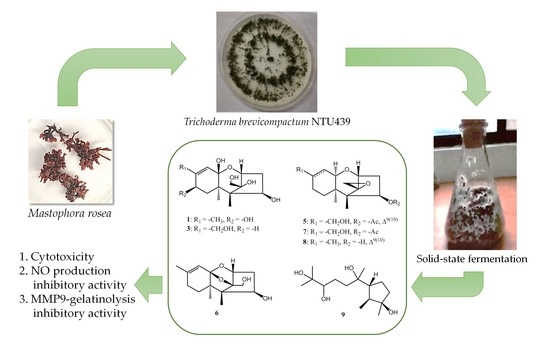
New Trichothecenes Isolated from the Marine Algicolous Fungus Trichoderma brevicompactum
Abstract
:1. Introduction
2. Results
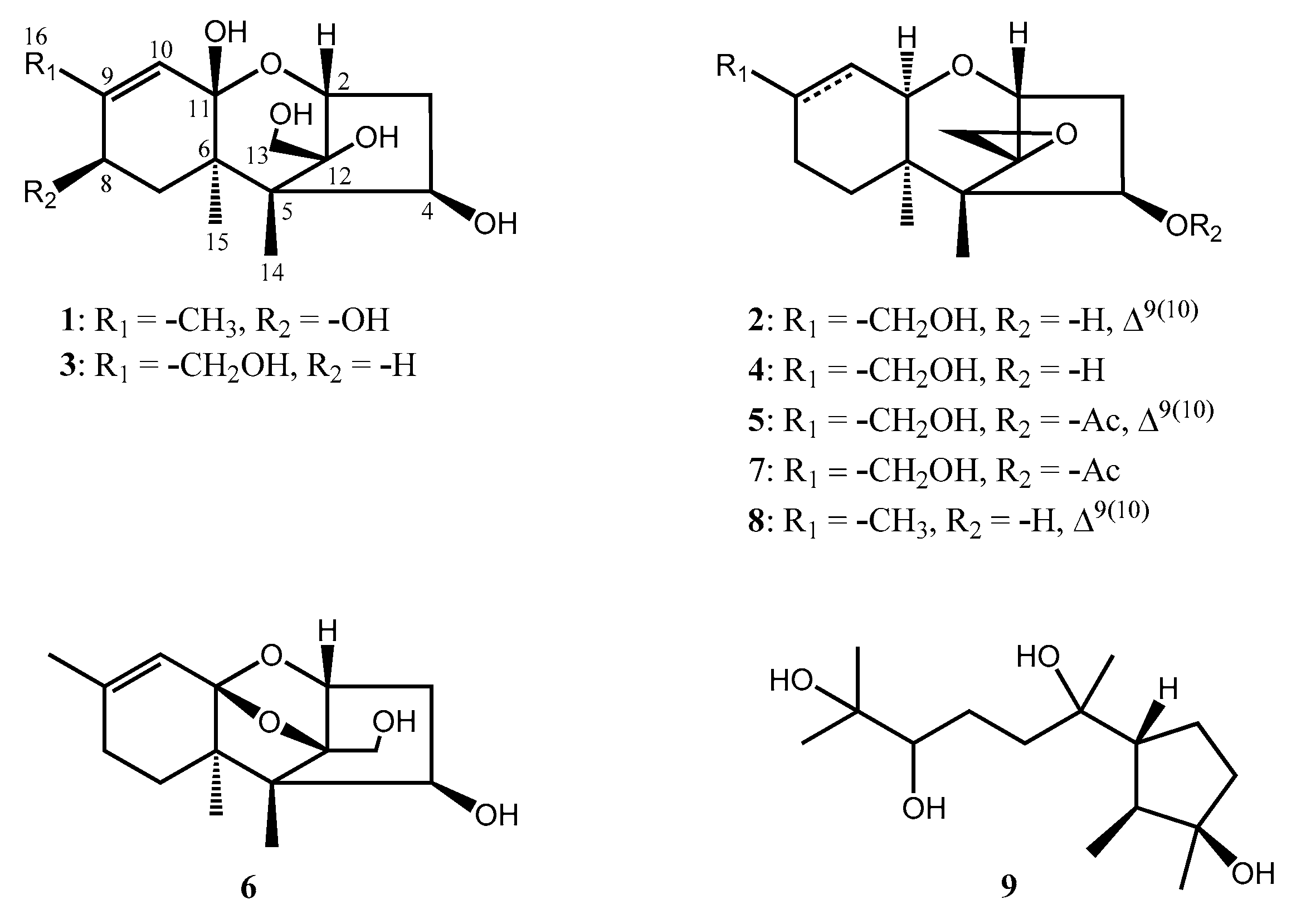
2.1. Chemical Characterization of the Produced Compound
2.2. Functional Characterization of the Produced Compounds
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Strain Isolation and Fermentation
4.3. Extraction and Purification of Secondary Metabolites
4.4. Cell Culture
4.5. Biologic Assay for Cytotoxic Activity
4.6. Biologic Assay for Relative Gelatinolysis by MMP-9
4.7. Biologic Assay for Anti-Neuroinflammatory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | nuclear magnetic resonance |
| MS | mass spectrometry |
| IR | infrared spectroscopy |
| HCT | human colorectal carcinoma cell |
| PC | human prostate cell |
| SK-Hep | human hepatic carcinoma cell |
| SBR | sulforhodamine B |
| LPS | lipopolysaccharide |
| NO | nitric oxide |
| MMP | matrix metalloproteinase |
| THP-1 | human leukemia monocytic cell line |
| COSY | correlation spectroscopy |
| NOESY | nuclear overhauser effect spectroscopy |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.H.; McCormick, S.P.; Kim, H.-S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amagata, T.; Rath, C.; Rigot, J.F.; Tarlov, N.; Tenney, K.; Valeriote, F.A.; Crews, P. Structures and cytotoxic properties of trichoverroids and their macrolide analogues produced by saltwater culture of Myrothecium verrucaria. J. Med. Chem. 2003, 46, 4342–4350. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Liu, X.-H.; Li, X.-N.; Ji, N.-Y. Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food Chem. 2020, 68, 15440–15448. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Hao, X.; Li, S.; Jia, J.; Guan, Y.; Peng, Z.; Bi, H.; Xiao, C.; Cen, S.; et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules 2019, 24, 2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-J.; Wang, S.-W.; Chiang, Y.-R.; Pang, K.-L.; Kuo, Y.-H.; Shih, T.-Y.; Lee, T.-H. Highly oxygenated constituents from a marine alga-derived fungus Aspergillus giganteus NTU967. Mar. Drugs 2020, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Huang, L.L.; Ye, Y.H.; Zou, W.X.; Guo, Z.J.; Tan, R.X. Antifungal and new metabolites of Myrothecium sp. Z16, a fungus associated with white croaker Argyrosomus argentatus. J. Appl. Microbiol. 2006, 100, 195–202. [Google Scholar] [CrossRef]
- Zou, J.-X.; Song, Y.-P.; Ji, N.-Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2021, 35, 216–221. [Google Scholar] [CrossRef]
- Hawas, U.W.; Farrag, A.R.H.; Ahmed, E.F.; Abou El-Kassem, L.T. Cytotoxic Effect of Fusarium equiseti Fungus metabolites Against N-Nitrosodiethylamine- and CCL4-Induced Hepatocarcinogenesis in Rats. Pharm. Chem. J. 2018, 52, 326–333. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, P.; Liu, T.; Wang, X.-F.; Li, Z.-X.; Li, W.; Wang, F.-L. Insecticidal activities of chloramphenicol derivatives isolated from a marine alga-derived endophytic fungus, Acremonium vitellinum, against the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Molecules 2018, 23, 2995. [Google Scholar] [CrossRef] [Green Version]
- Miao, F.; Zuo, J.; Liu, X.; Ji, N. Algicidal activities of secondary metabolites of marine macroalgal-derived endophytic fungi. J. Ocean. Limnol. 2019, 37, 112–121. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Sakayaroj, J. Trichothecenes from a Soil-Derived Trichoderma brevicompactum. J. Nat. Prod. 2019, 82, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-T.; Wang, Y.-J.; Ma, X.-Y.; Yin, X.-L.; Ji, N.-Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1-4. Nat. Prod. Res. 2019, 33, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-X.; Ai, H.-L.; Feng, T.; Wang, W.-X.; Wu, B.; Zheng, Y.-S.; Sun, H.; He, J.; Li, Z.-H.; Liu, J.-K. Trichothecrotocins A–C, antiphytopathogenic agents from potato endophytic fungus Trichothecium crotocinigenum. Org. Lett. 2018, 20, 8069–8072. [Google Scholar] [CrossRef]
- Aitken, A.; Miller, J.D.; McMullin, D.R. Isolation, chemical characterization and hydrolysis of the trichothecene 7α-Hydroxy, 15-Deacetylcalonectrin (3ANX) from Fusarium graminearum DAOMC 242077. Tetrahedron Lett. 2019, 60, 852–856. [Google Scholar] [CrossRef]
- Ryu, S.M.; Lee, H.M.; Song, E.G.; Seo, Y.H.; Lee, J.; Guo, Y.; Kim, B.S.; Kim, J.J.; Hong, J.S.; Ryu, K.H.; et al. Antiviral Activities of Trichothecenes Isolated from Trichoderma albolutescens against Pepper Mottle Virus. J. Agric. Food Chem. 2017, 65, 4273–4279. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Tamez, P.A.; Aydogmus, Z.; Tan, G.T.; Saikawa, Y.; Hashimoto, K.; Nakata, M.; Hung, N.V.; Xuan, L.T.; Cuong, N.M.; et al. Antimalarial agents from plants. III. trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med. 2002, 68, 1088–1091. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Jarvis, B.B.; Dailey, R.G.; Bright, W.; Bryan, R.F.; Shizuri, Y. Baccharin, a novel potent antileukemic trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem. Soc. 1976, 98, 7092–7093. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, J.; Zhou, Y.; Zhang, C.; Ou, X.; Su, W.; Zhao, J.; Zhu, G. Synthesis and antifungal activities of trichodermin derivatives as fungicides on rice. Chem. Biodivers. 2013, 10, 600–611. [Google Scholar] [CrossRef]
- Song, Y.-P.; Miao, F.-P.; Liu, X.-H.; Yin, X.-L.; Ji, N.-Y. Cyclonerane derivatives from the algicolous endophytic fungus Trichoderma asperellum A-YMD-9-2. Mar. Drugs 2019, 17, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barúa, J.E.; de la Cruz, M.; de Pedro, N.; Cautain, B.; Hermosa, R.; Cardoza, R.E.; Gutiérrez, S.; Monte, E.; Vicente, F.; Collado, I.G. Synthesis of trichodermin derivatives and their antimicrobial and cytotoxic activities. Molecules 2019, 24, 3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, I.; Varsavky, E.; Haidukowski, M.; Frade, H. Macrocyclic trichothecenes in Baccharis coridifolia plants and endophytes and Baccharis artemisioides plants. Toxicon 1997, 35, 753–757. [Google Scholar] [CrossRef]
- García, C.C.; Rosso, M.L.; Bertoni, M.D.; Maier, M.S.; Damonte, E.B. Evaluation of the antiviral activity against junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia. Planta Med. 2002, 68, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Nishiyama, M.; Maeda, H.; Tonouchi, A.; Konno, K.; Hashimoto, M. Structure-activity relationships of trichothecenes against COLO201 cells and Cochliobolus miyabeanus: The role of 12-epoxide and macrocyclic moieties. Bioorg. Med. Chem. Lett. 2019, 29, 982–985. [Google Scholar] [CrossRef]
- Lin, T.; Wang, G.; Zhou, Y.; Zeng, D.; Liu, X.; Ding, R.; Jiang, X.; Zhu, D.; Shan, W.; Chen, H. Structure elucidation and biological activity of two new trichothecenes from an endophyte, Myrothecium roridum. J. Agric. Food Chem. 2014, 62, 5993–6000. [Google Scholar] [CrossRef]
- Ramu, A.; Yagen, B.; Ramu, N. The cytotoxicity of T-2 toxin and related 12,13-epoxytrichothecenes to adriamycin-sensitive and-resistant P388 leukemia cells. Cancer Chemother. Pharm. 1989, 24, 264–267. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Sheu, J.-R.; Chung, C.-L.; Chen, C.-Y.; Lin, F.-L.; Hsu, M.-J.; Kuo, Y.-H.; Hsiao, G. Nuclear-targeted inhibition of NF-κB on MMP-9 production by N-2-(4-bromophenyl) ethyl caffeamide in human monocytic cells. Chem.-Biol. Interact. 2010, 184, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-H.; Hsiao, G.; Chang, C.-H.; Yang, Y.-L.; Ju, Y.-M.; Kuo, Y.-H.; Lee, T.-H. Polyketides with anti-neuroinflammatory activity from Theissenia cinerea. J. Nat. Prod. 2021, 84, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Hsiao, C.-J.; Lin, Y.-N.; Wu, J.-W.; Kuo, Y.-C.; Lee, C.-K.; Hsiao, G. Carbamazepine attenuates inducible nitric oxide synthase expression through Akt inhibition in activated microglial cells. Pharm. Biol. 2014, 52, 1451–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, G.; Fong, T.H.; Tzu, N.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. A Potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo 2004, 18, 351–356. [Google Scholar] [PubMed]
 ) and COSY (
) and COSY (  ) correlations of compounds 1–4.
) correlations of compounds 1–4.

| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 2 | 4.15, d (5.3) | 3.67, d (5.3) | 4.18, d (5.3) | 3.66, d (5.3) |
| 3a | 2.47, dd (16.3, 7.5) | 2.49, dd (15.1, 7.6) | 2.50, dd, (16.4, 7.6) | 2.37, dd (15.0, 7.8) |
| 3b | 1.77, ddd (16.3, 5.3, 1.6) | 1.87, ddd (15.1, 5.3, 3.2) | 1.80, ddd, (16.4, 5.3, 1.8) | 1.80, ddd (15.0, 5.3, 3.5) |
| 4 | 4.04, dd (7.5, 1.6) | 4.41, dd (7.6, 3.5) | 4.05, dd (7.6, 1.5) | 4.29, dd (7.8, 3.5) |
| 7a | 2.05, dd (14.2, 5.6) | 1.91, dd (12.6, 9.1) | 1.88, dt (12.9, 5.9) | 1.98, dd (13.8, 4.4) |
| 7b | 1.67, dd (14.2, 1.3) | 1.51, m (12.6) | 1.50, ddd (12.9, 5.3, 1.5) | 1.17, br, dd (13.8, 4.4) |
| 8a | 4.01, d (5.6) | 2.05, m | 2.18, m | 1.70, m |
| 8b | 2.03, m | 2.05, m | 1.64, dt (14.1, 4.4) | |
| 9 | 1.75, m | |||
| 10a | 5.39, q (1.2) | 5.59, dt (5.6, 1.5) | 5.60, br | 1.92, ddd (15.3, 6.5, 3.8) |
| 10b | 1.59, m | |||
| 11 | 3.64, d (5.6) | 3.39, br | ||
| 13a | 3.85, d (12.0) | 2.99, d (4.1) | 3.89, d (11.4) | 3.04, d (4.0) |
| 13b | 3.83, d (12.0) | 2.80, d (4.1) | 3.85, d (11.4) | 2.83, d (4.0) |
| 14 | 0.99, s | 0.78, s | 1.01, s | 0.73, s |
| 15 | 1.04, s | 0.88, s | 0.89, s | 0.99, s |
| 16a | 1.87, d (1.2) | 3.97, br | 3.98, br | 3.74, dd (10.9, 5.6) |
| 16b | 3.94, br | 3.47, dd (10.9, 5.6) |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | |
| 2 | 81.2, CH | 79.3, CH | 82.9, CH | 79.2, CH |
| 3 | 40.9, CH2 | 38.4, CH2 | 42.4, CH2 | 38.4, CH2 |
| 4 | 74.6, CH | 72.5, CH | 76.3, CH | 72.3, CH |
| 5 | 54.2, C | 48.7, C | 55.5, C | 49.2, C |
| 6 | 46.9, C | 40.2, C | 48.0, C | 40.7, C |
| 7 | 38.9, CH2 | 23.8, CH2 | 31.6, CH2 | 22.7, CH2 |
| 8 | 66.7, CH | 23.0, CH2 | 25.3, CH2 | 20.9, CH2 |
| 9 | 144.7, C | 142.9, C | 149.5, C | 33.9, CH |
| 10 | 118.2, CH | 118.1, CH | 116.7, CH | 27.9, CH2 |
| 11 | 107.5, C | 69.9, CH | 108.9, C | 71.8, CH |
| 12 | 95.0, C | 65.2, C | 96.9, C | 65.4, C |
| 13 | 58.1, CH2 | 46.5, CH2 | 59.8, CH2 | 46.9, CH2 |
| 14 | 9.6, CH3 | 4.9, CH3 | 14.7, CH3 | 4.6, CH3 |
| 15 | 16.0, CH3 | 14.6, CH3 | 10.9, CH3 | 16.3, CH3 |
| 16 | 19.1, CH3 | 64.8, CH2 | 65.6, CH2 | 64.3, CH2 |
| Compounds | Cytotoxicity (IC50, μM) | NO (μM) ± SD | Cell Viability (%) ± SD in BV-2 Cell | ||
|---|---|---|---|---|---|
| HCT-116 | PC-3 | SK-Hep-1 | |||
| 1 | >10 | >10 | >10 | 10.8 ± 2.1 *** | 95.4 ± 5.7 |
| 2 | >10 | >10 | >10 | 8.1 ± 0.7 *** | 99.9 ± 1.6 |
| 3 | >10 | >10 | >10 | 12.4 ± 1.7 ** | 105.8 ± 2.9 |
| 4 | >10 | >10 | >10 | 9.2 ± 1.2 *** | 102.8 ± 9.4 |
| 5 | 5.4 ± 0.3 | 6.4 ± 0.1 | 5.0 ± 0.3 | 1.9 ± 0.5 *** | 62.9 ± 3.7 *** |
| 6 | >10 | >10 | >10 | 12.1 ± 1.5 ** | 104.9 ± 12.2 |
| 7 | 7.5 ± 0.3 | 9.3 ± 0.4 | 5.9 ± 0.2 | 4.2 ± 1.1 *** | 94.7 ± 17.8 |
| 8 | 3.3 ± 0.3 | 5.3 ± 0.3 | 1.8 ± 0.8 | 1.9 ± 0.1 *** | 63.3 ± 6.4 *** |
| 9 | >10 | >10 | >10 | 12.5 ± 0.8 ** | 103.5 ± 4.5 |
| Trichodermin a | 0.5 ± 0.0 | 0.9 ± 0.1 | 0.4 ± 0.1 | - | - |
| Resting | - | - | - | 2.4 ± 0.2 | 100 ± 0.0 |
| Vehicle | - | - | - | 16.4 ± 0.5 ### | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safwan, S.; Wang, S.-W.; Hsiao, G.; Hsiao, S.-W.; Hsu, S.-J.; Lee, T.-H.; Lee, C.-K. New Trichothecenes Isolated from the Marine Algicolous Fungus Trichoderma brevicompactum. Mar. Drugs 2022, 20, 80. https://doi.org/10.3390/md20020080
Safwan S, Wang S-W, Hsiao G, Hsiao S-W, Hsu S-J, Lee T-H, Lee C-K. New Trichothecenes Isolated from the Marine Algicolous Fungus Trichoderma brevicompactum. Marine Drugs. 2022; 20(2):80. https://doi.org/10.3390/md20020080
Chicago/Turabian StyleSafwan, Safwan, Shih-Wei Wang, George Hsiao, Sui-Wen Hsiao, Su-Jung Hsu, Tzong-Huei Lee, and Ching-Kuo Lee. 2022. "New Trichothecenes Isolated from the Marine Algicolous Fungus Trichoderma brevicompactum" Marine Drugs 20, no. 2: 80. https://doi.org/10.3390/md20020080
APA StyleSafwan, S., Wang, S.-W., Hsiao, G., Hsiao, S.-W., Hsu, S.-J., Lee, T.-H., & Lee, C.-K. (2022). New Trichothecenes Isolated from the Marine Algicolous Fungus Trichoderma brevicompactum. Marine Drugs, 20(2), 80. https://doi.org/10.3390/md20020080