AsKC11, a Kunitz Peptide from Anemonia sulcata, Is a Novel Activator of G Protein-Coupled Inward-Rectifier Potassium Channels
Abstract
:1. Introduction
2. Results
2.1. Enhancement of K+ Currents through GIRK1/2 Channels by the Venom Fraction from Anemonia Sulcata
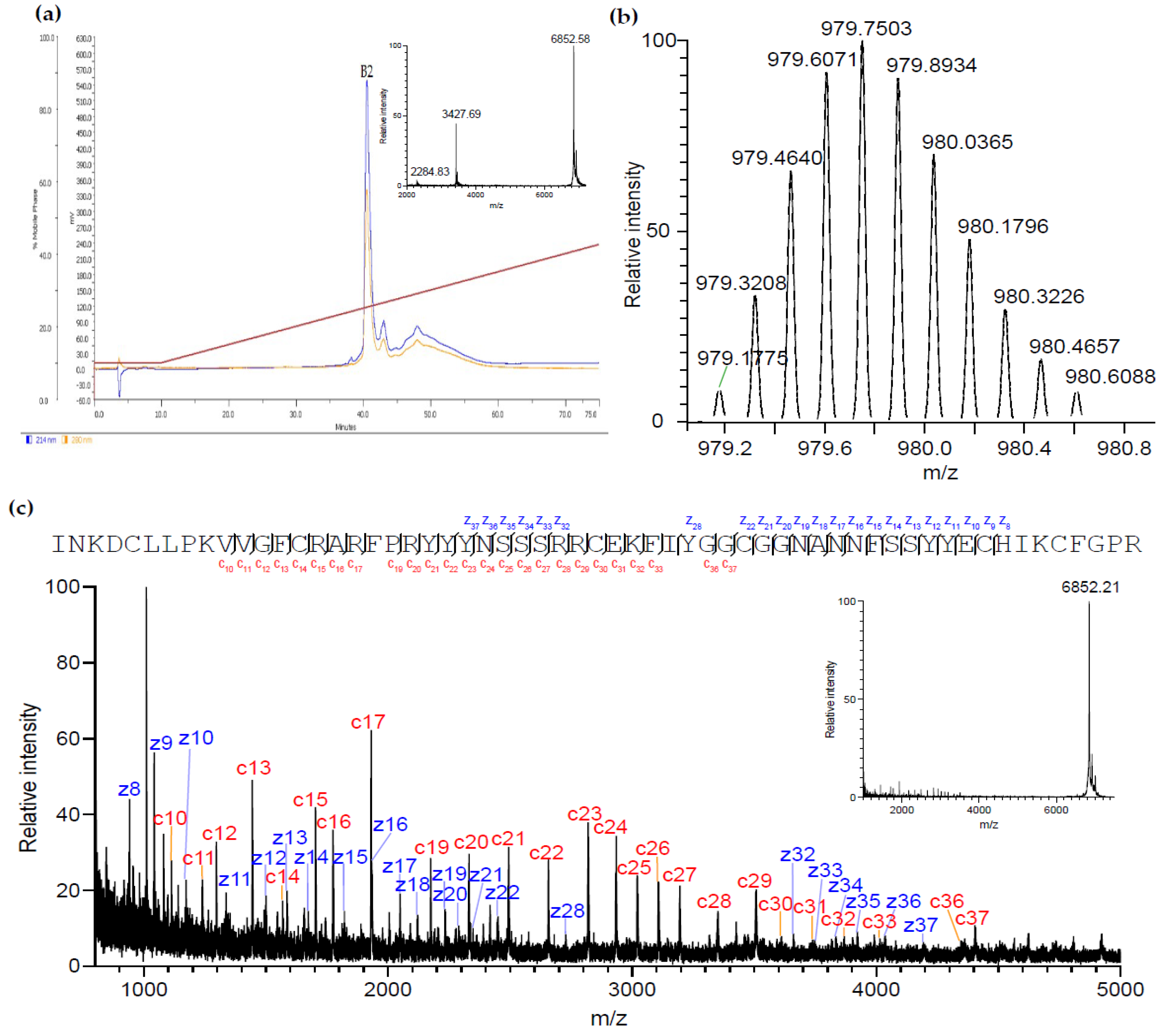
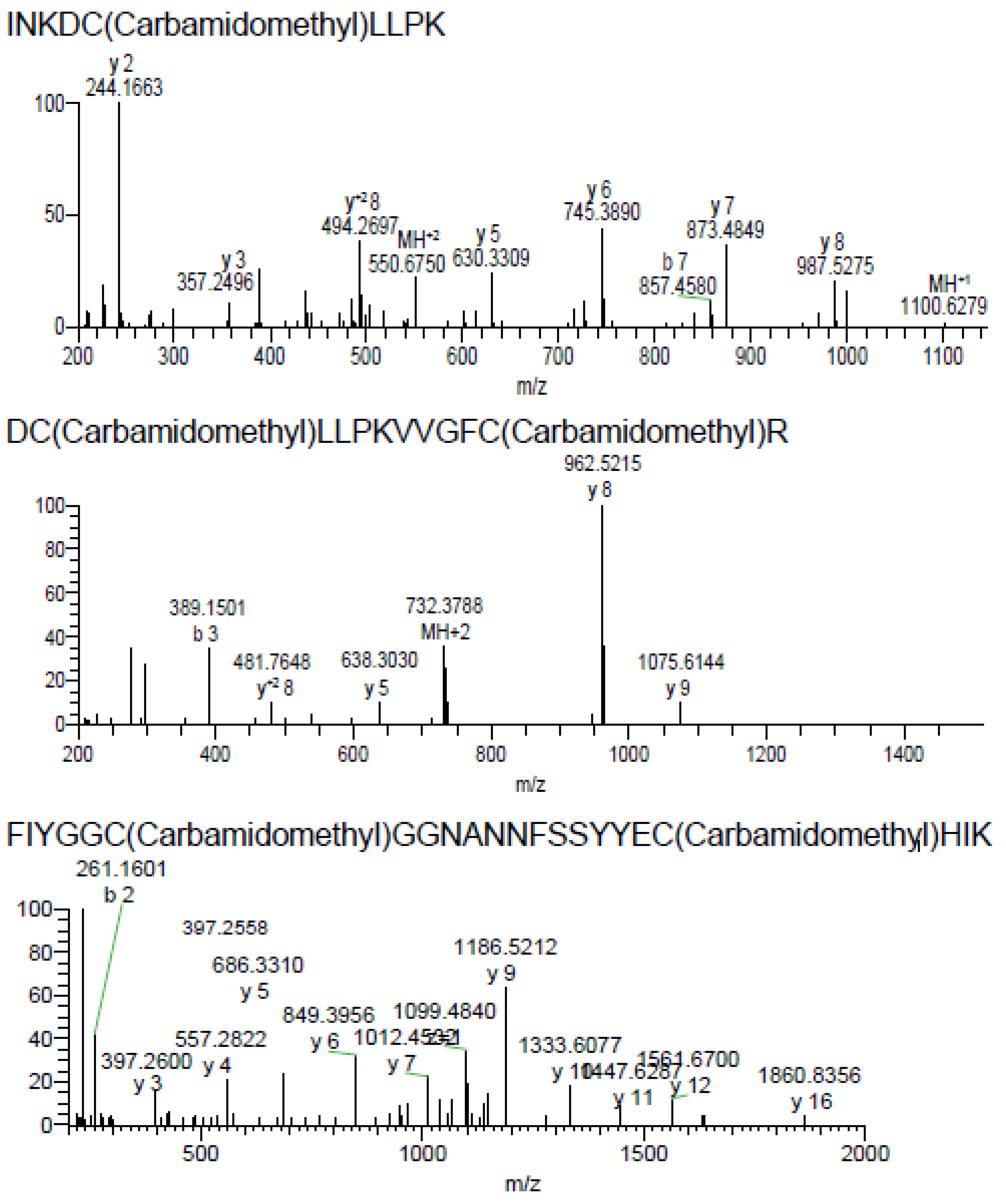
2.2. Purification and Identification of AsKC11 from the Active Venom Fraction
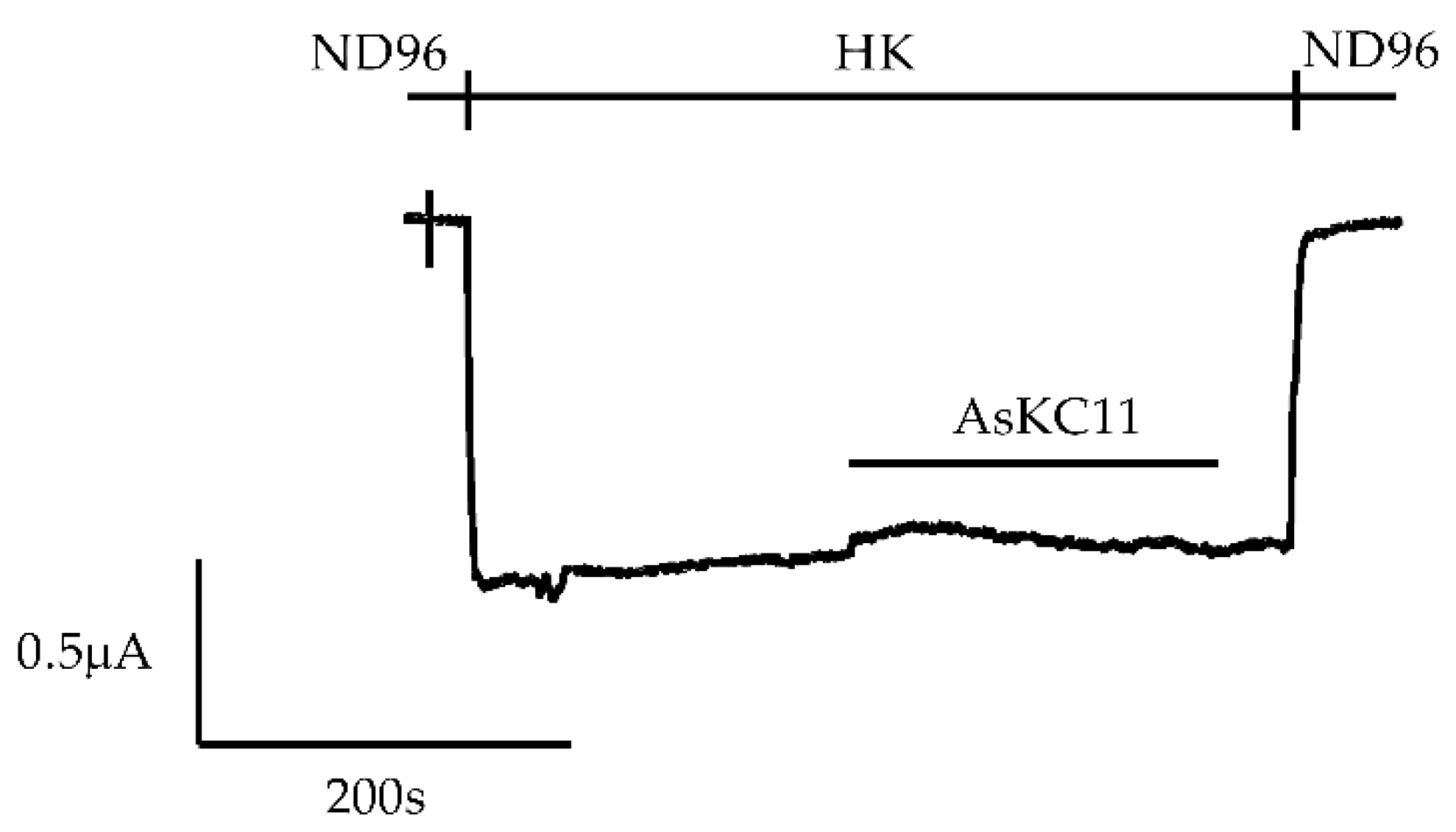
2.3. Potency of AsKC11 at Enhancing GIRK1/2 Channel-Carried Inward K+ Currents
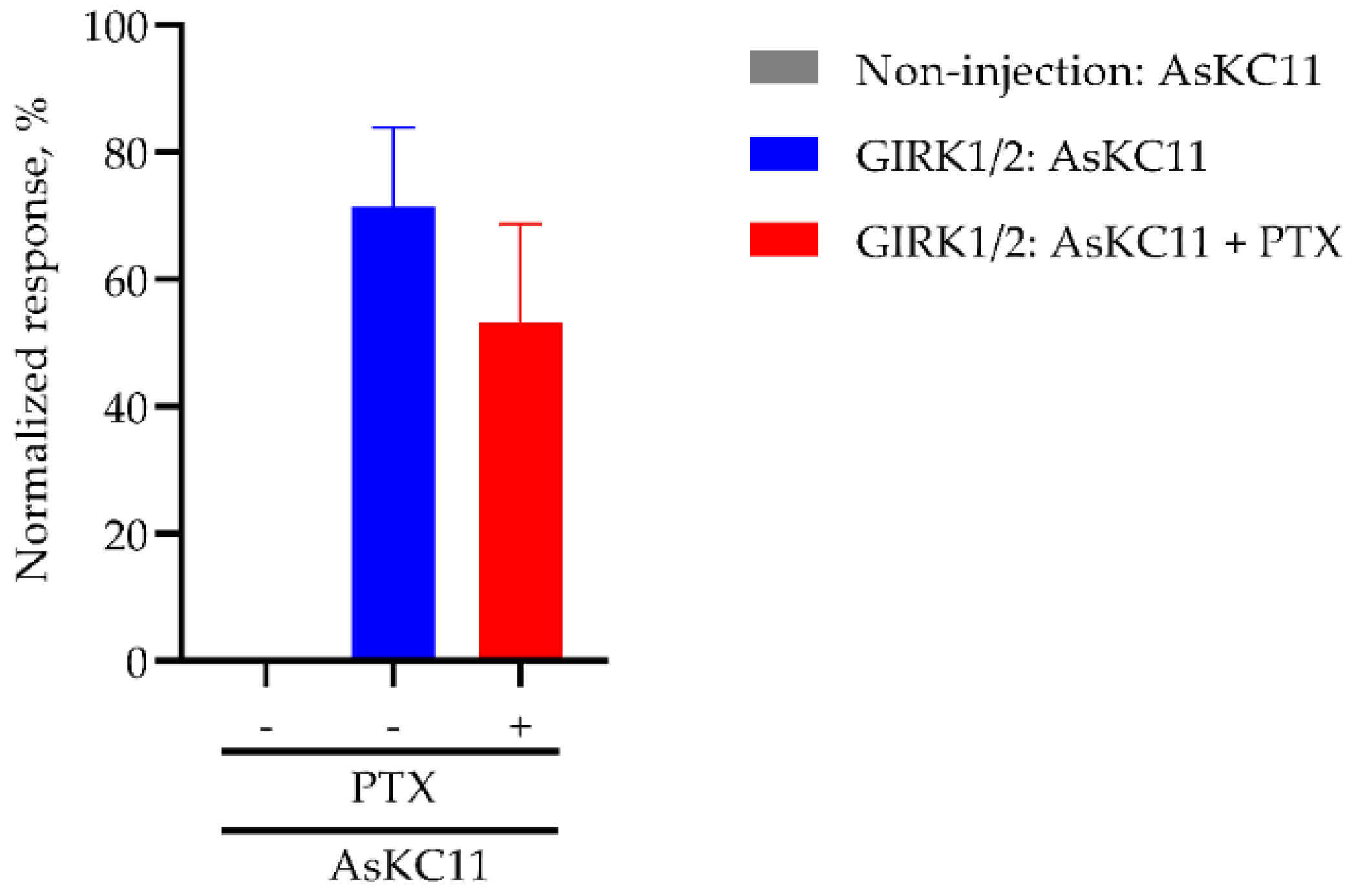
2.4. AsKC11-Evoked Activation of GIRK1/2 Channels Independent from Gi/o
2.5. Affinity of AsKC11 to GIRK1/2 Channels
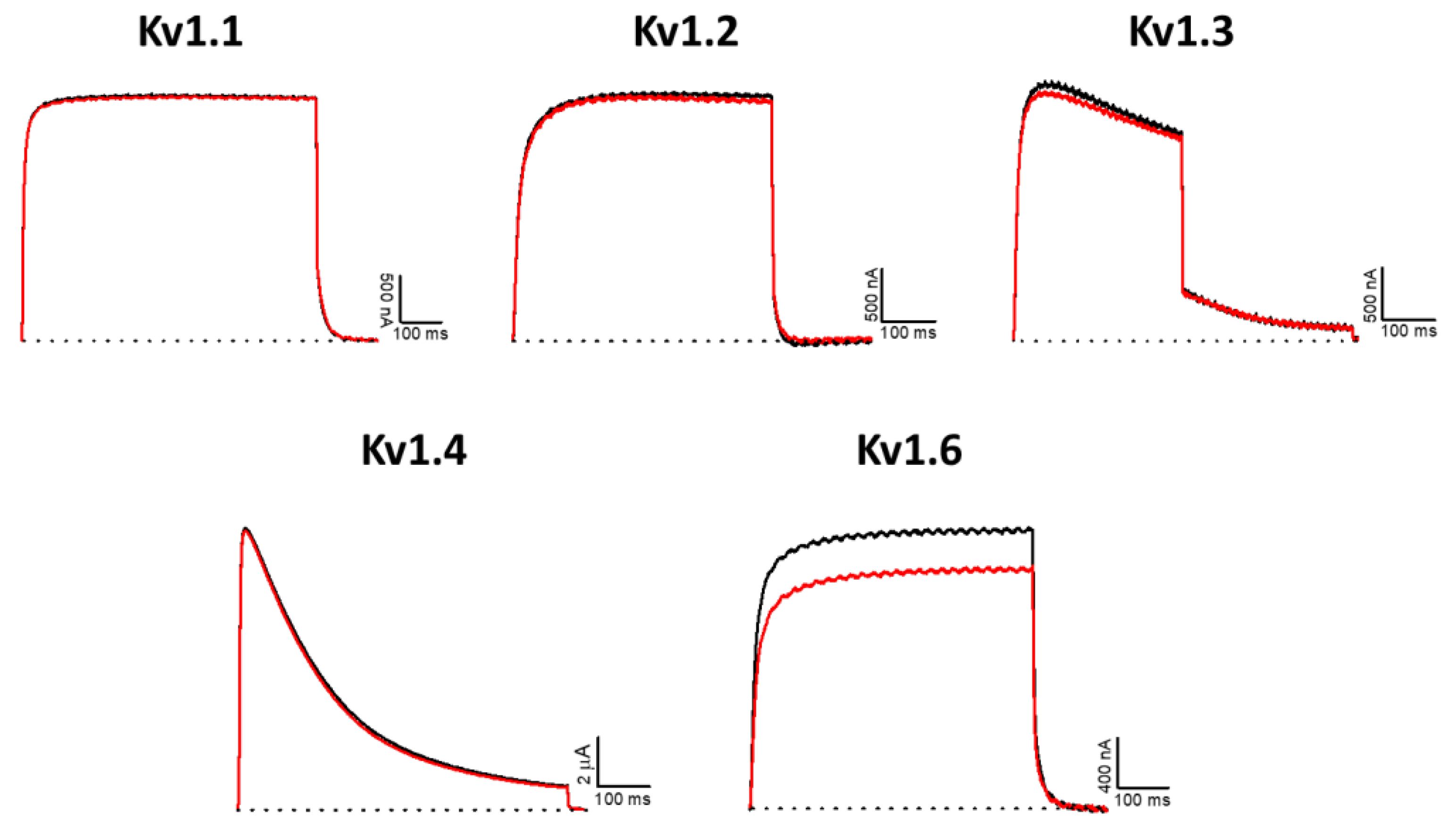
2.6. Effect of AsKC11 on Different Potassium Ion Channels
3. Discussion
4. Materials and Methods
4.1. AsKC11 Identification
4.2. Recombinant Synthesis of rAsKC11
4.3. Oocyte Preparation
4.4. Electrophysiological Measurements
4.5. Data Analysis

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeremic, D.; Sanchez-Rodriguez, I.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Therapeutic Potential of Targeting G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in the Central Nervous System. Pharmacol. Ther. 2021, 223, 107808. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.; Romaine, I.; Days, E.; Pascual, C.; Malik, A.; Yang, L.; Zou, B.; Du, Y.; Sliwoski, G.; Morrison, R.D.; et al. ML297 (VU0456810), the First Potent and Selective Activator of the GIRK Potassium Channel, Displays Antiepileptic Properties in Mice. ACS Chem. Neurosci. 2013, 4, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.D.; Denton, J.S. Next-Generation Inward Rectifier Potassium Channel Modulators: Discovery and Molecular Pharmacology. Am. J. Physiol.-Cell Physiol. 2021, 320, C1125–C1140. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Tao, X.; Touhara, K.K.; Mackinnon, R. Cryo-Em Analysis of Pip2 Regulation in Mammalian Girk Channels. eLife 2020, 9, e60552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gameiro-Ros, I.; Glaaser, I.W.; Slesinger, P.A. Advances in Targeting GIRK Channels in Disease. Trends Pharmacol. Sci. 2021, 42, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Cantwell, L.; Zorn, A.; Logothetis, D.E. Kir Channel Molecular Physiology, Pharmacology, and Therapeutic Implications. Handb. Exp. Pharmacol. 2021, 267, 277–356. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Tytgat, J. WIN55, 212-2, a Dual Modulator of Cannabinoid Receptors and G Protein-Coupled Inward Rectifier Potassium Channels. Biomedicines 2021, 9, 484. [Google Scholar] [CrossRef]
- Lüscher, C.; Slesinger, P.A. Emerging Roles for G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in Health and Disease. Nat. Rev. Neurosci. 2010, 11, 301–315. [Google Scholar] [CrossRef]
- Alfaro-Ruiz, R.; Martín-Belmonte, A.; Aguado, C.; Hernández, F.; Moreno-Martínez, A.E.; Ávila, J.; Luján, R. The Expression and Localisation of G-Protein-Coupled Inwardly Rectifying Potassium (GIRK) Channels Is Differentially Altered in the Hippocampus of Two Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 11106. [Google Scholar] [CrossRef]
- Djebari, S.; Iborra-Lázaro, G.; Temprano-Carazo, S.; Sánchez-Rodríguez, I.; Nava-Mesa, M.O.; Múnera, A.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. G-Protein-Gated Inwardly Rectifying Potassium (Kir3/Girk) Channels Govern Synaptic Plasticity That Supports Hippocampal-Dependent Cognitive Functions in Male Mice. J. Neurosci. 2021, 41, 7086–7102. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, I.; Temprano-Carazo, S.; Nájera, A.; Djebari, S.; Yajeya, J.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Activation of G-Protein-Gated Inwardly Rectifying Potassium (Kir3/GirK) Channels Rescues Hippocampal Functions in a Mouse Model of Early Amyloid-β Pathology. Sci. Rep. 2017, 7, 14658. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, I.; Djebari, S.; Temprano-Carazo, S.; Vega-Avelaira, D.; Jiménez-Herrera, R.; Iborra-Lázaro, G.; Yajeya, J.; Jiménez-Díaz, L.; Navarro-López, J.D. Hippocampal Long-Term Synaptic Depression and Memory Deficits Induced in Early Amyloidopathy Are Prevented by Enhancing G-Protein-Gated Inwardly Rectifying Potassium Channel Activity. J. Neurochem. 2020, 153, 362–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abney, K.K.; Bubser, M.; Du, Y.; Kozek, K.A.; Bridges, T.M.; Linsdley, C.W.; Daniels, J.S.; Morrison, R.D.; Wickman, K.; Hopkins, C.R.; et al. Analgesic Effects of the GIRK Activator, VU0466551, Alone and in Combination with Morphine in Acute and Persistent Pain Models. ACS Chem. Neurosci. 2019, 10, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Y.; Kong, S.; Zang, K.; Jiang, S.; Wan, L.; Chen, L.; Wang, G.; Jiang, M.; Wang, X.; et al. GIRK1-Mediated Inwardly Rectifying Potassium Current Suppresses the Epileptiform Burst Activities and the Potential Antiepileptic Effect of ML297. Biomed. Pharmacother. 2018, 101, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ung, P.M.U.; Zahoránszky-Kőhalmi, G.; Zakharov, A.V.; Martinez, N.J.; Simeonov, A.; Glaaser, I.W.; Rai, G.; Schlessinger, A.; Marugan, J.J.; et al. Identification of a G-Protein-Independent Activator of GIRK Channels. Cell Rep. 2020, 31, 107770. [Google Scholar] [CrossRef] [PubMed]
- Wydeven, N.; Marron Fernandez De Velasco, E.; Du, Y.; Benneyworth, M.A.; Hearing, M.C.; Fischer, R.A.; Thomas, M.J.; Weaver, C.D.; Wickman, K. Mechanisms Underlying the Activation of G-Protein-Gated Inwardly Rectifying K+ (GIRK) Channels by the Novel Anxiolytic Drug, ML297. Proc. Natl. Acad. Sci. USA 2014, 111, 10755–10760. [Google Scholar] [CrossRef] [Green Version]
- Vo, B.N.; Abney, K.K.; Anderson, A.; Marron Fernandez de Velasco, E.; Benneyworth, M.A.; Daniels, J.S.; Morrison, R.D.; Hopkins, C.R.; Weaver, C.D.; Wickman, K. VU0810464, a Non-Urea G Protein-Gated Inwardly Rectifying K+ (Kir 3/GIRK) Channel Activator, Exhibits Enhanced Selectivity for Neuronal Kir 3 Channels and Reduces Stress-Induced Hyperthermia in Mice. Br. J. Pharmacol. 2019, 176, 2238–2249. [Google Scholar] [CrossRef]
- Xu, Y.; Cantwell, L.; Molosh, A.I.; Plant, L.D.; Gazgalis, D.; Fitz, S.D.; Dustrude, E.T.; Yang, Y.; Kawano, T.; Garai, S.; et al. The Small Molecule GAT1508 Activates Brain-Specific GIRK1/2 Channel Heteromers and Facilitates Conditioned Fear Extinction in Rodents. J. Biol. Chem. 2020, 295, 3614–3634. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Holford, M.; Daly, M.; King, G.F.; Norton, R.S. Venoms to the Rescue: Insights into the Evolutionary Biology of Venoms Are Leading to Therapeutic Advances. Science 2018, 361, 842–843. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Finol-Urdaneta, R.K.; Belovanovic, A.; Micic-Vicovac, M.; Kinsella, G.K.; McArthur, J.R.; Al-Sabi, A. Marine Toxins Targeting Kv1 Channels: Pharmacological Tools and Therapeutic Scaffolds. Mar. Drugs 2020, 18, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Fernández, R.; Peigneur, S.; Pons, T.; Alvarez, C.; González, L.; Chávez, M.A.; Tytgat, J. The Kunitz-Type Protein ShPI-1 Inhibits Serine Proteases and Voltage-Gated Potassium Channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type Peptide HCRG21 from the Sea Anemone Heteractis Crispa Is a Full Antagonist of the TRPV1 Receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.M.; Béress, L.; Lazdunski, M. Kalicludines and Kaliseptine. Two Different Classes of Sea Anemone Toxins for Voltage Sensitive K+ Channels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M. Evolutionary Aspects of the Structural Convergence and Functional Diversification of Kunitz-Domain Inhibitors. J. Mol. Evol. 2020, 88, 537–548. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Monastyrnaya, M.; Tabakmakher, V.; Yurchenko, E.; Menchinskaya, E.; Pislyagin, E.; Andreev, Y.; Kozlov, S.; Peigneur, S.; et al. Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model. Biomedicines 2021, 9, 283. [Google Scholar] [CrossRef]
- Mans, B.J.; Louw, A.I.; Neitz, A.W.H. Savignygrin, a Platelet Aggregation Inhibitor from the Soft Tick Ornithodoros Savignyi, Presents the RGD Integrin Recognition Motif on the Kunitz-BPTI Fold. J. Biol. Chem. 2002, 277, 21371–21378. [Google Scholar] [CrossRef] [Green Version]
- Ciolek, J.; Reinfrank, H.; Quinton, L.; Viengchareun, S.; Stura, E.A.; Vera, L.; Sigismeau, S.; Mouillac, B.; Orcel, H.; Peigneur, S.; et al. Green Mamba Peptide Targets Type-2 Vasopressin Receptor against Polycystic Kidney Disease. Proc. Natl. Acad. Sci. USA 2017, 114, 7154–7159. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 Activation Power Can Switch an Action Mode for Its Polypeptide Ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef]
- Stotz, S.C.; Spaetgens, R.L.; Zamponi, G.W. Block of Voltage-Dependent Calcium Channel by the Green Mamba Toxin Calcicludine. J. Membr. Biol. 2000, 174, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Béress, L.; Tytgat, J. A Bifunctional Sea Anemone Peptide with Kunitz Type Protease and Potassium Channel Inhibiting Properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.L. Twenty Years of Dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- You, D.; Hong, J.; Rong, M.; Yu, H.; Liang, S.; Ma, Y.; Yang, H.; Wu, J.; Lin, D.; Lai, R. The First Gene-Encoded Amphibian Neurotoxin. J. Biol. Chem. 2009, 284, 22079–22086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitz, H.; Heurteaux, C.; Bois, P.; Moinier, D.; Romey, G.; Lazdunski, M. Calcicludine, a Venom Peptide of the Kunitz-Type Protease Inhibitor Family, Is a Potent Blocker of High-Threshold Ca2+ Channels with a High Affinity for L-Type Channels in Cerebellar Granule Neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 878–882. [Google Scholar] [CrossRef] [Green Version]
- Báez, A.; Salceda, E.; Fló, M.; Graña, M.; Fernández, C.; Vega, R.; Soto, E. α-Dendrotoxin Inhibits the ASIC Current in Dorsal Root Ganglion Neurons from Rat. Neurosci. Lett. 2015, 606, 42–47. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins Into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [Green Version]
- Yow, T.T.; Pera, E.; Absalom, N.; Heblinski, M.; Johnston, G.A.; Hanrahan, J.R.; Chebib, M. Naringin Directly Activates Inwardly Rectifying Potassium Channels at an Overlapping Binding Site to Tertiapin-Q. Br. J. Pharmacol. 2011, 163, 1017–1033. [Google Scholar] [CrossRef] [Green Version]
- Bhave, G.; Lonergan, D.; Chauder, B.A.; Denton, J.S. Small-Molecule Modulators of Inward Rectifier K+ Channels: Recent Advances and Future Possibilities. Future Med. Chem. 2010, 2, 757–774. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Liao, Y.; Jin, A.-H.; Gao, B. Discovery of Novel Peptide Neurotoxins from Sea Anemone Species. Front. Biosci. (Landmark Ed.) 2021, 26, 1256. [Google Scholar] [CrossRef]
- Madio, B.; King, G.F.; Undheim, E.A.B. Sea Anemone Toxins: A Structural Overview. Mar. Drugs 2019, 17, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourão, C.B.F.; Schwartz, E.F. Protease Inhibitors from Marine Venomous Animals and Their Counterparts in Terrestrial Venomous Animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladkikh, I.; Peigneur, S.; Sintsova, O.; Pinheiro-Junior, E.L.; Klimovich, A.; Menshov, A.; Kalinovsky, A.; Isaeva, M.; Monastyrnaya, M.; Kozlovskaya, E.; et al. Kunitz-Type Peptides from the Sea Anemone Heteractis Crispa Demonstrate Potassium Channel Blocking and Anti-Inflammatory Activities. Biomedicines 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Robertson, B. Dendrotoxins: Structure-Activity Relationships and Effects on Potassium Ion Channels. Curr. Med. Chem. 2004, 11, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical Reactive Center Kunitz-Type Inhibitor from the Sea Anemone Heteractis Crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Ikeda, K.; Kojima, H.; Niki, H.; Yano, R.; Yoshioka, T.; Kumanishi, T. Ethanol Opens G-Protein-Activated Inwardly Rectifying K+ Channels. Nat. Neurosci. 1999, 2, 1091–1097. [Google Scholar] [CrossRef]
- You, C.; Savarese, A.; Vandegrift, B.J.; He, D.; Pandey, S.C.; Lasek, A.W.; Brodie, M.S. Ethanol Acts on KCNK13 Potassium Channels in the Ventral Tegmental Area to Increase Firing Rate and Modulate Binge–like Drinking. Neuropharmacology 2019, 144, 29–36. [Google Scholar] [CrossRef]
- Saponara, S.; Testai, L.; Iozzi, D.; Martinotti, E.; Martelli, A.; Chericoni, S.; Sgaragli, G.; Fusi, F.; Calderone, V. (+/−)-Naringenin as Large Conductance Ca2+-Activated K+ (BKCa) Channel Opener in Vascular Smooth Muscle Cells. Br. J. Pharmacol. 2006, 149, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Tytgat, J.; Debont, T.; Carmeliet, E.; Daenens, P. The Alpha-Dendrotoxin Footprint on a Mammalian Potassium Channel. J. Biol. Chem. 1995, 270, 24776–24781. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.; Pinheiro-Junior, E.L.; Béress, L.; Gladkikh, I.; Leychenko, E.; Undheim, E.A.B.; Peigneur, S.; Tytgat, J. AsKC11, a Kunitz Peptide from Anemonia sulcata, Is a Novel Activator of G Protein-Coupled Inward-Rectifier Potassium Channels. Mar. Drugs 2022, 20, 140. https://doi.org/10.3390/md20020140
An D, Pinheiro-Junior EL, Béress L, Gladkikh I, Leychenko E, Undheim EAB, Peigneur S, Tytgat J. AsKC11, a Kunitz Peptide from Anemonia sulcata, Is a Novel Activator of G Protein-Coupled Inward-Rectifier Potassium Channels. Marine Drugs. 2022; 20(2):140. https://doi.org/10.3390/md20020140
Chicago/Turabian StyleAn, Dongchen, Ernesto Lopes Pinheiro-Junior, László Béress, Irina Gladkikh, Elena Leychenko, Eivind A. B. Undheim, Steve Peigneur, and Jan Tytgat. 2022. "AsKC11, a Kunitz Peptide from Anemonia sulcata, Is a Novel Activator of G Protein-Coupled Inward-Rectifier Potassium Channels" Marine Drugs 20, no. 2: 140. https://doi.org/10.3390/md20020140
APA StyleAn, D., Pinheiro-Junior, E. L., Béress, L., Gladkikh, I., Leychenko, E., Undheim, E. A. B., Peigneur, S., & Tytgat, J. (2022). AsKC11, a Kunitz Peptide from Anemonia sulcata, Is a Novel Activator of G Protein-Coupled Inward-Rectifier Potassium Channels. Marine Drugs, 20(2), 140. https://doi.org/10.3390/md20020140







