Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits
Abstract
1. Introduction
2. Specialized Metabolites with Anti-Inflammatory Activity
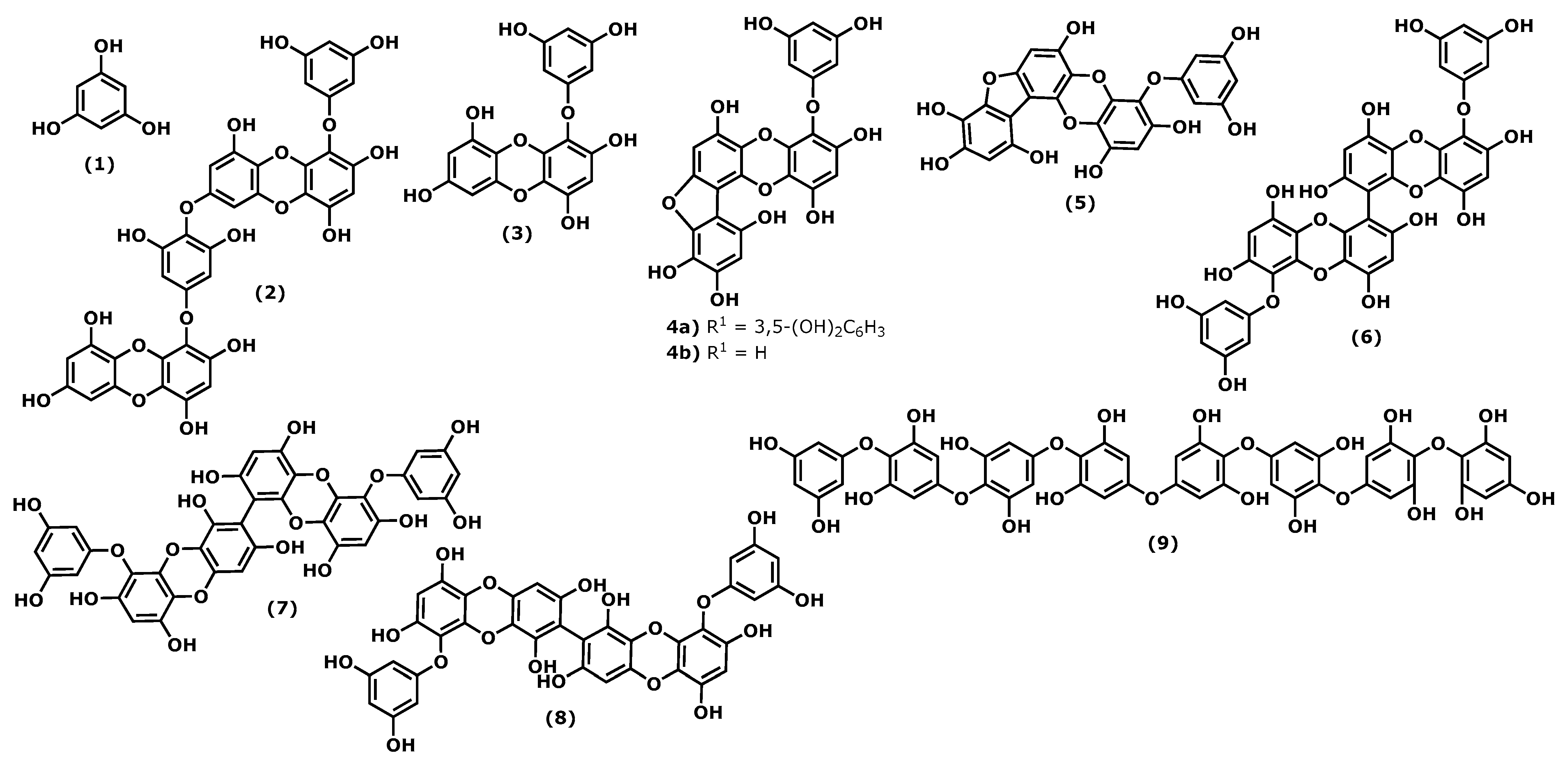
2.1. Phlorotannins
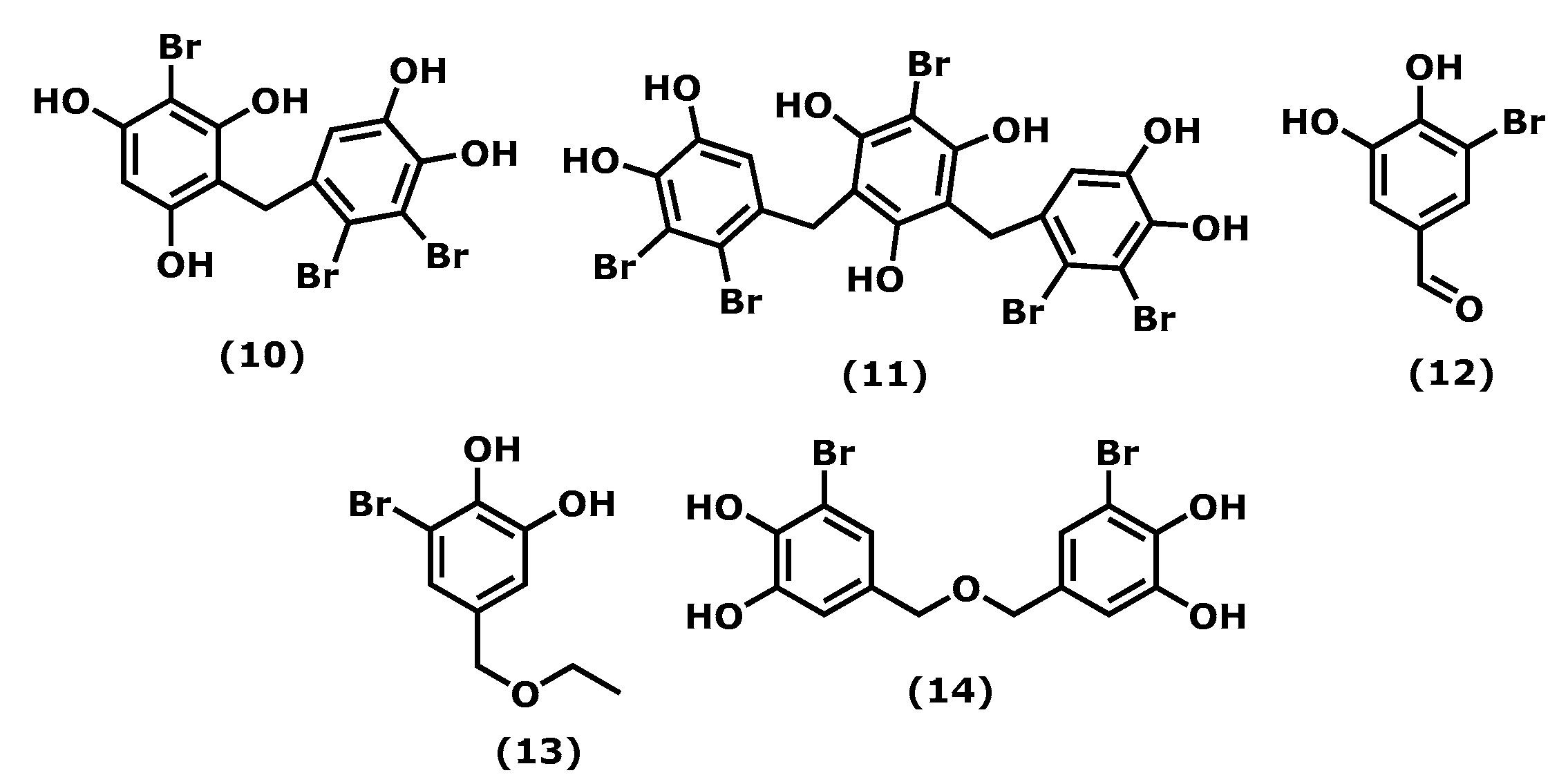
2.2. Bromophenols
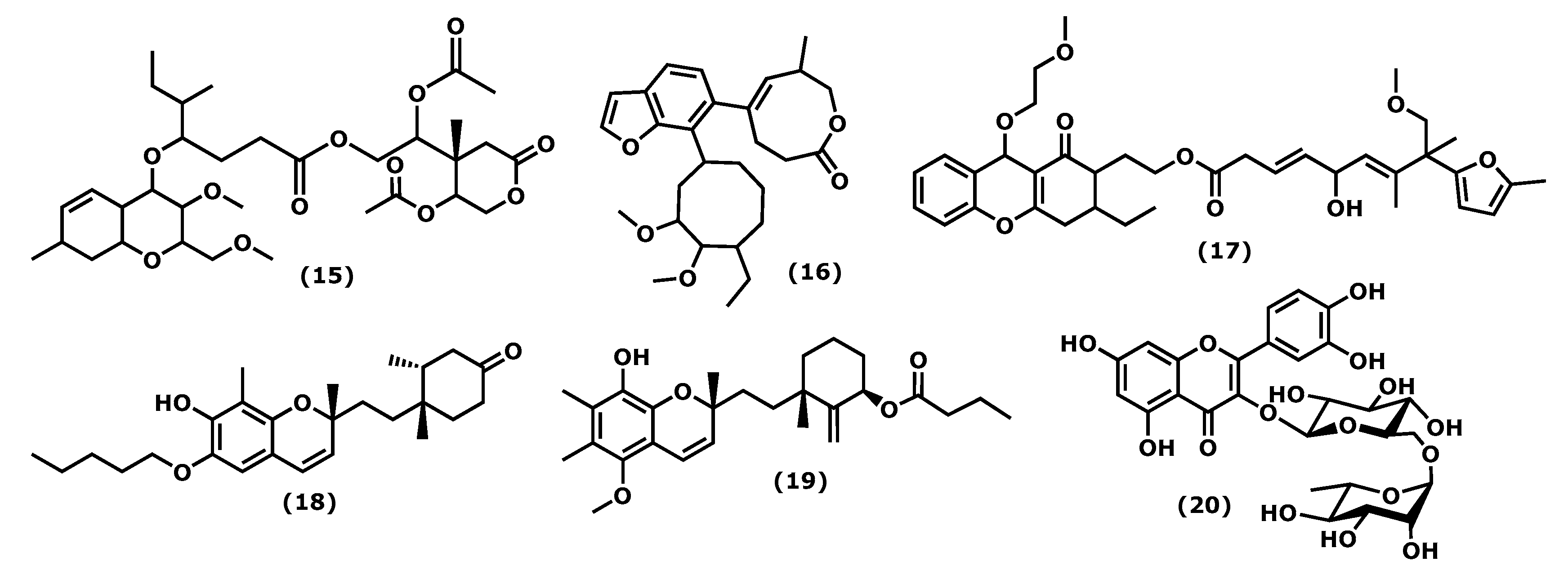
2.3. Chromenes
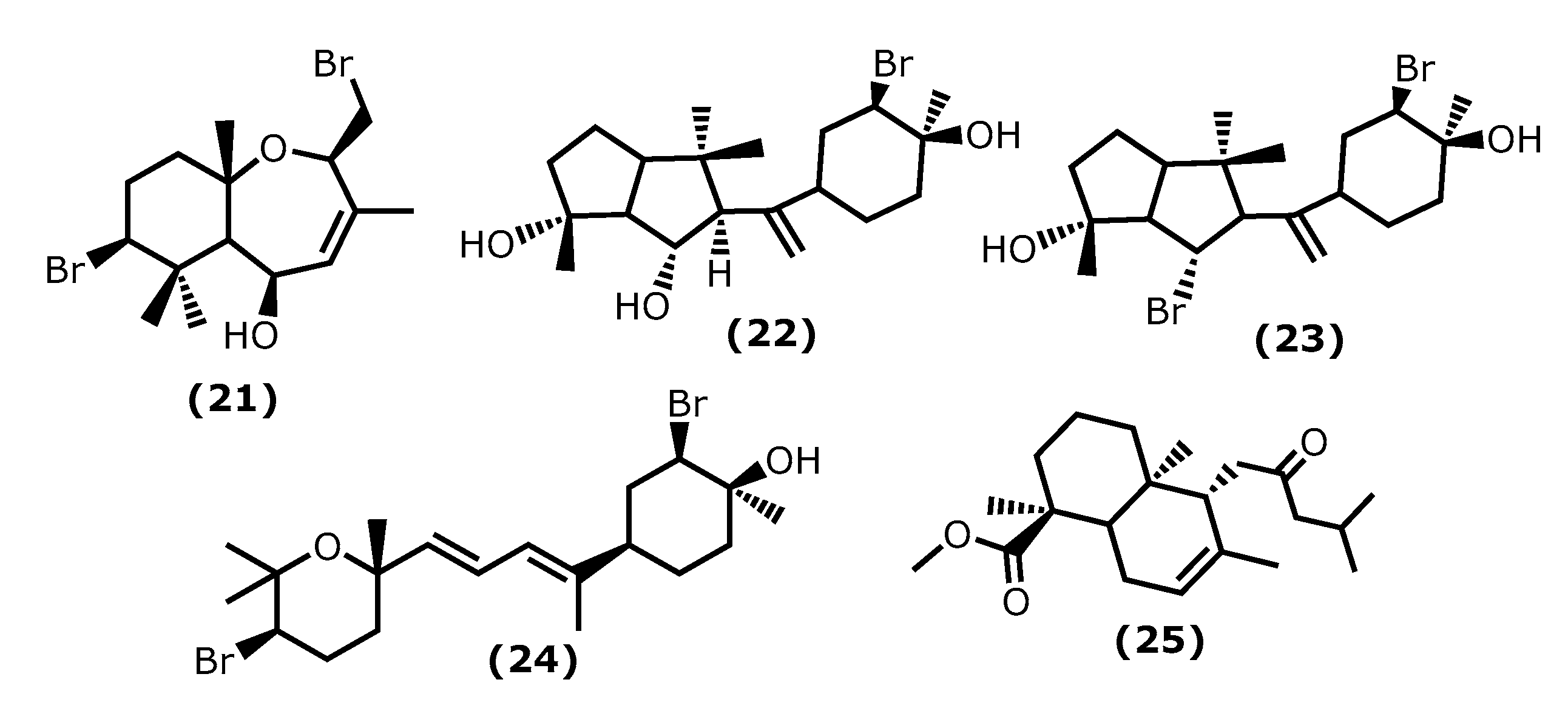
2.4. Terpenoids
2.5. Fucoxanthin
2.6. Fucosterol
2.7. Caulerpin
2.8. Fatty Acids
3. Macroalgae Commercially Available Products
4. Conclusions
| Compound (Number) | Source * | Concentration Tested | Experimental Model | Pharmacological Markers |
|---|---|---|---|---|
| Phloroglucinol (1) 1 [40] | Ecklonia cava | 10 μΜ | RAW 264.7 cells HT1080 cells | ↓ TNF-α, IL-1β e IL-6, PGE2 Inhibit MMP-2 and MMP-9 |
| Dieckol (2) 2 [42,47] | Eisenia sp. | 10 and 20 μΜ | HUVECs Mice treated by high mobility group box 1 protein (HMGB1) | ↓ LPS-mediated hyperpermeability (74.9%) ↓ LPS-induced HMGB1 release ↓ acetic acid induced-hyperpermeability and carboxymethylcellulose-induced leukocytes migration (55%) |
| Eckol (3) 3 [43] | Eisenia sp. Eckonia sp. | 1–10 μΜ | Propionibacterium acnes induced HaCaT cells | ↓ TNF-α ↓ COX-2, iNOS |
| Phlorofucofuroeckol B 4 (4a) [45] | Eisenia arborea | 75 μΜ | ICR strain mouse | inhibition of ear edema induced by AA (42.2%), by TPA (38.4%), and by OXA (41.0%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively |
| Phlorofucofuroeckol A 5 (4b) [41,45] | Eisenia arborea Ecklonia stolonifera Okamura 1913 a | 40 μΜ 10 μΜ 75 μΜ | LPS-stimulated BV-2 cells RBL-2H3 cells ICR strain mouse | ↓ TNF-α, IL-1β e IL-6 ↓ COX-2, NO ↓ phosphorylation Akt, ERK, JNK ↓ histamine, leukotriene B4, PEG2 inhibition of ear edema induced by AA (30.5%), TPA (31.7%), and OXA (23.4%). EGCG inhibits 12.9%, 13.8%, and 5.7% of by AA, TPA, and OXA, respectively |
| Fucofuroeckol-A (5) 6 [39] | Eisenia bicyclis (Kjellman) Setchell 1905 | 1–100 μΜ | LPS-induced RAW 264.7 cells | ↓ NO, PGE2, iNOS ↓ TNF-α, IL-1β, IL-6 ↓ COX-2 |
| 6,6′-Bieckol (6) 7 [44,45] | Ecklonia cava Eisenia arborea | 100 and 200 μΜ 75 μΜ | LPS-induced RAW 264.7 cells ICR strain mouse | ↓ NO, PGE2, iNOS ↓ TNF-α, IL-6 ↓ COX-2 inhibition of ear oedema induced by AA (41.9%), TPA (34.2%), and OXA (17.8%). EGCG inhibits 12.9%, 13.8%, and 5.7% of by AA, TPA, and OXA, respectively |
| 6,8′-Bieckol (7) 8 [45] | Eisenia arborea | 10 μΜ 75 μΜ | RBL-2H3 cells ICR strain mouse | ↓ COX-2 mRNA expression inhibition of ear oedema induced by AA (39.8%), TPA (49.4%), and OXA (77.8%). EGCG inhibits 12.9%, 13.8%, and 5.7% of by AA, TPA, and OXA, respectively |
| 8,8′-Bieckol (8) 9 [45] | Eisenia arborea | 10 μΜ 75 μΜ | RBL-2H3 cells ICR strain mouse | ↓ histamine, leukotriene B4, PEG2 inhibition of ear oedema induced by AA (21.0%), TPA (31.7%), and OXA (32.3%). EGCG inhibits 12.9%, 13.8%, and 5.7% of by AA, TPA, and OXA, respectively |
| Octaphlorethol A (9) 10 [46] | Ishige foliacea | 6.2 and 12.5 μΜ | CpG-stimulated BMCD and BMDM | ↓ TNF-α, IL-6, IL12 p40 |
| Vidalol A (10) 11 [52] | Vidalia obtusiloba | n. r. | phorbol ester (PMA)—induced swelling of the mouse ear Enzymatic activity | ↓ eodema (58–82%) ↓ phospholipase A2 |
| Vidalol B (11)12 [52] | Vidalia obtusiloba | n. r. | phorbol ester (PMA)—induced swelling of the mouse ear Enzymatic activity | ↓ eodema (58–82%) ↓ phospholipase A2 |
| 3-BDB (12) 13 [53,54] | Polysiphonia morrowii Polysiphonia urceolata Rhodomela confervoides | 12.5, 25, 50, and 100 μM 100 mg/kg | LPS-stimulated RAW 264.7 BALB/c mice induced by DNCB | ↓ IL-6, phosphorylation NF-ΚB ↓ STAT1; Tyr 701 ↓ edema inflammation, AD symptoms, Ig2 |
| BEMB (13) 14 [56] | Polysiphonia morrowii | 12.5–50 μM | LPS-stimulated RAW 264.7 and zebrafish embryos | ↓ NO, iNOS, COX-2, NF-ƘB |
| BBDE (14) 15 [57] | Polysiphonia morrowii | 0.1, 1, 2 μM | LPS-stimulated RAW 264.7 | ↓ NO, iNOS, COX-2, PGE2, TNF-α, IL-6, IL-1β |
| Compound (15) 16 [58] | Gracilaria opuntia | n. r. | Enzymatic activity | ↓ COX-2, 5-LOX |
| Compound (16) 17 [59] | Gracilaria opuntia | n. r. | Enzymatic activity | ↓ 5-LOX |
| Compound (17) 18 [59] | Gracilaria opuntia | n. r. | Enzymatic activity | ↓ 5-LOX |
| Compound (18) 19 [60] | Gracilaria salicornia | n. r. | Enzymatic activity | ↓ COX-2, 5-LOX |
| Compound (19) 20 [60] | Gracilaria salicornia | n. r. | Enzymatic activity | ↓ COX-2, 5-LOX |
| Rutin (20) 21 [61] | Porphyra dentata | 80–250 μM | LPS-stimulated RAW 264.7 | ↓ NO, iNOS, NF-ƘB |
| 5β-Hydroxypalisadin B 22 (21) [64] | Laurencia snackeyi | 50 μM 0.25, 0.1 and 1 µg/mL | LPS-induced RAW 264.7 LPS-induced zebrafish embryo | ↓ NO, COX-2, iNOS ↓ NO, Improved survival, heart rate and yolk sac oedema size |
| Neorogioltriol (22)23 [66,67] | Laurencia glandulifera | 8 μM 1 mg/kg | LPS-induced RAW 264.7 Writhing test in mice Formalin test in rats | ↓ NO, iNOS ↓ macrophage activation induce Arginase 1, MRC1, miRNA miR-146a ↓ writhing response induced by acetic acid by 88.9% ↓ 2° phase formalin test in 48.7% |
| Neorogioldiol (23) 24 [67] | Laurencia sp | 62.5 μM | LPS-induced RAW 264.7 C57BL/6 mice | ↓ NO, iNOS ↓ macrophage activation induce Arginase 1, MRC1, miRNA miR-146a ↓ tissue damage, TNF-α, IL-6, IL-12 |
| Compound (24) 25 [67] | Laurencia sp | 10 μM | LPS-induced RAW 264.7 C57BL/6 mice | ↓ NO, iNOS ↓ macrophage activation induce Arginase 1, MRC1, miRNA miR-146a ↓ tissue damage, TNF-α, IL-6, IL-12 |
| Compound (25) 26 [68] | Gracilaria Salicornia | n. r. | Enzymatic activity | ↓ 5-LOX |
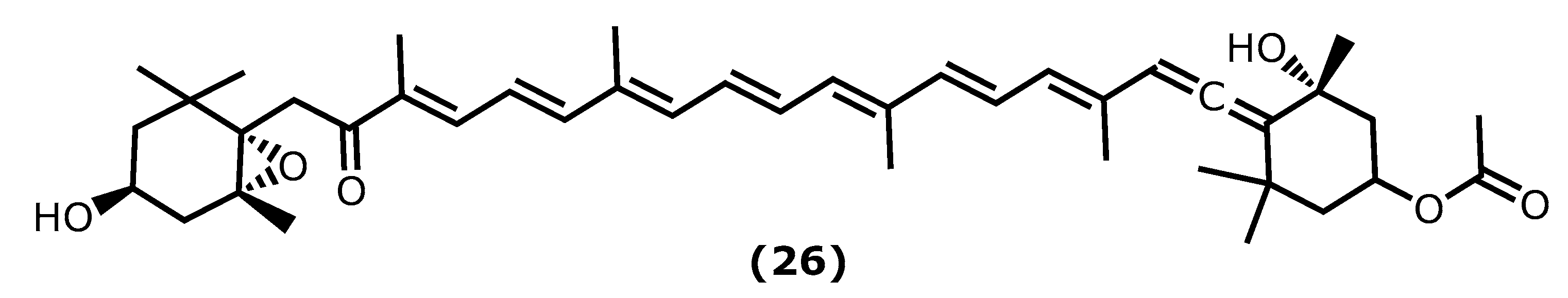
| Fucoxanthin (26) 27 [74,77,82] | Sargassum siliquastrum (Mertens ex Turner) C.Agardh 1820 | 0.1–1 mg/kg 15, 30, 60 μM | LPS-induced sepsis in mice LPS-induced RAW 264.7 LPS-activated BV-2 microglia | ↓ TNF-α, IL-6, IL-12, NF-ƘB ↑ rate of survival ↓ iNOS, COX-2, mRNA, TNF-α, IL-6 ↓ iNOS, COX-2, mRNA, TNF-α, IL-6 ↓ Akt, NF-Κb, ERK, p38 MAPK |
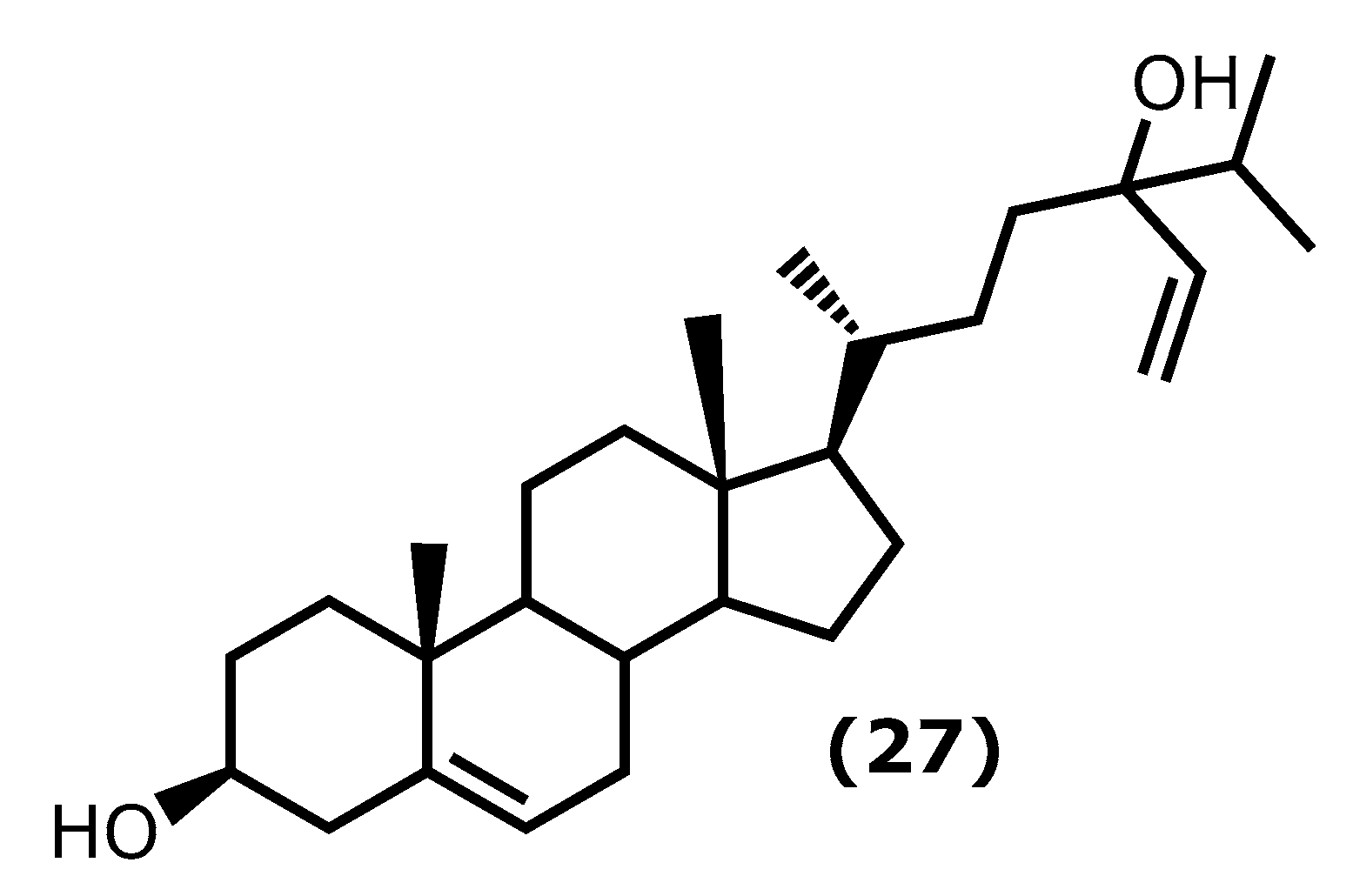
| Fucosterol (27) 28 [90,91,93] | Undaria pinnatifida Hizikia fusiformis (Harvey) Okamura 1932 b Panida australis c | 15, 30, 60 mg/kg 1–10 µM 0.004, 0.2, 10 µM | LPS-induced ALI in mise CoCl2-induced hypoxia in keratinocytes LPS or Aβ-induced BV2 (microglial) cells | ↓ lung histopathologic changes, wet-to-dry ratio ↓ TNF-α, IL-6, IL-1β, NF-κB ↓ IL-6, IL-1β, TNF-α, pPI3K and pAkt and HIF1-α accumulation ↓ L-6, IL-1β, TNF-α, NO, PGE2 |
| Caulerpin (28) 29 [97,98] | Caulerpa racemosa (Forsskål) J.Agardh 1873 Caulerpa sertularioide | 100 μmol/kg 4 mg/kg | Swiss albino mice C57BL/6 mice with colitis induced DSS | ↓ formalin effects in both phases by 35.4% and 45.6%. reduction 55.8% on capsaicin-induced ear oedema model ↓ recruit cells (48.3%) on carrageenan-induced peritonitis triggering improvement of DAI and attenuating the colon shortening/ damage ↓ TNF-α, IFN-γ, IL-6, IL-17, NFκB p65 ↑ IL-10 in the colon tissue |
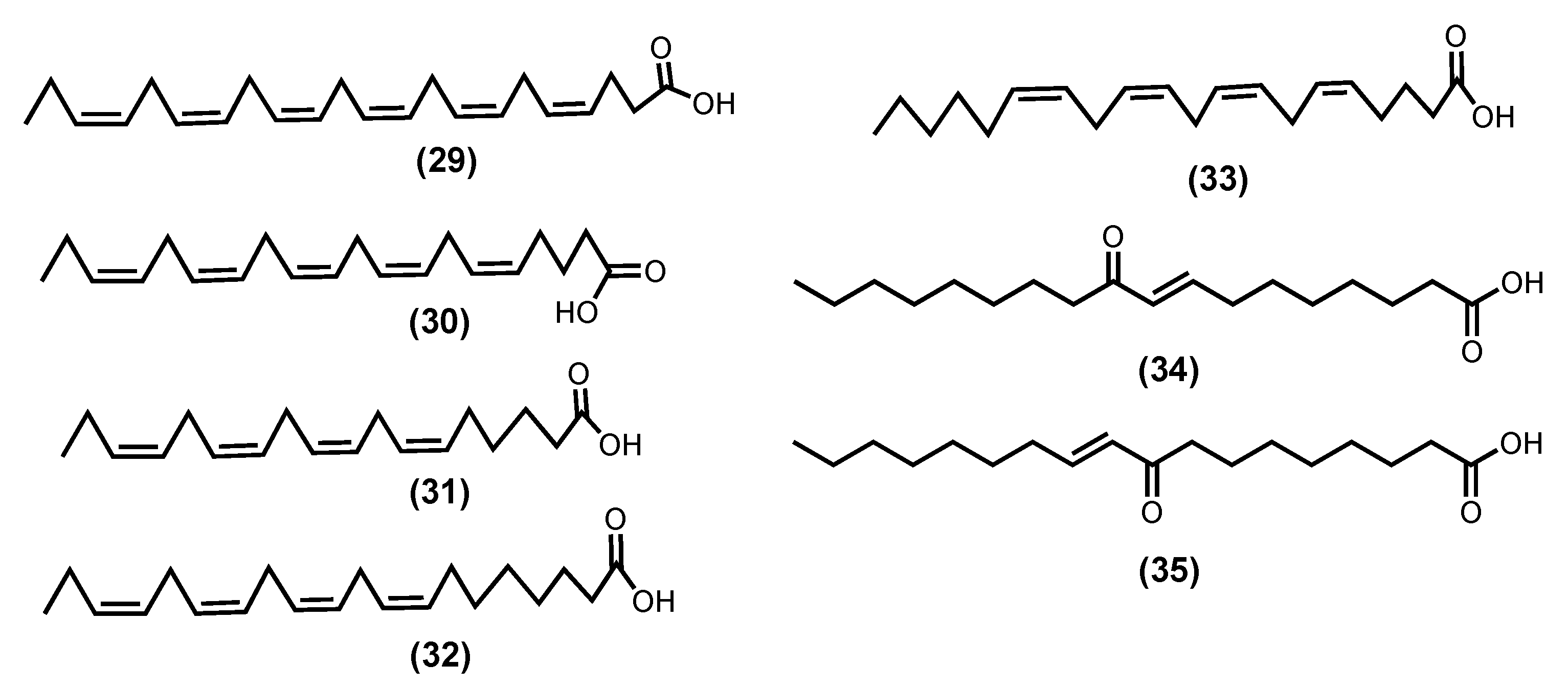
| Z-4,7,10,13,16,19-Docosahexaenoic acid-DHA (29) [102,104,106] | Sargassum natans (Linnaeus) Gaillon 1828 | n. r. 3 g/day | 21 volunteers (9 men and 12 postmenopausal women) with chronic inflammation and some characteristics of metabolic syndrome | RvE1 protect tissue counterregulates pro-inflammatory gene expression ↓ fumarate, pyruvate, citrate, isocitrate, malate, α-ketoglutarate ↑ succinate, glucuronate |
| Z-5,8,11,14,17-Eicosapentaenoic acid-EPA (30) [99,104,106] | Vertebrata lanosa (Linnaeus) T.A.Christensen 1967 Palmaria palmata (Linnaeus) F.Weber and D.Mohr 1805 Laminaria digitata (Hudson) J.V.Lamouroux 1813 | n. r. 3 g/day | 21 volunteers (9 men and 12 postmenopausal women) with chronic inflammation and some characteristics of metabolic syndrome | RvE1 protect tissue counterregulates pro-inflammatory gene expression ↓ fumarate, α-ketoglutarate ↑ UDP-glucuronate, glucuronate |
| E-9-Oxooctadec-10-enoic acid (34) [105] | Gracilaria verrucose (Hudson) Papenfuss, nom. Rejic. 1950 d | 50–100 μM | LPS-induced RAW 264.7 | ↓ NO, TNF-α, IL-6 ↓ NF-ƘB, JAK/STAT |
| E-10-Oxooctadec-8-enoic acid (35) [105] | Gracilaria verrucosed | 50–100 μM | LPS-induced RAW 264.7 | ↓ NO, TNF-α, IL-6 ↓ NF-ƘB, JAK/STAT |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kachhwaha, N.; Sharma, M.K.; Khandelwal, R.; Kaushik, P. Marine algal bioactive metabolites and their pharmacological applications. In Therapeutic Implications of Natural Bioactive Compounds, Chapter 6; Bentham Books: Singapore, 2022; Volume 3, pp. 118–134. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The seaweed resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernades, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor activity of Fucus vesiculosus- Derived phlorotannins through activation of apoptotic signals in gastric and colorectal tumor cell lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef]
- Matos, J.; Gomes, A.; Cardoso, C.; Afonso, C.; Campos, A.M.; Gomes, R.; Falé, P.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Commercial red seaweed in Portugal (Gelidium sesquipedale and Pterocladiella capillacea, Florideophyceae): Going beyond a single-purpose product approach by valorizing bioactivity. Int. J. Mar. Sci. 2020, 36, 213–224. [Google Scholar] [CrossRef]
- Freitas, M.V.; Inácio, L.G.; Martins, M.; Afonso, C.; Pereira, L.; Mouga, T. Primary composition and pigments of 11 red seaweed species from the Center of Portugal. J. Mar. Sci. Eng. 2022, 10, 1168. [Google Scholar] [CrossRef]
- Melo, R.; Sousa-Pinto, I.; Antunes, S.C.; Costa, I.; Borges, D. Temporal and spatial variation of seaweed biomass and assemblages in Northwest Portugal. J. Sea Res. 2021, 174, 102079. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Lesenfants, M.L.; Rosa, G.P.; Barreto, M.C.; Silva, A.M.S.; Seca, A.M.L. GC- and UHPLC-MS profiles as a tool to valorize the red alga Asparagopsis armata. Appl. Sci. 2022, 12, 892. [Google Scholar] [CrossRef]
- Bamaniya, P.K.; Joshi, N.H.; Tiwari, A.; Shaji, S. Seaweed-Classification, Source and Uses. In Agri-India Today (Montly e-Newsletter); Laha, S.K., Devi, M.S., Eds.; Darjeeling, Agri-India Today: Darjeeling, India, 2022; Volume 2, pp. 54–57. ISSN 2583-0910. [Google Scholar]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red seaweed pigments from a biotechnological perspective. Phycology 2022, 2, 1–29. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in In vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingrediente for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.M.; Petrovski, Ž. Highlighting the biological potential of the brown seaweed Fucus spiralis for skin applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Rabecca, R.; Doss, A.; Praveen, R.P.; Satheesh, S. Phytochemical and anti-inflammatory properties of green macroalga Codium tomentosum. Biocatal. Agric. Biotechnol. 2022, 45, 102492. [Google Scholar] [CrossRef]
- Inotai, A.; Hankó, B.; Mészáros, Á. Trends in the non-steroidal anti-inflammatory drugs market in six Central-Eastern European countries based on retail information. Pharmacoepidemiol. Drug Saf. 2010, 19, 183–190. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Conforti, A.; Cola, I.D.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Fernando, I.P.S.; Nah, J.-W.; Jeon, Y.-J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Will, C.L.S.E.P.; Gomes, C.A.J.; da Nascimento, S.M.; de Sousa, P.T.; de Quevedo, F.K.; Alves, L.G.; Soriano, E.M.; Araújo, R.M.; Leite, E.L. Effect of galactofucan sulfate of a Brown seaweed on induced hepatotoxicity in rats, sodium pentobarbital-induced sleep, and anti-inflammatory activity. J. Appl. Phycol. 2016, 28, 2005–2017. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agarth ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-κB signalling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Teichmann, A.T.; Wieland, F.H.; Wang, A.; Lei, X.; Zhu, Y.; Yin, J.; Fan, T.; Zhou, L.; et al. Development of a novel nitric oxide (NO) production inhibitor with potential therapeutic effect on chronic inflammation. Eur. J. Med. Chem. 2020, 193, 112216. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Heo, S.-J.; Jeon, Y.-J.; Lee, C.-M.; Park, Y.-M.; Byun, H.-G.; Choi, Y.H.; Park, S.-G.; Choi, I.-W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.H.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signalling. Biomed. Pharm. 2007, 6, 289–296. [Google Scholar] [CrossRef]
- Ah Jung, H.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Lee, S.-H.; Eom, S.-H.; Yoon, N.-Y.; Kim, M.-M.; Li, Y.-X.; Ha, S.-K.; Kim, S.-K. Fucofuroeckol-A from Eisenia bicyclis inhibits inflammation in lipopolysaccharide-induced mouse macrophages via downregulation of the MAPK/NF-κB signaling pathway. J. Chem. 2016, 2016, 6509212. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol B supresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.-K.; Lee, T.; Bae, J.-S. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 2188–2195. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, E.-H.; Park, K.; Kwon, J.-Y.; Kim, P.-H.; Jung, W.-K.; Kim, Y.-M. Eckol from Eisenia bicyclis inhibits inflammation through the AKT/NF-Κb signaling in Propionibacterium acnes-induced human keratinocyte HACAT cells. J. Food Biochem. 2016, 41, e12312. [Google Scholar] [CrossRef]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6’-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE(2) production and inflammatory cytokine expression in macrophages: The inhibition of NF-kB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally administered phlorotannins from Eisenia arborea supress chemical mediator release and cyclooxygenase-2 signaling to alleviate mouse ear swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, Z.; Mathema, V.B.; Chae, D.; Kang, H.-K.; Yoo, E.-S.; Koh, Y.S. Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-KB pathways. Biosci. Biotechnol. Biochem. 2013, 77, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, T.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of eckol and its derivatives. Biorg. Med. Chem. Lett. 2012, 22, 3710–3712. [Google Scholar] [CrossRef]
- Stout, E.P.; Kubanek, J. Marine macroalgal natural products. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands; Volume 2, Chapter 2.03; pp. 41–65. [CrossRef]
- Mandrekar, V.K.; Gawas, U.B.; Majik, M.S. Brominated molecules from marine algae and their pharmacological importance. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands; Volume 61, Chapter 13; pp. 461–490. [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Katsui, N.; Suzuki, Y.; Irie, T. 5,6-Dibromoprotocatechualdehyde and 2,3-dibromo-4,5-dihydroxybenzyl methyl ether: New dibromophenols from Rhodomela larix. Tetrahedrn 1967, 23, 1185–1188. [Google Scholar] [CrossRef]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine red alga Vidalia obtusiloba. Cell. Mol. Life Sci. 1991, 47, 851–853. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoon, W.J.; Kang, H.K.; Yoo, E.-S. Anti-inflammatory effect of 3-bromo-4,5-dihydroxybenzaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-Y.; Heo, S.-J.; Cho, S.-H.; Lee, W.W.; Kim, S.-Y.; Yang, H.-W.; Ahn, G.; Cha, S.-H.; Kwon, S.-H.; Jeong, M.S.; et al. 3-Bromo-5-(ethoxymethyl)-1,2-benzenediol inhibits LPS-induced pro-inflammatory responses by preventing ROS production and downregulating NF-κB in vitro and in a zebrafish model. Int. Immunopharmacol. 2019, 67, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Ye, B.-R.; Kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2017, 32, 2756–2765. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Novel furanyl derivatives from the red seaweed Gracilaria opuntia with pharmacological activities using different in vitro models. Med. Chem. Res. 2018, 27, 1245–1259. [Google Scholar] [CrossRef]
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2020, 34, 3470–3482. [Google Scholar] [CrossRef]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Algal terpenoids: A potential source of antioxidants for cancer therapy. In Terpenes and Terpenoids—Recent Advances; Perveen, S., Al-Taweel, A.M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Kang, M.-C.; Lee, W.-W.; Lee, H.-W.; Kamada, T.; Vairappan, C.S.; Jeon, Y.-J. 5β-Hydroxypalisadin B isolated from red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Kim, E.-A.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Vairappan, C.S.; Jeon, Y.-J. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef]
- Chatter, R.; Kladi, M.; Tarhouni, S.; Maatoug, R.; Kharrat, R.; Vagias, C.; Roussis, V. Neorogioltriol: A brominated diterpene with analgesic activity from Laurencia glandulifera. Phytochem. Lett. 2009, 2, 25–28. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and related diterpenes from the red alga Laurencia inhibit inflammatory bowel disease in mice by suppressing M1 and promoting M2-like macrophage responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. First report of antioxidant abeo-labdane type diterpenoid from intertidal red seaweed Gracilaria salicornia with 5-lipoxygenase inhibitory potential. Nat. Prod. Res. 2018, 34, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improve obesity-induced inflammation in a dipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef]
- Miyahita, K.; Beppu, F.; Hosokawa, M.; Wang, S. Nutraceutical characteristics of the Brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells Oxidative. Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial. Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217. [Google Scholar] [CrossRef]
- Rodriguez-Luna, A.; Avila-Roman, J. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Chengye, Z.; Daixing, Z.; Qiang, Z.; Shusheng, L. PGC-1-related coactivator (PRC) negatively regulates endothelial adhesion of monocytes via inhibition of NF-kB activity. Biochem. Biophys. Res. Commun. 2013, 439, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.C.; Chou, W.C.; Chu, P.M.; Hsieh, P.L.; Hung, C.H.; Tsai, K.L. Fucoxanthin protects against oxLDL-induced endothelial damage via activating the AMPK-Akt-CREB-PGC1alpha pathway. Mol. Nutr. Food Res. 2019, 63, e1801353. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammatory-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef]
- Wu, J.; Qin, Z.; Jiang, X.; Fang, D.; Lu, Z.; Zheng, L.; Zhao, J. ROS-responsive PPGF nanofiber membrane as a drug delivery system for long-term drug release in attenuation of osteoarthritis. NPJ Regen. Med. 2022, 7, 66. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]
- Hannana, M.A.; Sohag, A.; Al, M.; Dasha, R.; Haque, M.N.; Mohibbullahd, M.; Oktaviania, D.F.; Hossainb, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol isolated from dietary brown alga Sargassum horneri protects TNF-α/IFN-γ-stimulated human dermal fibroblasts via regulating Nrf2/HO-1 and NF-κB/MAPK pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The Protective Effect of Mycosporine-Like Amino Acids (MAAs) from Porphyra yezoensis in a Mouse Model of UV Irradiation-Induced Photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protect cobalt chloride induced inflammatory by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 2018, 2824139. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 171, 150–158. [Google Scholar] [CrossRef]
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef]
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine natural product bis-indole alkaloid caulerpin: Chemistry and biology, Mini-Rev. Med. Chem. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.C.; de Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; de Miranda, G.E.C.; de Araújo-Júnior, J.X.; Chaves, M.C.; et al. The antinociceptive and anti-Inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689. [Google Scholar] [CrossRef] [PubMed]
- Lucena, A.M.M.; Souza, C.R.M.; Jales, J.T.; Guedes, P.M.M.; de Miranda, G.E.C.; de Moura, A.M.A.; Araújo-Júnior, J.X.; Nascimento, G.J.; Scortecci, K.C.; Santos, B.V.O.; et al. The bisindole alkaloid caulerpin, from seaweeds of the genus Caulerpa, attenuated colon damage in murine colitis model. Mar. Drugs 2018, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.-S. Omega-3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochim 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.T.; Helsper, J.P.F.G.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Jaswir, I.; Hammed, A.M. Anti-inflammatory compounds of macroalgae origin: A review. J. Med. Plants Res. 2011, 5, 7146–7154. [Google Scholar] [CrossRef]
- Arita, M.; Clish, C.B.; Serhan, C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: Novel oxygenase products from x-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005, 338, 149–157. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Chang, W.-C.; So, J.; Lamon-Fava, S. Differential and shared effects of eicosapentaenoic acid and docosahexaenoic acid on serum metabolome in subjects with chronic inflammation. Sci. Rep. 2021, 11, 16324. [Google Scholar] [CrossRef]
- Lee, H.-J.; Dang, H.-T.; Kang, G.-J.; Yang, E.-J.; Park, S.-S.; Yoon, W.-J.; Jung, J.H.; Kang, H.-K.; Yoo, E.-S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NFκB and STAT1 activity in lipopolysaccharide-stimulated RAW 264.7 Cells. Arch. Pharm. Res. 2009, 32, 453–462. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Manickam, S.; Chang, J.S.; Show, P.L. Emerging algal nanotechnology for high-value compounds: A direction to future food production. Trends Food Sci. Technol. 2021, 116, 290–302. [Google Scholar] [CrossRef]
- Seaweed Market by Product (Red, Brown, and Green) and Application (Human Food, Hydrocolloids, Fertilizers, Animal Feed Additives, and Others)—Global Opportunity Analysis and Industry Forecast, 2018–2024, Report Code: A04296. Available online: https://www.alliedmarketresearch.com/seaweed-market (accessed on 9 December 2022).
- Kite-Powell, J. See How Algae Could Change Our World. Forbes 2018. Available online: https://www.forbes.com (accessed on 9 December 2022).
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://activheal.com/wound-care-dressing-range/hydrogel-dressing/ (accessed on 9 December 2022).
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Russo, D.; Penna, I.; Ceseracciu, L.; Palazon, F.; Scarpellini, A.; Cingolani, R.; Bertorelli, R.; Bayer, I.S.; Heredia-Guerrero, J.A.; et al. Facile production of seaweed-based biomaterials with antioxidant and anti-inflammatory activities. Algal Res. 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. App. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garci-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Mar. Drugs 2022, 20, 789. https://doi.org/10.3390/md20120789
Rocha DHA, Pinto DCGA, Silva AMS. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Marine Drugs. 2022; 20(12):789. https://doi.org/10.3390/md20120789
Chicago/Turabian StyleRocha, Djenisa H. A., Diana C. G. A. Pinto, and Artur M. S. Silva. 2022. "Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits" Marine Drugs 20, no. 12: 789. https://doi.org/10.3390/md20120789
APA StyleRocha, D. H. A., Pinto, D. C. G. A., & Silva, A. M. S. (2022). Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Marine Drugs, 20(12), 789. https://doi.org/10.3390/md20120789








