Sulfated Polysaccharides from Seaweed Strandings as Renewable Source for Potential Antivirals against Herpes simplex Virus 1
Abstract
:1. Introduction
2. Results
2.1. Enzyme-Assisted Extraction Increased the SPs Yields
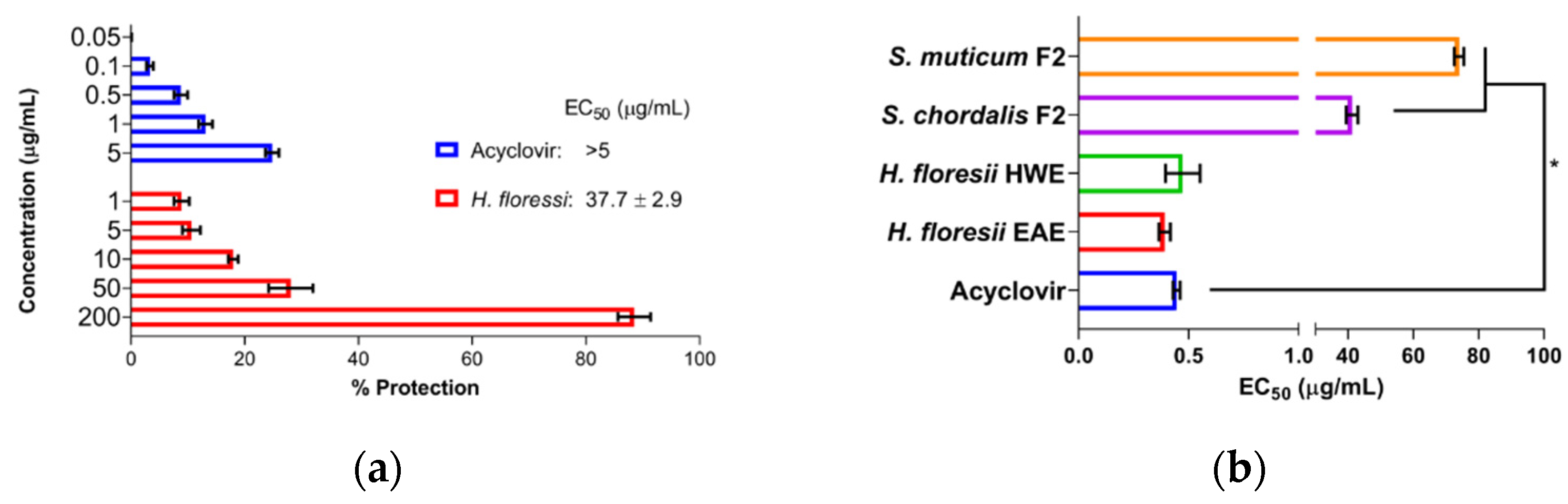
2.2. Screening for Antiviral Activity and Cytotoxicity of Sulfated Polysaccharides
2.3. Antiviral Activity at Different Treatment Schemes from Selected Polysaccharides
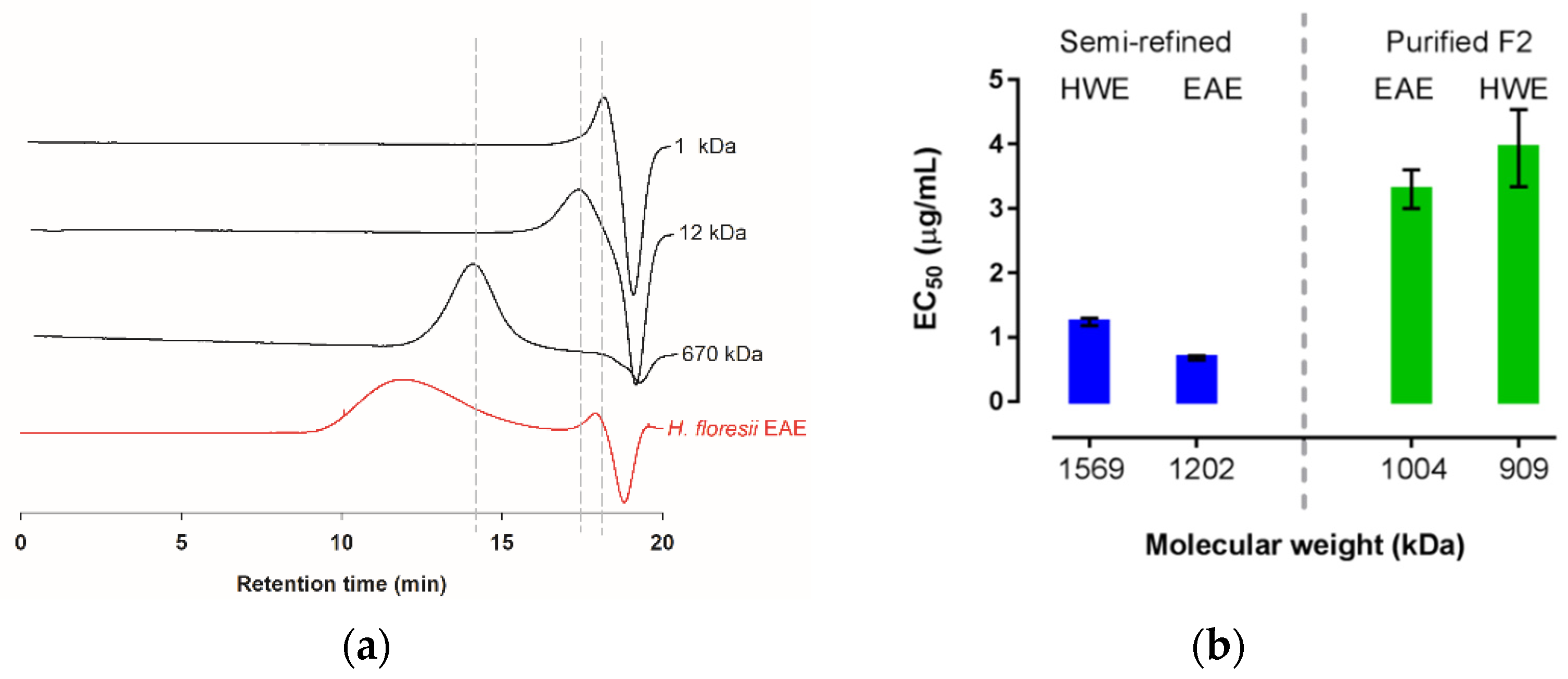
2.4. Biochemical Composition of Purified Polysaccharides
2.5. Preliminary Characterization and Molecular Mass of H. floresii Polysaccharides
3. Discussion
3.1. The Enzyme-Assisted Extraction Produced Higher Yields of Polysaccharides
3.2. Antiviral Activity of Sulfated Polysaccharides
3.3. Carrageenan from Halymenia floresii
3.4. Seaweed Stranding as Renewable Source for Potential Antivirals Polysaccharides
4. Materials and Methods
4.1. Algal Material
4.2. Extraction of Sulfated Polysaccharides
4.3. Purification of Semi-Refined Sulfated Polysaccharides (sr-SPs)
4.4. Screening for Antiviral Activity In Vitro and Cytotoxicity of Algal Polysaccharides
4.5. Antiviral Activity at Different Treatment Schemes from Selected Polysaccharides
4.6. Biochemical Composition of Polysaccharides
4.7. Characterization of H. floresii Polysaccharide
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, P.; Segondy, M.; Foulongne, V.; Ouedraogo, A.; Konate, I.; Huraux, J.-M.; Mayaud, P.; Nagot, N. Herpes Simplex Virus and HIV-1: Deciphering Viral Synergy. Lancet Infect. Dis. 2008, 8, 490–497. [Google Scholar] [CrossRef]
- Agelidis, A.M.; Shukla, D. Cell Entry Mechanisms of HSV: What We Have Learned in Recent Years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Chiu, L.C.M.; Ooi, V.E.C.; Chan, P.K.S.; Ang, P.O. Antiviral Property and Mode of Action of a Sulphated Polysaccharide from Sargassum Patens against Herpes Simplex Virus Type 2. Int. J. Antimicrob. Agents 2004, 24, 279–283. [Google Scholar] [CrossRef]
- Itzhaki, R.F. Overwhelming Evidence for a Major Role for Herpes Simplex Virus Type 1 (HSV1) in Alzheimer’s Disease (AD); Underwhelming Evidence Against. Vaccines 2021, 9, 679. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Lin, W.-R.; Shang, D.; Wilcock, G.K.; Faragher, B.; Jamieson, G.A. Herpes Simplex Virus Type 1 in Brain and Risk of Alzheimer’s Disease. Lancet 1997, 349, 241–244. [Google Scholar] [CrossRef]
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A.; Henríquez, A.; Agurto-Muñoz, C.; González, P.A. Current Antivirals and Novel Botanical Molecules Interfering With Herpes Simplex Virus Infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral Resistance in Herpes Simplex Virus and Varicella-Zoster Virus Infections: Diagnosis and Management. Curr. Opin. Infect. Dis. 2016, 29, 654–662. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Chapter 8 - Carbohydrates From Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, 2016; pp. 223–274. ISBN 978-0-12-802772-1. [Google Scholar]
- Terme, N.; Hardouin, K.; Cortès, H.P.; Peñuela, A.; Freile-Pelegrín, Y.; Robledo, D.; Bedoux, G.; Bourgougnon, N. Chapter 10 - Emerging Seaweed Extraction Techniques: Enzyme-Assisted Extraction a Key Step of Seaweed Biorefinery. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Advances in Geen and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–256. ISBN 978-0-12-817943-7. [Google Scholar]
- Bourgougnon, N.; Lahaye, M.; Quemener, B.; Chermann, J.-C.; Rimbert, M.; Cormaci, M.; Furnari, G.; Kornprobst, J.-M. Annual Variation in Composition Andin Vitro Anti-HIV-1 Activity of the Sulfated Glucuronogalactan FromSchizymenia Dubyi (Rhodophyta, Gigartinales). J. Appl. Phycol. 1996, 8, 155–161. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.A.; Córdoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomón, H.; Turk, G. Antiretroviral Activity of Fucoidans Extracted from the Brown Seaweed Adenocystis Utricularis. Phytother. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV Activity of Fucoidans from Three Brown Seaweed Species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.P.; Keller, M.J.; Gradissimo, A.; Chen, Z.; Morgan, S.L.; Xue, X.; Strickler, H.D.; Fernández-Romero, J.A.; Burk, R.; Einstein, M.H. In Vitro Inhibition of Human Papillomavirus Following Use of a Carrageenan-Containing Vaginal Gel. Gynecol. Oncol. 2016, 143, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Bourgougnon, N.; Lahaye, M.; Chermann, J.-C.; Kornprobst, J.-M. Composition and Antiviral Activities of a Sulfated Polysaccharide from Schizymenia Dubyi (Rhodophyta, Gigartinales). Bioorg. Med. Chem. Lett. 1993, 3, 1141–1146. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus Coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella Thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Hardouin, K.; Burlot, A.-S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and Antiviral Activities of Enzymatic Hydrolysates from Different Invasive French Seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-Assisted Extraction of Bioactive Material from Chondrus Crispus and Codium Fragile and Its Effect on Herpes Simplex Virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef] [Green Version]
- Anne-Sophie, B.; Gilles, B. Response Surface Methodology for Enzyme-Assisted Extraction of Water- Soluble Antiviral Compounds from the Proliferative Macroalga Solieria Chordalis. Enz. Eng. 2016, 5, 1000148. [Google Scholar] [CrossRef] [Green Version]
- Bedoux, G.; Caamal-Fuentes, E.; Boulho, R.; Marty, C.; Bourgougnon, N.; Freile-Pelegrín, Y.; Robledo, D. Antiviral and Cytotoxic Activities of Polysaccharides Extracted from Four Tropical Seaweed Species. Nat. Prod. Commun. 2017, 12, 1934578X1701200602. [Google Scholar] [CrossRef] [Green Version]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) Activity of Carrageenans from the Red Seaweed Solieria Chordalis (Rhodophyta, Gigartinales) Extracted by Microwave-Assisted Extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An Analysis of the Nutritional and Health Values of Caulerpa Racemosa (Forsskål) and Ulva Fasciata (Delile)—Two Chlorophyta Collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef] [PubMed]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural Analysis and Antiviral Activity of a Sulfated Galactan from the Red Seaweed Schizymenia Binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and Anticoagulant Properties of Carrageenans from the Red Seaweed Gigartina Skottsbergii and Their Cyclized Derivatives: Correlation between Structure and Biological Activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from Chilean Samples of Stenogramme Interrupta (Phyllophoraceae): Structural Analysis and Biological Activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-Herpes Simplex Virus Activity of Sulfated Galactans from the Red Seaweeds Gymnogongrus Griffithsiae and Cryptonemia Crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal Activity of Polysaccharide Extracts from Four Algal Species against Herpes Simplex Virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Navid, M.H.; Bandyopadhyay, S.S.; Schnitzler, P.; Ray, B. Sulfated Polysaccharides from Laminaria Angustata: Structural Features and in Vitro Antiviral Activities. Carbohydr. Polym. 2012, 87, 123–130. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on Antivirally Active Sulfated Polysaccharides: From Structure–Activity Analysis to Clinical Evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-Assisted Extraction (EAE) for the Production of Antiviral and Antioxidant Extracts from the Green Seaweed Ulva Armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic Activity and Mode of Action of Natural Carrageenans of Diverse Structural Types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- González, M.E.; Alarcón, B.; Carrasco, L. Polysaccharides as Antiviral Agents: Antiviral Activity of Carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.-B.; Sankawa, U. An Antivirally Active Sulfated Polysaccharide from Sargassum Horneri (TURNER) C.AGARDH. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, K.; Mateu, C.G.; Mandal, P.; Pujol, C.A.; Damonte, E.B.; Ray, B. Galactan Sulfate of Grateloupia Indica: Isolation, Structural Features and Antiviral Activity. Phytochemistry 2007, 68, 1428–1435. [Google Scholar] [CrossRef]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus Evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [Green Version]
- Michel, G.; Nyval-Collen, P.; Barbeyron, T.; Czjzek, M.; Helbert, W. Bioconversion of Red Seaweed Galactans: A Focus on Bacterial Agarases and Carrageenases. Appl. Microbiol. Biotechnol. 2006, 71, 23–33. [Google Scholar] [CrossRef]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Robledo, D.; Freile-Pelegrín, Y. Prospects for the Cultivation of Economically Important Carrageenophytes in Southeast Mexico. J. Appl. Phycol. 2011, 23, 415–419. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Azamar, J.A.; Robledo, D. Preliminary Characterization of Carrageenan from the Red Seaweed Halymenia Floresii. J. Aquat. Food Prod. Technol. 2011, 20, 73–83. [Google Scholar] [CrossRef]
- Stortz, C.A.; Cerezo, A.S. The Systems of Carrageenans from Cystocarpic and Tetrasporic Stages from Iridaea Undulosa: Fractionation with Potassium Chloride and Methylation Analysis of the Fractions. Carbohydr. Res. 1993, 242, 217–227. [Google Scholar] [CrossRef]
- Guibet, M.; Kervarec, N.; Génicot, S.; Chevolot, Y.; Helbert, W. Complete Assignment of 1H and 13C NMR Spectra of Gigartina Skottsbergii λ-Carrageenan Using Carrabiose Oligosaccharides Prepared by Enzymatic Hydrolysis. Carbohydr. Res. 2006, 341, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Guibet, M.; Colin, S.; Barbeyron, T.; Genicot, S.; Kloareg, B.; Michel, G.; Helbert, W. Degradation of λ-Carrageenan by Pseudoalteromonas Carrageenovora λ-Carrageenase: A New Family of Glycoside Hydrolases Unrelated to κ- and ι-Carrageenases. Biochem. J. 2007, 404, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus Distichus Subsp. Evanescens and Saccharina Latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva Sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A Review about Brown Algal Cell Walls and Fucose-Containing Sulfated Polysaccharides: Cell Wall Context, Biomedical Properties and Key Research Challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the Antiviral Activities and Mechanisms of Marine Polysaccharides from Seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An Overview of Their Most Important Sourcing and Applications as Natural Polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef] [Green Version]
- Peñuela, A.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomás, M.-S.; Yolanda, F.-P. Anti-Herpes Simplex Virus (HSV-1) Activity and Antioxidant Capacity of Carrageenan-Rich Enzymatic Extracts from Solieria Filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar] [CrossRef]
- Spivack, J.G.; Prusoff, W.H.; Tritton, T.R. A Study of the Antiviral Mechanism of Action of 2-Deoxy-d-Glucose: Normally Glycosylated Proteins Are Not Strictly Required for Herpes Simplex Virus Attachment but Increase Viral Penetration and Infectivity. Virology 1982, 123, 123–138. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, V. One-Pot Gram-Scale Synthesis of Virucidal Heparin-Mimicking Polymers as HSV-1 Inhibitors. ChemComm 2021, 57, 11948. [Google Scholar] [CrossRef] [PubMed]
- Montanha, J.; Bourgougnon, N.; Boustie, J.; Amoros, M. Antiviral Activity of Carrageenans from Marine Red Algae. Lat. Am. J. Pharm. 2009, 28, 443–448. [Google Scholar]
- Copeland, R.; Balasubramaniam, A.; Tiwari, V.; Zhang, F.; Bridges, A.; Linhardt, R.J.; Shukla, D.; Liu, J. Using a 3-O-Sulfated Heparin Octasaccharide To Inhibit the Entry of Herpes Simplex Virus Type 1. Biochemistry 2008, 47, 5774–5783. [Google Scholar] [CrossRef] [Green Version]
- Mese, K.; Bunz, O.; Volkwein, W.; Vemulapalli, S.P.B.; Zhang, W.; Schellhorn, S.; Heenemann, K.; Rueckner, A.; Sing, A.; Vahlenkamp, T.W.; et al. Enhanced Antiviral Function of Magnesium Chloride-Modified Heparin on a Broad Spectrum of Viruses. Int. J. Mol. Sci. 2021, 22, 10075. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Delattre, C.; Laroche, C.; Wadouachi, A.; Dulong, V.; Picton, L.; Andriamadio, P.; Michaud, P. Highly Sulphated Galactan from Halymenia Durvillei (Halymeniales, Rhodophyta), a Red Seaweed of Madagascar Marine Coasts. Int. J. Biol. Macromol. 2009, 45, 140–145. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular Weight Distribution of Polysaccharides from Edible Seaweeds by High-Performance Size-Exclusion Chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 1000. [Google Scholar] [CrossRef]
- Pliego-Cortés, H. Aquaculture and Antioxidant Capacity of Rhodymenia pseudopalmata (Rhodophyta) in an IMTA System. Ph.D. Thesis, CINVESTAV, Mérida, Mexico, 2017. [Google Scholar]
- Peñuela, A.; Robledo, D.; Bourgougnon, N.; Bedoux, G.; Hernández-Núñez, E.; Freile-Pelegrín, Y. Environmentally Friendly Valorization of Solieria Filiformis (Gigartinales, Rhodophyta) from IMTA Using a Biorefinery Concept. Mar. Drugs 2018, 16, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgougnon, N.; Burlot, A.-S.; Jacquin, A.-G. Chapter Five—Algae for Global Sustainability? In Advances in Botanical Research; Jacquot, J.-P., Ed.; Past, Current and Future Topics; Academic Press: Cambridge, MA, USA, 2021; Volume 100, pp. 145–212. [Google Scholar]
- Langlois, M.; Allard, J.P.; Nugier, F.; Aymard, M. A Rapid and Automated Colorimetric Assay for Evaluating the Sensitivity of Herpes Simplex Strains to Antiviral Drugs. J. Biol. Stand. 1986, 14, 201–211. [Google Scholar] [CrossRef]
- Damonte, E.; Neyts, J.; Pujol, C.A.; Snoeck, R.; Andrei, G.; Ikeda, S.; Witvrouw, M.; Reymen, D.; Haines, H.; Matulewicz, M.C.; et al. Antiviral Activity of a Sulphated Polysaccharide from the Red Seaweed Nothogenia Fastigiata. Biochem. Pharmacol. 1994, 47, 2187–2192. [Google Scholar] [CrossRef]
- Krylova, N.V.; Silchenko, A.S.; Pott, A.B.; Ermakova, S.P.; Iunikhina, O.V.; Rasin, A.B.; Kompanets, G.G.; Likhatskaya, G.N.; Shchelkanov, M.Y. In Vitro Anti-Orthohantavirus Activity of the High-and Low-Molecular-Weight Fractions of Fucoidan from the Brown Alga Fucus Evanescens. Mar. Drugs 2021, 19, 577. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jaques, L.B.; Wollin, A. A Modified Method for the Colorimetric Determination of Heparin. Can. J. Physiol. Pharmacol. 1967, 45, 787–794. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress Tolerance and Photoadaptation to Solar Radiation in Rhodymenia Pseudopalmata (Rhodophyta) through Mycosporine-like Amino Acids, Phenolic Compounds, and Pigments in an Integrated Multi-Trophic Aquaculture System. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Cerar, J.; Dogsa, I.; Jamnik, A.; Tomšič, M. Physicochemical Data on Aqueous Polymeric Systems of Methyl Cellulose and Lambda- and Kappa-Carrageenan: SAXS, Rheological, Densitometry, and Sound Velocity Measurements. Data Brief 2017, 15, 427–438. [Google Scholar] [CrossRef]






| Assay | H. floresii EAE | H. floresii HWE | S. chordalis F2 | S. muticum F2 | Acyclovir (Control) |
|---|---|---|---|---|---|
| Virus adsorption | |||||
| TA | 0.38 ± 0.06 | 0.60 ± 0.01 | 56.7 ± 2.3 * | 83.6 ± 3.3 * | 0.42 ± 0.04 |
| TB | 1.76 ± 0.2 | 7.65 ± 0.4 | 181 ± 9.1 | 143 ± 4.5 | 0.38 ± 0.04 * |
| TC | 0.18 ± 0.02 | 0.74 ± 0.03 * | 31.5 ± 3.2 * | 54.8 ± 4.6 * | 0.09 ± 0.03 |
| Post-infection | |||||
| 0 h | 1.28 ± 0.4 | 4.14 ± 0.4 | 46.5 ± 4.8 | 36.5 ± 0.9 | 0.31 ± 0.08 * |
| 1 h | 2.47 ± 0.1 | 4.62 ± 0.03 | 83.7 ± 2.2 | 57.0 ± 2.3 | 0.24 ± 0.02 * |
| 2 h | 6.59 ± 0.4 | 5.02 ± 0.1 | >200 | 75.5 ± 2.6 | 0.73 ± 0.01 * |
| 3 h | 5.42 ± 0.05 | 5.25 ± 0.5 | >200 | 102 ± 5.4 | 1.70 ± 0.3 * |
| 5 h | 19.1 ± 0.6 | 22.8 ± 2.07 | >200 | >200 | 0.83 ± 0.1 * |
| Species | Neutral Sugars | Sulfate Groups | Uronic Acids | Protein | 3,6-AG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EAE | HWE | EAE | HWE | EAE | HWE | EAE | HWE | EAE | HWE | |
| H. floresii | 36.9 ± 0.04 a | 37.0 ± 0.05 a | 9.4 ± 0.1a | 10.1 ± 0.02 b | 3.16 ± 0.05 a | 3.13 ± 0.04 a | 1.18 ± 0.03 a | 1.12 ± 0.03 a | 0.6 ± 0.04 a | 0.5 ± 0.02 a |
| S. chordalis | 22.8 ± 0.7 a | 23.7 ± 0.5 a | 15.4 ± 0.2a | 13.5 ± 0.4 b | 7.6 ± 0.1a | 6.7 ± 0.09 b | 7.5 ± 0.1 a | 6.2 ± 0.02 b | 8.4 ± 0.3 a | 8.8 ± 0.08 a |
| Ulva sp. | 23.1 ± 0.8 a | 28.4 ± 0.5 b | 5.9 ± 0.1a | 5.7 ± 0.1 a | 17.5 ± 0.4 a | 17.1 ± 0.02 a | 5.0 ± 0.03 a | 3.5 ± 0.3 b | ND | ND |
| S. muticum | 25.0 ± 0.8 a | 27.0 ± 0.8 a | 12.9 ± 0.08 a | 11 ± 0.4 b | 15.6 ± 0.7 a | 16.1 ± 0.5 a | 6.0 ± 0.05 a | 3.1 ± 0.07 b | ND | ND |
| Sample | Carbohydrates | Sulfate Groups | Uronic Acids | Protein | 3,6-AG |
|---|---|---|---|---|---|
| EAE | 24.7 ± 1.6 | 17.2 ± 1.9 | 1.9 ± 0.25 | 2.7 ± 0.3 | 1.0 ± 0.02 |
| HWE | 24.9 ± 2.1 | 18.6 ± 2.3 | 2.1 ± 0.3 | 2.9 ± 0.2 | 1.2 ± 0.01 |
| Sample | AMw (kDa) 1 | MW (kDa) 2 |
|---|---|---|
| EAE | 1202.8 ± 60.1 | 4.4 ± 0.6 3 |
| HWE | 1569.8 ± 40.01 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pliego-Cortés, H.; Hardouin, K.; Bedoux, G.; Marty, C.; Cérantola, S.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N. Sulfated Polysaccharides from Seaweed Strandings as Renewable Source for Potential Antivirals against Herpes simplex Virus 1. Mar. Drugs 2022, 20, 116. https://doi.org/10.3390/md20020116
Pliego-Cortés H, Hardouin K, Bedoux G, Marty C, Cérantola S, Freile-Pelegrín Y, Robledo D, Bourgougnon N. Sulfated Polysaccharides from Seaweed Strandings as Renewable Source for Potential Antivirals against Herpes simplex Virus 1. Marine Drugs. 2022; 20(2):116. https://doi.org/10.3390/md20020116
Chicago/Turabian StylePliego-Cortés, Hugo, Kévin Hardouin, Gilles Bedoux, Christel Marty, Stéphane Cérantola, Yolanda Freile-Pelegrín, Daniel Robledo, and Nathalie Bourgougnon. 2022. "Sulfated Polysaccharides from Seaweed Strandings as Renewable Source for Potential Antivirals against Herpes simplex Virus 1" Marine Drugs 20, no. 2: 116. https://doi.org/10.3390/md20020116