin encapsulated in liposomes. Biol. Pharm. Bull. 2012, 35, 2238–2242. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Chen, B.; Peng, X.; Wang, J.G.; Wu, X. Laminated microstructure of bivalva shell and research of biomimetic ceramic/polymer composite. Ceram. Int. 2004, 30, 2011–2014. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Checa, A.G. The dynamics of nacre self-assembly. J. R. Soc. Interface 2007, 4, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Tjandra, E.S.; Iyer, K.S.; Humfrey, B.; Fear, M.; Wood, F.M.; Dunlop, S.; Raston, C.L. Evaluating the effects of nacre on human skin and scar cells in culture. Toxicol. Res. 2014, 3, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Kim, H.; Kim, J.M.; Chung, Y.H.; Lee, T.Y.; Lim, H.S.; Lim, J.H.; Kim, T.; Bae, J.S.; Woo, C.H.; et al. Nacre-driven water-soluble factors promote wound healing of the deep burn porcine skin by recovering angiogenesis and fibroblast function. Mol. Biol. Rep. 2012, 39, 3211–3218. [Google Scholar] [CrossRef]
- Almeida, M.J.; Milet, C.; Peduzzi, J.; Pereira, L.; Haigle, J.; Barthélemy, M.; Lopez, E. Effect of water-soluble matrix fraction extracted from the nacre of Pinctada maxima on the alkaline phosphatase activity of cultured fibroblasts. J. Exp. Zool. 2000, 288, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Le Faou, A.; Borzeix, S.; Berland, S. Stimulation of rat cutaneous fibroblasts and their synthetic activity by implants of powdered nacre (mother of pearl). Tissue Cell 2000, 32, 95–101. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chang, C.H.; Huang, C.C.; Liu, H.W. Anti-inflammation and anti-apoptosis effects of pearl extract gel on UVB irradiation HaCaT cells. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), S139–S145. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, M.; Bedouet, L.; Lati, E.; Gasser, P.; Le Ny, K.; Lopez, E. Restoration of stratum corneum with nacre lipids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 145, 1–9. [Google Scholar] [CrossRef]
- Tanaka, S.; Hatano, H.; Itasaka, O. Biochemical studies on pearl. I. amino acid composition of conchiolin in pearl and shell. Bull. Chem. Soc. Jpn. 1960, 33, 543–545. [Google Scholar] [CrossRef]
- Latire, T.; Legendre, F.; Bigot, N.; Carduner, L.; Kellouche, S.; Bouyoucef, M.; Carreiras, F.; Marin, F.; Lebel, J.M.; Galera, P.; et al. Shell extracts from the marine bivalve Pecten maximus regulate the synthesis of extracellular matrix in primary cultured human skin fibroblasts. PLoS ONE 2014, 9, e99931. [Google Scholar] [CrossRef] [Green Version]
- Torita, A.; Miyamoto, A.; Hasegawa, Y. The effects of scallop shell extract on collagen synthesis. Fish. Sci. 2007, 73, 1388–1394. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Griffiths, M.; Harrison, S.T.; Smit, M.; Maharajh, D. Major commercial products from micro-and macroalgae. In Algae Biotechnology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 269–300. [Google Scholar]
- Kim, D.-H.; Eom, S.-H.; Kim, T.H.; Kim, B.-Y.; Kim, Y.-M.; Kim, S.-B. Deodorizing effects of phlorotannins from edible brown alga Eisenia bicyclis on methyl mercaptan. J. Agric. Sci. 2013, 5, 95. [Google Scholar] [CrossRef]
- M Cardoso, S.; G Carvalho, L.; J Silva, P.; S Rodrigues, M.; R Pereira, O.; Pereira, L. Bioproducts from seaweeds: A review with special focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Déléris, P.; Fleurence, J.; Nguyen-Le, C.T.; Vo, K.H.; Dumay, J. Purification of R-phycoerythrin from a marine macroalga Gracilaria gracilis by anion-exchange chromatography. J. Appl. Phycol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Gröniger, A.; Sinha, R.P.; Klisch, M.; Häder, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal variation of mycosporine-like amino acids in three subantarctic red seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Takashi, H. Cosmetic potential of boiled water of Hijiki (Sargassum fusiforme) grown in the ocean in Okinawa, Japan. Mapp. Intimacies 2021. [Google Scholar] [CrossRef]
- Naik, P.P.; Farrukh, S.N. Influence of ethnicities and skin color variations in different populations—A Review. Ski. Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H.J.E.; Medicine, t. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, N.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Ohnishi, T.; Mori, T. Melanin reduces ultraviolet-induced DNA damage formation and killing rate in cultured human melanoma cells. J. Investig. Dermatol. 1993, 101, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Mauro, G.D.; Martini, F.; Tognon, M.; Mattei, M.D. MicroRNAs in the regulation of melanogenesis. Int. J. Mol. Sci. 2021, 22, 6104. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechri, S.; Sellem, I.; Bouacem, K.; Jabeur, F.; Chamkha, M.; Hacene, H.; Bouanane-Darenfed, A.; Jaouadi, B. Antioxidant and enzyme inhibitory activities of Metapenaeus monoceros by-product hydrolysates elaborated by purified alkaline proteases. Waste Biomass Valorization 2020, 11, 6741–6755. [Google Scholar] [CrossRef]
- Mechri, S.; Sellem, I.; Bouacem, K.; Jabeur, F.; Laribi-Habchi, H.; Mellouli, L.; Hacène, H.; Bouanane-Darenfed, A.; Jaouadi, B. A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environ. Sci. Pollut. Res. 2020, 27, 15842–15855. [Google Scholar] [CrossRef] [PubMed]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In vitro antioxidant, antityrosinase, and cytotoxic activities of astaxanthin from shrimp waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Ju, X.; Cheng, S.; Li, H.; Xu, X.; Wang, Z.; Du, M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem. 2022, 390, 133146. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Zhang, L.; Zha, M.J.; Tu, Z.C. From fish scale gelatin to tyrosinase inhibitor: A novel peptides screening approach application. Front. Nutr. 2022, 9, 853442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Wu, H.-T.; Wang, G.-H.; Liang, C.-H. Improvement of skin condition on skin moisture and anti-melanogenesis by collagen peptides from milkfish (Chanos chanos) scales. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022067. [Google Scholar] [CrossRef]
- Xhauflaire-Uhoda, E.; Fontaine, K.; Piérard, G.E. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef]
- Himaya, S.; Ryu, B.; Ngo, D.-H.; Kim, S.-K. Peptide isolated from Japanese flounder skin gelatin protects against cellular oxidative damage. J. Agric. Food Chem. 2012, 60, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.-M.; Chi, C.-F.; Luo, H.-Y.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Bak, S.-S.; Kim, S.-K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Park, S.H.; Park, B.J.; Kim, S.K. Antiphotoaging effect of chitooligosaccharides on human dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2012, 28, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Z.; Li, D.-D.; Luo, H.; Li, W.-J.; Huang, Y.-M.; Li, J.-C.; Hu, Z.; Huang, N.; Guo, M.-H.; Chen, Y. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp. Gerontol. 2018, 103, 27–34. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Jia, P.; Cheng, H.; Wang, C.; Chen, S.; Huang, H.; Han, Z.; Han, Z.-C.; Marycz, K. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ali Karami, M.; Sharif Makhmalzadeh, B.; Pooranian, M.; Rezai, A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: An in vivo evaluation of skin protection against UVB. Pharm. Dev. Technol. 2021, 26, 209–219. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, Y.-T.; Byun, H.-G.; Nam, K.-S.; Joo, D.-S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Je, J.-Y.; Kim, S.-K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Manni, L.; Ghorbel-Bellaaj, O.; Jellouli, K.; Younes, I.; Nasri, M. Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl. Biochem. Biotechnol. 2010, 162, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.S.; Nazeer, R.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Phanturat, P.; Benjakul, S.; Visessanguan, W.; Roytrakul, S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT-Food Sci. Technol. 2010, 43, 86–97. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Ohba, R.; Deguchi, T.; Kishikawa, M.; Arsyad, F.; Morimura, S.; Kida, K. Physiological functions of enzymatic hydrolysates of collagen or keratin contained in livestock and fish waste. Food Sci. Technol. Res. 2003, 9, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.; Kim, E.-A.; Kim, J.; Lee, S.-H.; Heo, S.-J. Identifying potential antioxidant properties from the viscera of sea snails (Turbo cornutus). Mar. Drugs 2021, 19, 567. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.B.; More, P.R.; Sonawane, S.K.; Arya, S.S. Antioxidant and anti-hypertensive bioactive peptides from Indian mackerel fish waste. Int. J. Pept. Res. Ther. 2021, 27, 2671–2684. [Google Scholar] [CrossRef]
- Henriques, A.; Vázquez, J.A.; Valcarcel, J.; Mendes, R.; Bandarra, N.M.; Pires, C. Characterization of protein hydrolysates from fish discards and by-products from the North-West Spain fishing fleet as potential sources of bioactive peptides. Mar. Drugs 2021, 19, 338. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.; Lee, H.J.; Chung, D.K. Evaluation of the skin moisturizing efficacy of a collagen peptide isolated from fish scales, using HaCaT keratinocytes. J. Korean Soc. Food Sci. Nutr. 2020, 49, 454–461. [Google Scholar] [CrossRef]
- Oba, C.; Ohara, H.; Morifuji, M.; Ito, K.; Ichikawa, S.; Kawahata, K.; Koga, J. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Koyama, Y.-i.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Skin-moisturizing effect of collagen peptides taking orally. J. Nutr. Food Sci. 2018, 8, 2. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. In Sustainability in Action; FAO: Rome, Italy, 2020; 244p. [Google Scholar]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-d.A. Marine waste utilization as a source of functional and health compounds. Adv. Food Nutr. 2019, 87, 187–254. [Google Scholar]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Technology. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Chen, T.; Hou, H.; Lu, J.; Zhang, K.; Li, B. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean. Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Lee, H.-G.; Je, J.-G.; Wang, L.; Kim, H.-S.; Jeon, Y.-J. Evaluation of cosmeceutical properties of fish skin by-product hydrolysates collected during surimi manufacturing process. Korean J. Fish. Aquat. Sci. 2020, 53, 297–307. [Google Scholar]
- Han, S.H.; Ballinger, E.; Choung, S.Y.; Kwon, J.Y. Anti-photoaging effect of hydrolysates from pacific whiting skin via MAPK/AP-1, NF-kappaB, TGF-beta/Smad, and Nrf-2/HO-1 signaling pathway in UVB-induced human dermal fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef]
- Cho, S.; Choi, C.W.; Lee, D.H.; Won, C.H.; Kim, S.M.; Lee, S.; Lee, M.J.; Chung, J.H. High-dose squalene ingestion increases type I procollagen and decreases ultraviolet-induced DNA damage in human skin in vivo but is associated with transient adverse effects. Clin. Exp. Dermatol. 2009, 34, 500–508. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-beta/Smad signaling pathway. J. Photochem. Photobiol. B 2016, 162, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z.; et al. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, X.; Li, B.; Zhang, Z.; Zhuang, Y. Inhibition of melanogenic activity by gelatin and polypeptides from pacific cod skin in B16 melanoma cells. J. Food Biochem. 2011, 35, 1099–1116. [Google Scholar] [CrossRef]
- Kong, S.-Z.; Li, J.-C.; Li, S.-D.; Liao, M.-N.; Li, C.-P.; Zheng, P.-J.; Guo, M.-H.; Tan, W.-X.; Zheng, Z.-H.; Hu, Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y. Protective effect of chitin urocanate nanofibers against ultraviolet radiation. Mar. Drugs 2015, 13, 7463–7475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sungkharak, S.; Supasit, N.; Choopan, S.; Ungphaiboon, S. Antibacterial activity against acne involved bacteria of chitosan in a soluble state and as nanoparticles. Chiang Mai J. Sci. 2016, 43, 1149–1158. [Google Scholar]
- Chairopoulou, M.A.; Garcia-Triñanes, P.; Teipel, U. Oyster shell reuse: A particle engineering perspective for the use as emulsion stabilizers. Powder Technol. 2022, 408, 117721. [Google Scholar] [CrossRef]
- Latire, T.; Legendre, F.; Bouyoucef, M.; Marin, F.; Carreiras, F.; Rigot-Jolivet, M.; Lebel, J.M.; Galera, P.; Serpentini, A. Shell extracts of the edible mussel and oyster induce an enhancement of the catabolic pathway of human skin fibroblasts, in vitro. Cytotechnology 2017, 69, 815–829. [Google Scholar] [CrossRef]
- Nam, K.; You, S.; Kim, S. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008, 73, C249–C255. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, Z.-H.; Xie, H.-H.; Gong, J.-Y.; Lou, J.; Ge, Q.; Wang, Y.-J.; Wu, Y.-F.; Liu, S.-W.; Sun, P.-L. Effect of quaternization degree on physiochemical and biological activities of chitosan from squid pens. Int. J. Biol. Macromol. 2014, 70, 545–550. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Huang, H.; Ma, Y.; Wang, R.; Hu, Y.; Zheng, X.; Chen, C.; Tang, H. Squid ink polysaccharides protect human fibroblast against oxidative stress by regulating NADPH oxidase and connexin43. Front. Pharmacol. 2020, 10, 1574. [Google Scholar] [CrossRef]
- Finn Canada. Available online: https://www.finncanada.com/ (accessed on 29 September 2022).
- Kenney&Ross Limited. Kenney and Ross. Available online: https://www.kenneyandross.com/index.php?option=com_content&view=featured&Itemid=101 (accessed on 11 November 2022).
- Zheng, H.; Kannan, B.; Chand, N.A.; Blake, A.; Chong, J.; Hosie, I.; Lepe, P. ActiVLayr nanofiber technology. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–246. [Google Scholar]
- Nuwen. Marine Active. Available online: https://www.nuwen.com/en/gamme/ingredients-en/marine-actives-ingredients-en/ (accessed on 18 November 2022).
- OneOcean. Collagen Boosting Powerhouses. Available online: https://oneoceanbeauty.com/collections/collagen (accessed on 15 November 2022).
- Ostrealia. Available online: https://www.ostrealia.fr/en/categorie-produit/cosmetics/ (accessed on 11 November 2022).
- Moreno-Sader, K.A.; Martínez-Consuegra, J.; González-Delgado, Á.D. An integrated biorefinery approach via material recycle/reuse networks for the extraction of value-added components from shrimp: Computer-aided simulation and environmental assessment. Food Bioprod. Process. 2021, 127, 443–453. [Google Scholar] [CrossRef]
- Medina Uzcategui, L.U.; Vergara, K.; Martinez Bordes, G. Sustainable alternatives for by-products derived from industrial mussel processing: A critical review. Waste Manag. Res. 2022, 40, 123–138. [Google Scholar] [CrossRef]
- Daneluz, J.; Favero, J.d.S.; Santos, V.d.; Weiss-Angeli, V.; Gomes, L.B.; Mexias, A.S.; Bergmann, C.P. The influence of different concentrations of a natural clay material as active principle in cosmetic formulations. Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Cristiano, L.; Guagni, M. Zooceuticals and cosmetic ingredients derived from animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. https://doi.org/10.3390/md20120734
Siahaan EA, Agusman, Pangestuti R, Shin K-H, Kim S-K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Marine Drugs. 2022; 20(12):734. https://doi.org/10.3390/md20120734
Chicago/Turabian StyleSiahaan, Evi Amelia, Agusman, Ratih Pangestuti, Kyung-Hoon Shin, and Se-Kwon Kim. 2022. "Potential Cosmetic Active Ingredients Derived from Marine By-Products" Marine Drugs 20, no. 12: 734. https://doi.org/10.3390/md20120734
APA StyleSiahaan, E. A., Agusman, Pangestuti, R., Shin, K.-H., & Kim, S.-K. (2022). Potential Cosmetic Active Ingredients Derived from Marine By-Products. Marine Drugs, 20(12), 734. https://doi.org/10.3390/md20120734







