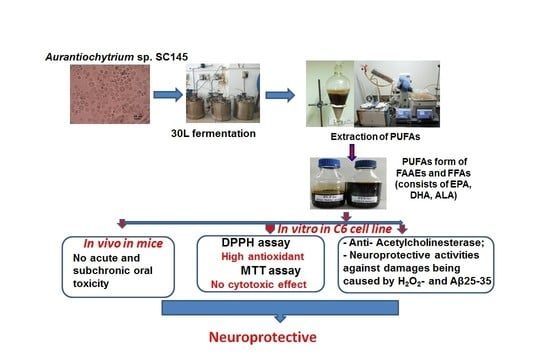
Characterization and Optimization of Culture Conditions for Aurantiochytrium sp. SC145 Isolated from Sand Cay (Son Ca) Island, Vietnam, and Antioxidative and Neuroprotective Activities of Its Polyunsaturated Fatty Acid Mixture
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Characterization of Aurantiochytrium sp. SC145
2.2. Optimization of Culture Conditions
2.2.1. Cultivation of Aurantiochytrium sp. SC145 in an Erlenmeyer flask
2.2.2. Cultivation of Aurantiochytrium sp. SC145 in a 30 L fermentor
2.3. Extraction of PUFAs Rich in Omega 3–6–9 Fatty Acids in the FFA Form
2.4. Extraction of PUFAs Rich in Omega 3–6–9 Fatty Acids in the FAAE Form
2.5. Acute Toxicity Test
2.6. Subchronic Toxicity Test
2.6.1. Behavioral Observations and Body Weight Trends
2.6.2. Hematological and Biochemical Parameters
2.7. Scavenging Activity of DPPH Radical
2.8. AChE Inhibitory Activity
2.9. Cytotoxicity Effect of Polyunsaturated Fatty Acids on C6 cells
2.10. Neuroprotective Effects of PUFAs against Damage Caused by Oxidative Stress Induced by H2O2 on C6 cells
2.11. PUFAs in the FAAE and FFA Forms Protect C6 Cell Line against Aβ25-35-Induced Cytotoxicity
3. Materials and Methods
3.1. Strain and Culture
3.2. Determination of Microalgal Growth
3.3. Morphological Observation
3.4. Transmission Electron Microscopy (TEM)
3.5. Scanning Electron Microscopy (SEM)
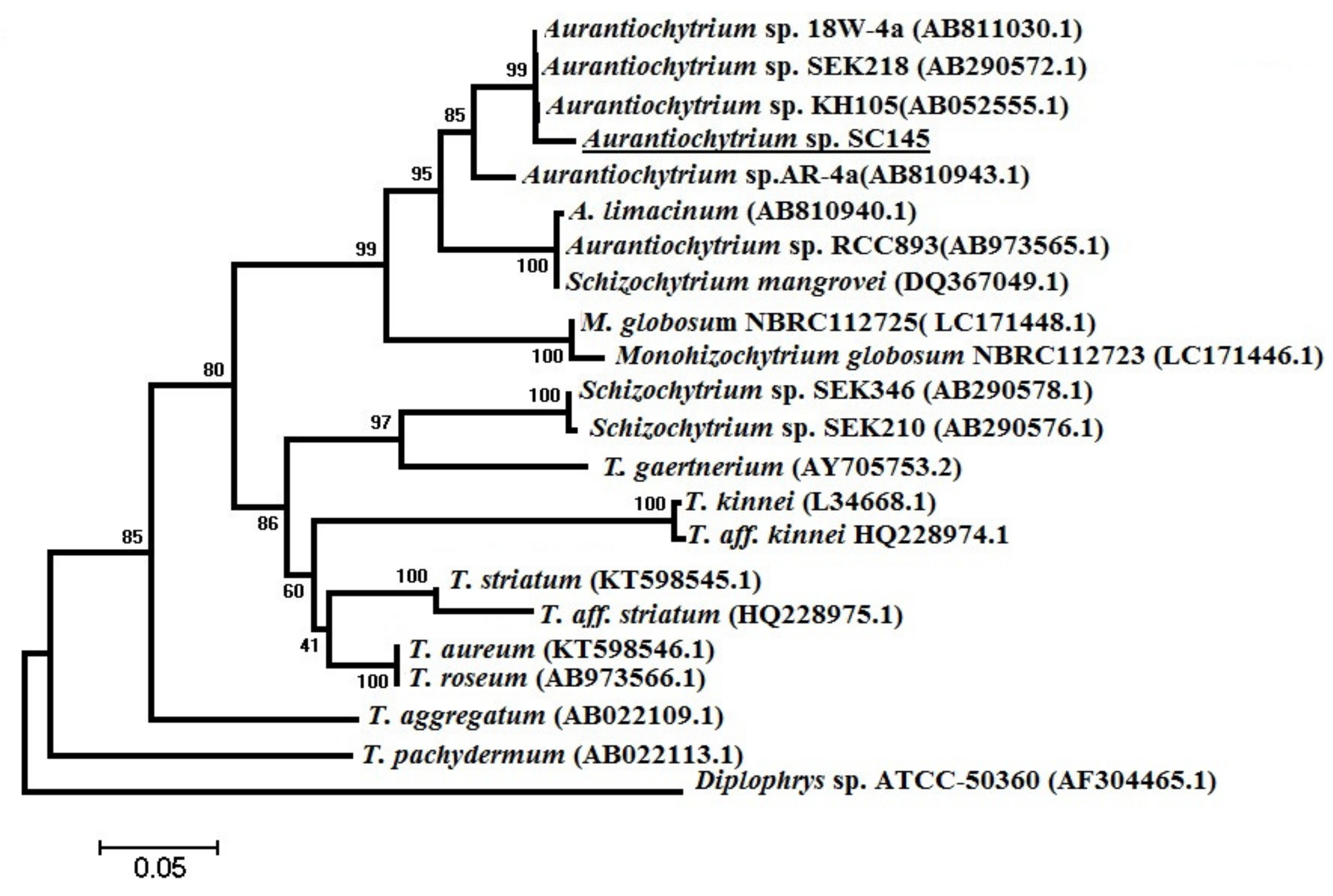
3.6. Phylogenetic Analysis
3.7. Methods for Determining Optimal Culture Conditions in Erlenmeyer Flask
3.8. Methods for Determining Optimal Culture Conditions in a 30 L Fermentor
3.9. Extraction and Determination of Total Carotenoid and Astaxanthin
3.10. Extraction and Determination of Total Protein and Carbohydrate Contents
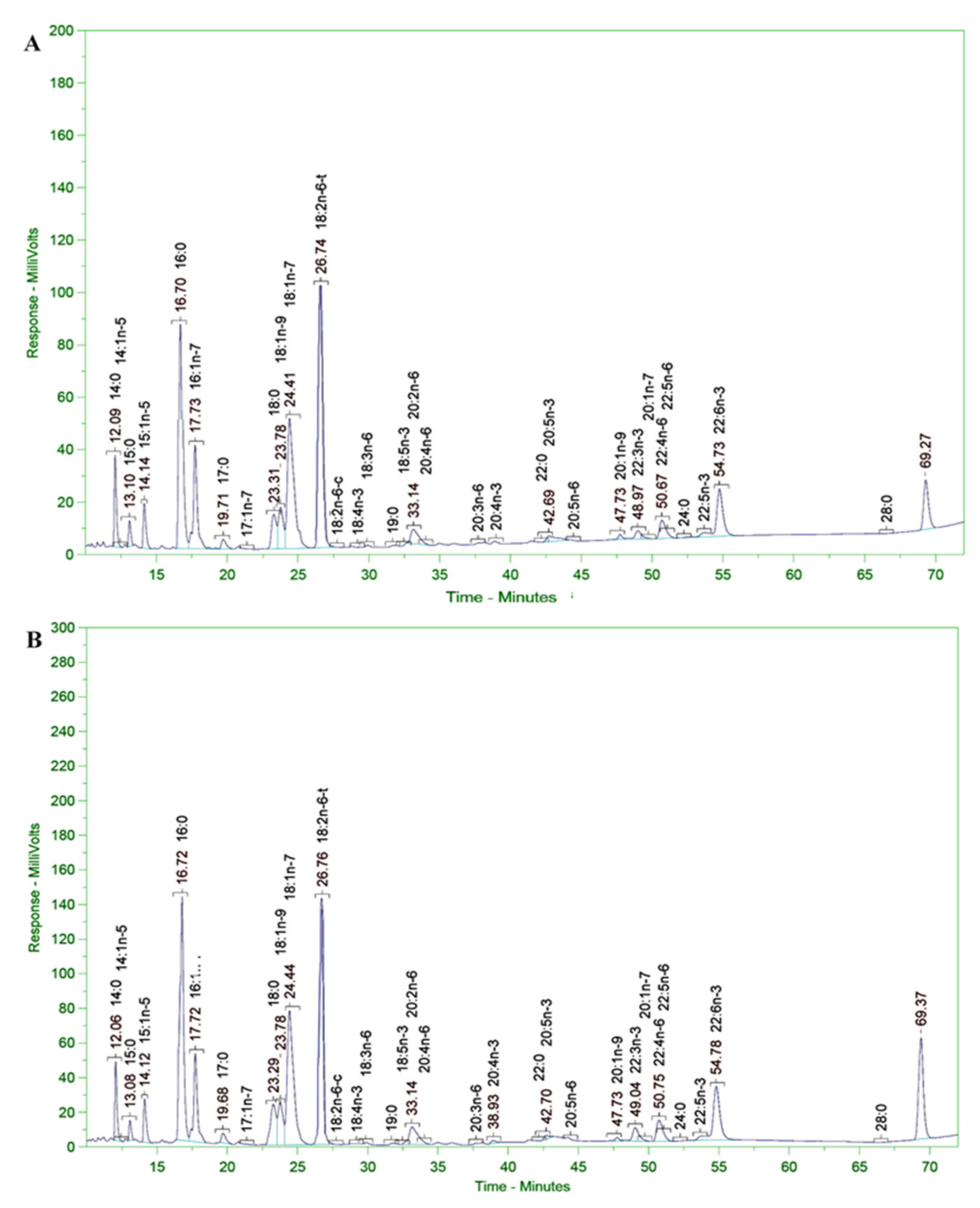
3.11. Analysis of Fatty Acid Composition of Microalgal Biomass
3.12. PUFAs Rich in Omega 3–6 Fatty Acids in the Free Form (FFA)
3.13. PUFAs Rich in Omega 3–6 Fatty Acids in the Alkyl Ester Form (FAAE)
3.14. Extraction and Purification of ALA, LA, EPA and DHA
3.15. Acute Oral Toxicity Study
3.16. Subchronic Oral Toxicity Study
3.17. DPPH Assay
3.18. AChE Inhibitory Activity Assay
3.19. Cell Culture and Treatment
3.20. MTT Assay
3.21. Statistics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of algal biomass to value-added metabolites: Emerging trends and opportunities. Phytochem. Rev. 2022, 2, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.H.; Hoang, T.L.A. New Heterotrophic marine microalgae of Labyrinthula, Schizochytrium, Thraustochytrium in Vietnam: Potential and Challenges; Natural Resources and Environment of Vietnam; Publisher of Science and Technology: Hanoi, Vietnam, 2016. (In Vietnamese) [Google Scholar]
- Kumar, A.; Singh, R.P.; Kumar, I.; Yadav, P.; Singh, S.K.; Kaushalendra; Singh, P.K.; Gupta, R.K.; Singh, S.M.; Kesawat, M.S.; et al. Algal Metabolites can be an immune Booster against COVID-19 Pandemic. Antioxidants 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Onyiaodike, C.C.; Brown, E.A.; Jordan, F.; Murray, H.; Nibbs, R.J.; Sattar, N.; Lyall, H.; Nelson, S.M.; Freeman, D.J. Maternal plasma DHA levels increase prior to 29 days post-lh surge in women undergoing frozen embryo transfer: A prospective, observational study of human pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does Consumption of LC Omega-3 PUFA Enhance Cognitive Performance in Healthy School-Aged Children and throughout Adulthood? Evidence from Clinical Trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.J.; Martínez, M.S.; Torres, W.; Chávez-Castillo, M.; Luzardo, E.; Villasmil, N.; Salazar, J.; Velasco, M.; Bermúdez, V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A molecular view into structure and function. Vessel Plus 2017, 1, 116–128. [Google Scholar] [CrossRef][Green Version]
- Arafiles, K.H.V.; Alcantara, J.C.O.; Cordero, P.R.F.; Batoon, J.A.L.; Galura, F.S.; Leano, E.M.; Dedeles, G.R. Cultural Optimization of Thraustochytrids for Biomass and Fatty Acid Production. Mycosphere 2011, 2, 521–531. [Google Scholar]
- Liu, Y.; Singh, P.; Sun, Y.; Luan, S.; Wang, G. Culturable diversity and biochemical features of thraustochytrids from coastal waters of Southern China. Appl. Microbiol. Biotechnol. 2014, 98, 3241–3255. [Google Scholar] [CrossRef]
- Hoang, T.L.A.; Nguyen, H.C.; Le, T.T.; Hoang, T.H.Q.; Pham, V.N.; Hoang, M.H.T.; Ngo, H.T.T.; Hong, D.D. Different fermentation strategies by Schizochytroium mangrovei strain PQ6 to produce feedstock for exploitation of squalene and omega-3 fatty acids. J. Phycol. 2018, 54, 550–556. [Google Scholar] [CrossRef]
- DSM Nutritional Products, Application for the Approval of DHA-Rich Algal Oil from Schizochytrium sp. (DHA-B) as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council of 27/2/1997 Concerning Novel Food and Novel Food Ingredient, DSM Nutritional Products. 6480 Dobbin Road, Columbia, MD 21045, USD. Available online: https://acnfp.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/dharichoildsm.pdf (accessed on 21 July 2022).
- Falk, M.C.; Zheng, X.; Chen, D.; Jiang, Y.; Liu, Z.; Lewis, K.D. Developmental and reproductive toxicological evaluation of arachidonic acid (ARA)-Rich oil and docosahexaenoic acid (DHA)-Rich oil. Food. Chem. Toxicol. 2017, 103, 270–278. [Google Scholar] [CrossRef]
- Fang, G.; Shi, B.; Wu, K.; Chen, S.; Gao, X.; Xiao, S.; Kang, J.X.; Li, W.; Huang, R. The protective role of endogenous n-3 polyunsaturated fatty acids in Tau Alzheimer’s disease mouse model. Int. J. Neurosci. 2019, 129, 325–336. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, H.; Li, Y.; Zhang, Y.; Wang, X.; Jin, Q.; Wang, X. Enrichment of DPAn-6 and DHA from Schizochytrium sp. Oil by Low-Temperature Solvent Crystallization. Ind. Eng. Chem. Res. 2016, 55, 737–746. [Google Scholar] [CrossRef]
- Le, T.T.; Dang, D.H. Cultivation and extraction of omega 3-6 fatty acids from the heterotrophic marine microalga Schizochytrium mangrovei TB17 to make a functional food. Res. J. Biotechnol. 2021, 16, 22–32. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Cauli, O.; Silveyra, M.X.; Rodrigo, R.; Candela, A.; Compañ, A.; Jover, R.; Pérez-Mateo, M.; Martínez, S.; Felipo, V. Brain cholinergic impairment in liver failure. Brain 2008, 131, 2946–2956. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Ointeus, S.; Mendes, S.; Pedrosa, R. Neuroprotective effects of seaweeds against 6-hydroxidopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complement. Altern. Med. 2018, 18, 58. [Google Scholar] [CrossRef]
- Murray, P.M.; Moane, S.; Collins, C.; Beletskaya, T.; Thomas, O.P.; Duarte, A.W.; Nobre, F.S.; Owoyemi, I.O.; Pagnocca, F.C.; Sette, L.D.; et al. Sustainable production of biologically active molecules of marine based origin. New Biotechnol. 2013, 30, 839–850. [Google Scholar] [CrossRef]
- Horta, A.; Pinteus, S.; Alves, C.; Fino, N.; Silva, J.; Fernandez, S.; Rodrigues, A.; Pedrosa, R. Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs 2014, 12, 1676–1689. [Google Scholar] [CrossRef]
- Hoang, T.M.H.; Nguyen, C.H.; Le, T.T.; Hoang, T.H.Q.; Ngo, T.T.; Hoang, L.A.; Dang, D.H. Squalene isolated from Schizochytrium mangrovei is a peroxisome proliferator-activated receptor-α agonist that regulates lipid metabolism in HepG2 cells. Biotechnol. Lett. 2016, 38, 1065–1071. [Google Scholar] [CrossRef]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practice in heterotrophic high-cell density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2001, 91, 31–46. [Google Scholar] [CrossRef]
- Aasen, I.M.; Ertesvåg, H.; Heggeset, T.M.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Samieri, C.; Feart, C.; Plourde, M. Dietary omega 3 poly-unsaturated fatty acids and Alzheimer’s disease: Interaction with apolipoprotein E gen-otype. Curr. Alzheimer. Res. 2011, 8, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Whelan, J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar]
- Yassine, H.N.; Feng, Q.; Azizkhanian, I.; Rawat, V.; Castor, K.; Fonteh, A.N.; Harrington, M.G.; Zheng, L.; Reed, B.R.; DeCarli, C.; et al. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016, 73, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- D’Ascoli, T.A.; Mursu, J.; Voutilainen, S.; Kauhanen, J.; Tuomainen, T.P.; Virtanen, J.K. Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and 528 women: The Kuopio Ischaemic Heart Dis-ease Risk Factor Study. Eur. J. Clin. Nutr. 2016, 70, 970. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Nadjar, A.; Lebbadi, M.; Calon, F.; Laye, S. n-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot Essent Fat. Acids 2014, 91, 1–20. [Google Scholar] [CrossRef]
- Arsenault, D.; Julien, C.; Tremblay, C.; Calon, F. DHA Improves Cognition and Prevents Dysfunction of Entorhinal Cortex Neurons in 3xTg-AD Mice. PLoS ONE 2011, 6, e17397. [Google Scholar] [CrossRef]
- Teng, E.; Taylor, K.; Bilousova, T.; Weiland, D.; Pham, T.; Zuo, X.; Yang, F.; Chen, P.P.; Glabe, C.G.; Takacs, A.; et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Aβ pathology and modulates Aβ oligomerization. Neurobiol. Dis. 2015, 82, 552–560. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Van der Zee, C.E.; Dederen, P.J.; Brouwer, K.M.; Reijmer, Y.D.; van Groen, T.; Broersen, L.M.; Lutjohann, D.; Heerschap, A.; Kiliaan, A.J. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol. Dis. 2009, 33, 482–498. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer 685 mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Oksman, M.; Iivonen, H.; Hogyes, E.; Amtul, Z.; Penke, B.; Leenders, I.; Broersen, L.; Lütjohann, D.; Hartmann, T.; Tanila, H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis. 2006, 23, 563–572. [Google Scholar] [CrossRef]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: Possible gender effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Takahashi, S.; Sakamaki, M.; Ferdousi, F.; Yoshida, M.; Demura, M.; Watanabe, M.M.; Isoda, H. Ethanol Extract of Aurantiochytrium mangrovei 18W-13a Strain Possesses Anti-inflammatory Effects on Murine Macrophage RAW264 Cells. Front. Physiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Sasaki, K.; Othman, M.B.; Ferdousi, F.; Yoshida, M.; Watanabe, M.; Tominaga, K.; Isoda, H. Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS ONE 2019, 14, e0218923. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Blasco, T.; Oliviero, M.; Sacchi, R.; Masi, P. Production of omega-3 oil by Aurantiochytrium mangrovei using spent osmotic solution from candied fruit industry as sole organic carbon source. Processes 2021, 9, 1834. [Google Scholar] [CrossRef]
- Abad, X.; Turon, X. Biotechnological production of docosahexaenoic acid using Aurantiochytrium limacinum: Carbon sources comparison and growth characterization. Mar. Drugs 2015, 13, 7275–7284. [Google Scholar] [CrossRef]
- Shen, X.F.; Chu, F.F.; Lam, P.K.; Zeng, R.J. Biosynthesis of high yield fatty acids from Chlorella vulgaris NIES-227 under nitrogen starvation stress during heterotrophic cultivation. Water Res. 2015, 81, 294–300. [Google Scholar] [CrossRef]
- Heggeset, T.; Ertesvåg, H.; Liu, B.; Ellingsen, T.E.; Vadstein, O.; Aasen, I.M. Lipid and DHA-production in Aurantiochytrium sp. Responses to nitrogen starvation and oxygen limitation revealed by analyses of production kinetics and global transcriptomes. Sci. Rep. 2019, 9, 1–3. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp. and Ulkenia sp. for production of biodiesel, long—chain omega-3 oils, and exopolysaccharide. Marine Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed Pharm. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Pace, R.D.; McElhenney, W.H. Green leafy vegetables in diets with a 25:1 omega-6/omega-3 fatty acid ratio modify the erythrocyte fatty acid profile of spontaneously hypertensive rats. Lipids Health Dis. 2018, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Brain lipids and ageing. In Technology and Nutrition, Food for the Ageing Population; Woodhead Publishing: Sawston, UK, 2009; pp. 219–251. [Google Scholar]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty. Acids: Implications for Cardiovascular and Other Diseases. J. Glycomics Lipidomics 2014, 4, 123. [Google Scholar] [CrossRef]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARgamma activation? Antioxid. Redox. Signal. 2014, 21, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Kotue, T.C.; Djote, W.N.B.; Marlyne, M.; Pieme, A.C.; Kansci, G.; Fokou, E. Antisickling and Antioxidant Properties of Omega-3 Fatty Acids EPA/DHA. Nutr. Food Sci. Int. J. 2019, 9, 1–6. [Google Scholar]
- Hajianfar, H.; Paknahad, Z.; Bahonar, A. The effect of omega-3 supplements on antioxidant capacity in patients with type 2 diabetes. Int. J. Prev. Med. 2013, 2, S234–S238. [Google Scholar]
- Zainal, Z.; Ong, A.; Yuen May, C.; Chang, S.K.; Abdul Rahim, A.; Khaza’ai, H. Acute and subchronic oral toxicity of oil palm puree in Sprague–Dawley rats. Int. J. Environ. Res. Public Health 2020, 17, 3404. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef]
- Zuliani, G.; Galvani, M.; Leitersdorf, E.; Volpato, S.; Cavalieri, M.; Fellin, R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr. Pharm. Des. 2009, 15, 4087–4093. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Pinto, M.E.A.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef]
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.P.; Pariante, C.M.; Zunszain, P.A. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 2017, 65, 230–238. [Google Scholar] [CrossRef]
- Borsini, A.; Stangl, D.; Jeffries, A.R.; Pariante, C.M.; Thuret, S. The role of omega-3 fatty acids in preventing glucocorticoid-induced reduction in human hippocampal neurogenesis and increase in apoptosis. Transl. Psychiatry 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Sagal, J.P.; Colombres, M. Acetylcholinesterase Interaction with Alzheimer Amyloid β. In Alzheimer’s Disease Subcellular Biochemistry; Harris, J.R., Fahrenholz, F., Eds.; Springer: Boston, MA, USA, 2005; pp. 299–317. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Coyle, C.H.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAIO J. 2007, 53, 17–22. [Google Scholar] [CrossRef]
- Dang, D.H.; Hoang, T.L.A.; Ngo, T.H.T. Study on biological characteristics of heterotrophic marine microalga—Schizochytrium mangrovei PQ6 isolated from Phu Quoc Island, Kien Giang province. J. Phycol. 2011, 47, 944–954. [Google Scholar]
- Inagaki, T.; Yamada, S.; Fujii, H.; Yoshikawa, T.; Shibamura, M.; Harada, S.; Fukushi, S.; Le, M.Q.; Nguyen, C.T.; Nguyen, T.T.T.; et al. Characterization of a Novel Alpha herpes virus Isolated from the Fruit Bat Pteropus lylei in Vietnam. J. Virol. 2020, 94, e00673-20. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huy, T.Q.; Nga, P.T.; Morita, K.; Dunia, I.; Benedetti, L. A new nidovirus (NamDinh virus NDiV): Its ultrastructural characterization in the C6/36 mosquito cell line. J. Virol. 2013, 444, 337–342. [Google Scholar]
- Mo, C.; Douek, J.; Rinkevich, B. Development of a PCR strategy for thraustochytrid identification based on 18S rDNA sequence. Mar. Biol. 2002, 140, 883–889. [Google Scholar] [CrossRef]
- Hassett, B.T. A Widely Distributed Thraustochytrid Parasite of Diatoms Isolated from the Arctic Represents a gen. and sp. nov. J. Eukaryot. Microbiol. 2020, 67, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.T.L.; Thu, N.T.H.; Hong, D.D. Identification of several marine strains isolated from Hai Phong and Nha Trang coasts based on morphology and 18S rRNA gene analysis. J. Biotechnol. 2010, 8, 387–396. (In Vietnamese) [Google Scholar]
- Furlan, V.J.M.; Batista, I.; Bandarra, N.; Mendes, R.; Cardoso, C. Conditions for the Production of Carotenoids by Thraustochytrium sp. ATCC 26185 and Aurantiochytrium sp. ATCC PRA-276. J. Aquat. Food Prod. Technol. 2019, 28, 465–477. [Google Scholar] [CrossRef]
- Tharek, A.; Yahya, A.; Salleh, M.M.; Jamaluddin, H.; Yoshizaki, S.; Hara, H.; Iwamoto, K.; Suzuki, I.; Mohamad, S.E. Improvement and screening of astaxanthin producing mutants of newly isolated Coelastrum sp. using ethyl methane sulfonate induced mutagenesis technique. Biotechnol. Rep. 2021, 15, e00673. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar] [CrossRef]
- Dang, D.H.; Dinh, T.N.M.; Le, T.T.; Nguyen, C.H.; Bui, D.L.; Luu, T.T.; Hoang, T.L.A.; Ngo, T.H.T. Biodiesel production from Vietnam heterotrophic marine microalga Schizochytrium mangrovei PQ6. J. Biosci. Bioeng. 2013, 116, 180–185. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Ener. Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Hang, N.T.M.; Giap, T.H.; Thanh, L.N.; Oanh, N.T.T.; Hong, D.D.; Minh, C.V. Metabolites from Microalgae Schizochytrium mangrovei. Chem. Nat. Compd. 2019, 55, 978–981. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Koulman, A.; van Rijssel, M.; Lützen, A.; de Boer, M.K.; Tyl, M.R.; Liebezeit, G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 2004, 43, 355–363. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, W.; Zhou, X.; Xie, Y.; Wang, S. Anti-thrombotic effects of α-linolenic acid isolated from Zanthoxylum bungeanum Maxim seeds. BMC Cent. Complement. Altern. Med. 2014, 14, 348. [Google Scholar] [CrossRef]
- World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Drug Safety Evaluation I: Acute and subchronic toxicity assessement; Academy Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]



| Culture Conditions | Cell Density (×106 Cells/mL) | DCW (g/L) | Lipid (% DCW) | |
|---|---|---|---|---|
| Nutritional media | Bajpai | 45.48 ± 1.23 b | 7.13 ± 0.31 b | 17.32 ± 1.37 b |
| M1 | 120.17 ± 2.76 a | 12.27 ± 0.53 a | 27.41 ± 0.73 a | |
| GPY | 10.20 ± 1.01 c | 2.25 ± 0.05 c | 4.25 ± 1.06 c | |
| Temperatures (°C) | 15 | 45.13 ± 0.51 f | 6.28 ± 0.31 c | 8.12 ± 0.43 c |
| 20 | 96.15 ± 1.04 d | 9.89 ± 1.02 b | 21.65 ± 1.63 b | |
| 25 | 117.43 ± 1.89 b | 12.10 ± 1.33 a | 27.84 ± 1.27 a | |
| 28 | 123.68 ± 2.73 a | 12.39 ± 1.62 a | 27.82 ± 1.67 a | |
| 30 | 112.71 ± 1.22 c | 8.03 ± 0.82 c | 20.56 ± 0.86 b | |
| 35 | 85.35 ± 0.67 e | 4.29 ± 0.37 d | 7.19 ± 0.31 c | |
| Carbon sources | Maltose | 32.37 ± 0.61 c | 3.95 ± 0.04 c | 10.74 ± 1.16 c |
| Glucose | 121.48 ± 1.06 a | 12.79 ± 1.15 a | 26.63 ± 1.18 a | |
| Starch | 30.67 ± 0.19 d | 3.73 ± 0.13 d | 4.61 ± 0.76 d | |
| Maltodextrin | 73.42 ± 0.53 b | 8.78 ± 1.11 b | 15.37 ± 1.03 b | |
| Concentrations of glucose (%) | 1 | 53.29 ± 0.62 d | 5.09 ± 0.12 b | 16.13 ± 1.21 c |
| 3 | 127.62 ± 2.03 a | 12.69 ± 1.12 a | 26.76 ± 0.24 a | |
| 5 | 121.15 ± 1.65 b | 11.73 ± 1.17 a | 24.37 ± 0.25 b | |
| 7 | 118.57 ± 1.18 bc | 11.78 ± 0.64 a | 23.85 ± 0.36 b | |
| 9 | 117.02 ± 1.43 c | 11.21 ± 0.21 a | 23.51 ± 0.51 b | |
| Nitrogen sources | (NH4)2SO4 | 68.03 ± 0.88 d | 6.32 ± 0.78 d | 10.37 ± 0.72 c |
| CH3COONH4 | 86.69 ± 1.71 c | 9.53 ± 0.34 c | 21.69 ± 0.30 b | |
| Yeast extract | 125.48 ± 1.89 a | 12.36 ± 0.75 a | 28.32 ± 1.54 a | |
| NaNO3 | 114.37 ± 1.34 b | 10.82 ± 0.51 b | 20.87 ± 1.29 b | |
| Concentrations of yeast extract (%) | 0.5 | 8.15 ± 0.41 d | 8.77 ± 0.31 b | 22.14 ± 0.15 c |
| 1.0 | 128.47 ± 1.08 a | 12.85 ± 1.37 a | 28.34 ± 1.21 a | |
| 1.5 | 119.93 ± 1.38 b | 12.18 ± 0.23 a | 28.02 ± 1.43 a | |
| 2.0 | 97.34 ± 0.56 c | 9.37 ± 0.64 b | 23.52 ± 0.76 b |
| Culture Conditions | Cell Density (×106 Cells/mL) | DCW (g/L) | Lipid (% DCW) | |
|---|---|---|---|---|
| Concentration of glucose (%) | 3 | 115.43 ± 2.48 d | 22.36 ± 1.45 c | 16.34 ± 0.15 d |
| 6 | 132.12 ± 2.04 c | 28.82 ± 1.02 b | 22.01 ± 0.26 c | |
| 9 | 154.75 ± 1.14 a | 31.15 ± 1.13 a | 24.92 ± 0.31 a | |
| 12 | 147.02 ± 1.10 b | 31.12 ± 0.18 a | 24.11 ± 0.43 b | |
| Nitrogen source | Industrial yeast extract | 148.51 ± 0.67 | 31.18 ± 2.63 | 25.29 ± 1.43 |
| Pure yeast extract | 145.27 ± 1.83 | 31.89 ± 1.17 | 26.15 ± 1.56 |
| Parameter | Content |
|---|---|
| Lipid (% DCW) | 25.29 ± 1.43 |
| Protein (% DCW) | 7.93 ± 0.17 |
| Carbohydrate (% DCW) | 15.21 ± 0.02 |
| Carotenoid (µg/L) | 143.67 ± 0.35 |
| Astaxanthin (µg/L) | 8.07 ± 0.17 |
| No | Fatty Acid Composition | Fatty Acid Content (g/100 g DCW) | No | Fatty Acid Composition | Fatty Acid Content (g/100 g DCW) |
|---|---|---|---|---|---|
| 1 | C4:0 Butyric acid | 0.000 | 28 | C20:1 Gondoic acid | 0.019 |
| 2 | C6:0 Caproic acid | 0.002 | 29 | C20:2 Eicosadienoic acid | 0.009 |
| 3 | C8:0 Capryllic acid | 0.003 | 30 | C20:3 gamma -Eicosatrienoic acid | 0.000 |
| 4 | C10:0 Capric acid | 0.000 | 31 | C20:3 Eicosatrienoic acid | 0.000 |
| 5 | C11:0 Undecanoic acid | 0.000 | 32 | C20:4 Arachidonic acid (ARA) | 0.000 |
| 6 | C12:0 Lauric acid | 0.003 | 33 | C20:5 Eicosapentaenoic acid (EPA) | 0.051 |
| 7 | C13:0 Tridecanoic acid | 0.000 | 34 | C21:0 Heneicosanoic acid | 0.000 |
| 8 | C14:0 Myristic acid | 0.016 | 35 | C22:0 Behenic acid | 0.147 |
| 9 | C14:1 Myristoleic acid | 0.000 | 36 | C22:1 Erucic acid | 0.003 |
| 10 | C15:0 Pentadecanoic acid | 0.008 | 37 | C22:2 Docosadienoic acid | 0.000 |
| 11 | C15:1 Pentadecenoic acid | 0.000 | 38 | C22:4 Docosatetraenoic acid | 0.000 |
| 12 | C16:0 Palmitic acid | 1.288 | 39 | C22:5 all cis-4, 7, 10, 13, 16 | 0.000 |
| 13 | C16:1 cis-7-Hexadecenoic acid | 0.001 | 40 | C22:5 all cis-7, 10, 13, 16 | 0.000 |
| 14 | C16:1 Palmitoleic acid | 0.037 | 41 | C22:6 Docosahexaenoic acid | 0.129 |
| 15 | C17:0 Heptadecanoic acid | 0.058 | 42 | C23:0 Tricosanoic acid | 0.005 |
| 16 | C17:1 10-Heptadecenoic acid | 0.000 | 43 | C24:0 Lignoceric acid | 0.120 |
| 17 | C18:0 Stearic acid | 0.738 | 44 | C24:1 Nevonic acid | 0.000 |
| 18 | C18:1 Tran fatty acids | 0.028 | 45 | Other fatty acids | 0.549 |
| 19 | C18:1 ω-9 Oleic acid | 2.134 | 46 | Total fatty acids | 6.655 |
| 20 | C18:2 Trans fatty acids | 0.025 | 47 | Total fat (as triglyceride fat) | 6.758 |
| 21 | C18:2 Rumenic acid (CLA) | 0.000 | 48 | Saturated fatty acids | 2.566 |
| 22 | C18:2 ω-6 Linoleic acid (LA) | 0.016 | 49 | Trans fatty acids | 0.060 |
| 23 | C18:3 Trans fatty acids | 0.007 | 50 | Monounsaturated fatty acids | 2.193 |
| 24 | C18:3 ω-3 Linolenic acid (ALA) | 1.076 | 51 | Polyunsaturated fatty acids | 1.318 |
| 25 | C18:3 gamma-Linolenic acid | 0.000 | 52 | Omega-3 | 1.261 |
| 26 | C18:4 Octadecatetraenoic acid | 0.005 | 53 | Omega-6 | 0.025 |
| 27 | C20:0 Arachidic acid | 0.178 | 54 | Omega-9 | 2.157 |
| No | Acid Name | Scientific Name | Common Name | PUFAs Contents (% TFA) | |
|---|---|---|---|---|---|
| In FFAs Form | In FAAEs Form | ||||
| 1 | 14:0 | Tetradecanoic acid | Myristic | 1.38 ± 0.01 | 1.36 ± 0.01 |
| 2 | 15:0 | Pentadecanoic acid | 1.31 ± 0.10 | 0.92 ± 0.01 | |
| 3 | 15:1 ω-5 | Hexadecanoic acid | 2.44 ± 0.05 | 2.45 ± 0.02 | |
| 4 | 16:0 | Hexandecanoic acid | Palmitic | 22.93 ± 0.22 | 22.26 ± 0.14 |
| 5 | 16:1 ω-7 | 9-hexadecenoic acid | Palmitoleic | 2.97 ± 0.02 | 0.71 ± 0.01 |
| 6 | 17:0 | Heptadecanoic acid | Margnic | 1.04 ± 0.01 | 0.88 ± 0.02 |
| 7 | 18:0 | Octadecanoic acid | Stearic | 3.75 ± 0.01 | 4.66 ± 0.01 |
| 8 | 18:1 ω-9 | Cis 9- octadecenoic acid | Oleic | 4.57 ± 0.02 | 4.32 ± 0.01 |
| 9 | 18:1 ω-7 | 11- octadecenoic acid | 19.28 ± 0.06 | 17.74 ± 0.05 | |
| 10 | 18:2 ω-6-t | 9,12- octadecenoic acid | Linoleic (LA) | 5.63 ± 0.07 | 5.12 ± 0.08 |
| 11 | 18:3 ω-6 | Alpha—Linolenic acid | ALA | 20.13 ± 0.01 | 22.02± 0.02 |
| 12 | 20:2 ω-6 | 11,14-eicosadienoic acid | 2.56 ± 0.01 | 3.34 ± 0.01 | |
| 13 | 20:5 ω-3 | 5,8,11,14,17 eicosapentaenoic acid | EPA | 1.40 ± 0.02 | 0.36 ± 0.01 |
| 14 | 20:1 ω-9 | 11-eicosenoic acid | 0.43 ± 0.02 | 0.24 ± 0.01 | |
| 15 | 22:3 ω-3 | 11,14,17-eicosatrienoic acid | 1.27 ± 0.01 | 1,81 ± 0.02 | |
| 16 | 22:4 ω-6 | 7,10,13,16-docosatetraenoic acid | 2.38 ± 0.01 | 2,80 ± 0.02 | |
| 17 | 22:6 ω-3 | 4,7,10,13,16,19 docosahexaenoic acid | DHA | 7.36 ± 0.03 | 8.99 ± 0.03 |
| Omega 3 | 24.53 ± 0.05 | 25.66 ± 0.06 | |||
| Omega 6 | 11.20 ± 0.10 | 13.76 ± 0.13 | |||
| Omega 9 | 5.00 ± 0.04 | 4.56 ± 0.02 | |||
| Total omega 3, 6, 9 | 40.73 ± 0.19 | 44.00 ± 0.21 | |||
| Groups | Body Weight | ||
|---|---|---|---|
| Before Test | After 45 Days | After 90 Days | |
| Control | 178.24 ± 5.38 | 205.19 ± 5.39 | 225.89 ± 5.59 |
| Group 1 | 179.26 ± 5.19 | 207.07 ± 4.77 | 227.28 ± 5.89 |
| Group 2 | 179.57 ± 4.50 | 208.67 ± 9.18 | 228.51 ± 9.61 |
| Group 3 | 173.43 ± 5.14 | 204.20 ± 5.05 | 219.99 ± 5.34 |
| Group 4 | 175.40 ± 4.96 | 207.99 ± 4.56 | 224.33 ± 5.63 |
| Parameter | Control | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| Hematological parameters | |||||
| RBC (×1012 g/l) | 8.57 ± 1.57 | 8.82 ± 2.11 | 8.42 ± 0.05 | 8.58 ± 1.57 | 8.42 ± 0.05 |
| Hemoglobin (g/dL) | 135.54 ± 18.58 | 133.24 ± 10.00 | 136.56 ± 13.72 | 135.67 ± 18.60 | 136.69 ±13.73 |
| Hematocrit (%) | 43.61 ± 6.17 | 42.37 ± 6.58 | 42.12 ±2.72 | 43.65 ± 6.18 | 42.16 ±2.73 |
| Average volume off erythrocytes (fl) | 52.28 ± 2.02 | 51.39 ± 8.12 | 51.51 ± 2.67 | 52.33 ± 2.02 | 51.56 ± 2.68 |
| WBC (g/L) | 11.63 ± 3.11 | 11.41 ± 4.68 | 11.42 ± 3.25 | 11.64 ± 3.23 | 11.44 ± 3.26 |
| Platelet count (g/L) | 687.36 ± 154.06 | 702.53 ± 249.72 | 638.78 ± 88.95 | 688.03 ± 154.21 | 639.40 ± 89.04 |
| Biological parameters in liver | |||||
| AST (UI/L) | 85.43 ± 13.42 | 78.03 ± 20.95 | 80.71 ± 11.55 | 85.51 ± 13.44 | 82.79 ± 11.56 |
| ALT (UI/L) | 71.28 ± 10.51 | 64.14 ± 22.58 | 67.71 ± 11.52 | 71.35 ± 10.52 | 70.77 ± 11.53 |
| Biological parameters in plasma | |||||
| Albumin (g/L) | 33.28 ± 1.44 | 33.66 ± 2.67 | 34.56 ± 2.96 | 33.32 ± 1.44 | 33.59 ± 2.96 |
| TC (mmol/L) | 1.25 ± 0.49 | 1.25 ± 0.45 | 1.22 ± 0.27 | 1.25 ± 0.27 | 1.21 ± 0.49 |
| Biological parameters in kidney | |||||
| Creatinine (g/L) | 64.65 ± 28.40 | 63.63 ± 22.58 | 58.27 ± 14.10 | 64.71 ± 28.42 | 62.33 ± 14.11 |
| Sample | DPPH Assay | AChE Inhibitory Activity |
|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | |
| FAAE | 294.98 ± 4.03 | 2.65 ± 0.16 |
| FFA | 239.21 ± 5.01 | 4.9 ± 0.65 |
| Ascorbic acid | 19.43 ± 1.44 | nd |
| Galantamine | nd | 1.78 ± 0.15 |
| IC50 (μM) | IC50 (μM) | |
| Linoleic acid (LA) | nd | nd |
| α-Linolenic acid (ALA) | 262.54 ± 5.47 | 10.80 ± 0.66 |
| Eicosapentaenoic acid (EPA) | 197.87 ± 3.63 | 5.03 ± 0.27 |
| Docosahexaenoic acid (DHA) | 285.04 ± 2.71 | 4.40 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hien, H.T.M.; Thom, L.T.; Ha, N.C.; Tam, L.T.; Thu, N.T.H.; Nguyen, T.V.; Loan, V.T.; Dan, N.T.; Hong, D.D. Characterization and Optimization of Culture Conditions for Aurantiochytrium sp. SC145 Isolated from Sand Cay (Son Ca) Island, Vietnam, and Antioxidative and Neuroprotective Activities of Its Polyunsaturated Fatty Acid Mixture. Mar. Drugs 2022, 20, 780. https://doi.org/10.3390/md20120780
Hien HTM, Thom LT, Ha NC, Tam LT, Thu NTH, Nguyen TV, Loan VT, Dan NT, Hong DD. Characterization and Optimization of Culture Conditions for Aurantiochytrium sp. SC145 Isolated from Sand Cay (Son Ca) Island, Vietnam, and Antioxidative and Neuroprotective Activities of Its Polyunsaturated Fatty Acid Mixture. Marine Drugs. 2022; 20(12):780. https://doi.org/10.3390/md20120780
Chicago/Turabian StyleHien, Hoang Thi Minh, Le Thi Thom, Nguyen Cam Ha, Luu Thi Tam, Ngo Thi Hoai Thu, Tru Van Nguyen, Vu Thi Loan, Nguyen Trong Dan, and Dang Diem Hong. 2022. "Characterization and Optimization of Culture Conditions for Aurantiochytrium sp. SC145 Isolated from Sand Cay (Son Ca) Island, Vietnam, and Antioxidative and Neuroprotective Activities of Its Polyunsaturated Fatty Acid Mixture" Marine Drugs 20, no. 12: 780. https://doi.org/10.3390/md20120780
APA StyleHien, H. T. M., Thom, L. T., Ha, N. C., Tam, L. T., Thu, N. T. H., Nguyen, T. V., Loan, V. T., Dan, N. T., & Hong, D. D. (2022). Characterization and Optimization of Culture Conditions for Aurantiochytrium sp. SC145 Isolated from Sand Cay (Son Ca) Island, Vietnam, and Antioxidative and Neuroprotective Activities of Its Polyunsaturated Fatty Acid Mixture. Marine Drugs, 20(12), 780. https://doi.org/10.3390/md20120780