Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches
Abstract
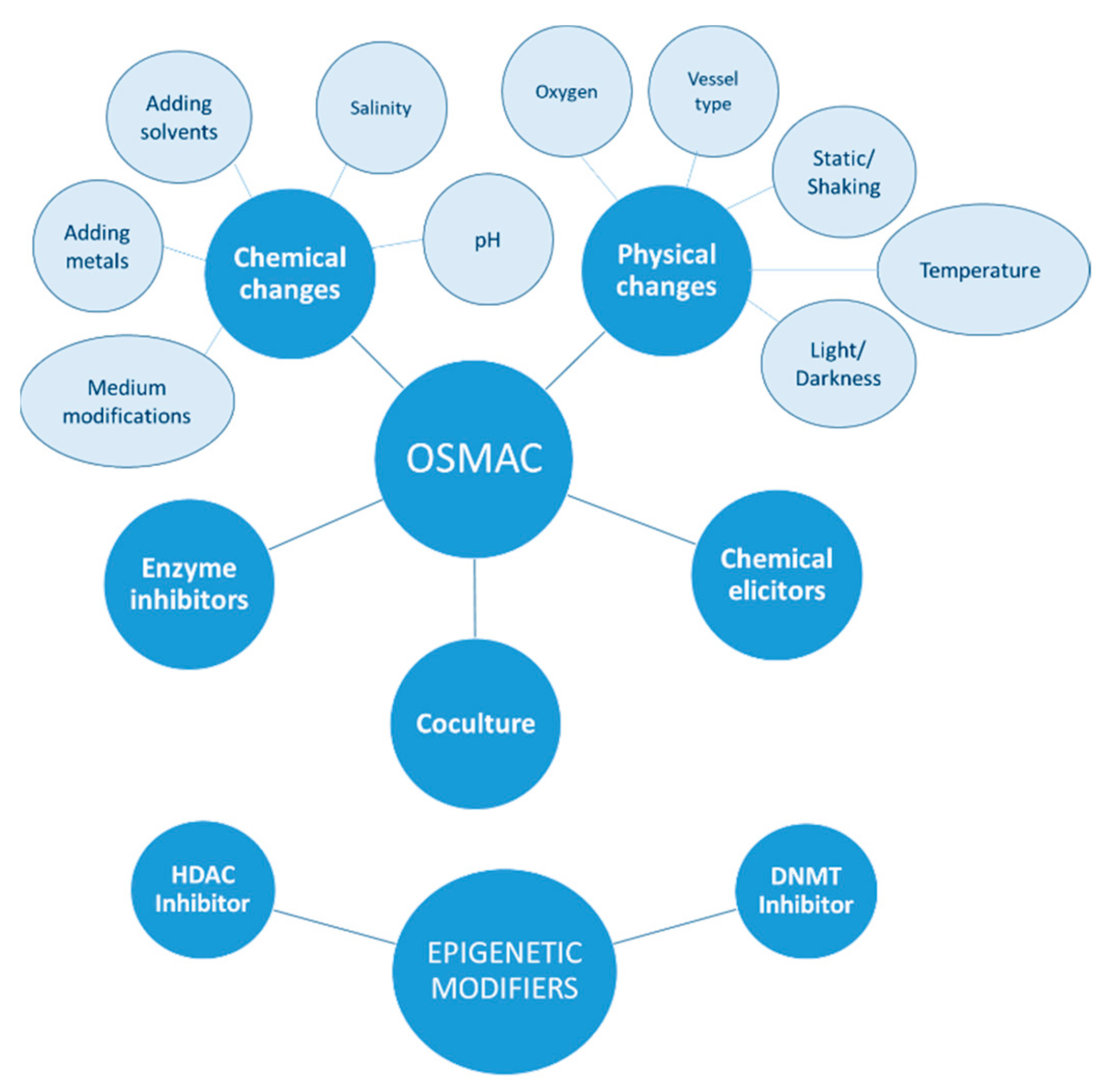
:1. Introduction
2. Alkaloids
2.1. OSMAC Approach
2.2. Epigenetic Approach
3. Peptides
3.1. OSMAC Approach
3.2. Epigenetic Approach
4. Polyketides
4.1. OSMAC Approach
4.2. Epigenetic Approach
5. Terpenes
5.1. OSMAC Approach
5.2. Epigenetic Approach
6. Miscellaneous
6.1. OSMAC Approach
6.2. Epigenetic Approach
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jensen, P.R.; Fenical, W. Marine Bacterial Diversity as a Resource for Novel Microbial Products. J. Ind. Microbiol. Biotechnol. 1996, 17, 346–351. [Google Scholar] [CrossRef]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rateb, M.E.; Ebel, R. Secondary Metabolites of Fungi from Marine Habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherlach, K.; Hertweck, C. Triggering Cryptic Natural Product Biosynthesis in Microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Zarins-Tutt, J.S.; Barberi, T.T.; Gao, H.; Mearns-Spragg, A.; Zhang, L.; Newman, D.J.; Goss, R.J.M. Prospecting for New Bacterial Metabolites: A Glossary of Approaches for Inducing, Activating and Upregulating the Biosynthesis of Bacterial Cryptic or Silent Natural Products. Nat. Prod. Rep. 2016, 33, 54–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, E.; Brachmann, A.O.; Kegler, C.; Simsek, R.; Dauth, C.; Zhou, Q.; Kaiser, M.; Klemmt, P.; Bode, H.B. Simple “On-Demand” Production of Bioactive Natural Products. ChemBioChem 2015, 16, 1115–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Xu, S.; Wang, B.; Ju, J.; Tan, H.; Li, W. Activation and Enhancement of Fredericamycin A Production in Deepsea-Derived Streptomyces somaliensis SCSIO ZH66 by Using Ribosome Engineering and Response Surface Methodology. Microb. Cell Fact. 2015, 14, 64. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, Y.; Lu, C.; Zhang, J.; Zhu, J.; Wang, H.; Shen, Y. Activating a Cryptic Ansamycin Biosynthetic Gene Cluster to Produce Three New Naphthalenic Octaketide Ansamycins with N-Pentyl and n-Butyl Side Chains. Org. Lett. 2015, 17, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhong, Y.; Shen, L.; Wu, X.; Ye, Y.; Chen, C.-T.A.; Wu, B. Stress-Driven Discovery of Natural Products from Extreme Marine Environment-Kueishantao Hydrothermal Vent, a Case Study of Metal Switch Valve. Curr. Org. Chem. 2014, 18, 925–934. [Google Scholar] [CrossRef]
- Almeida, E.L.; Kaur, N.; Jennings, L.K.; Rincón, A.F.C.; Jackson, S.A.; Thomas, O.P.; Dobson, A.D.W. Genome Mining Coupled with OSMAC-Based Cultivation Reveal Differential Production of Surugamide A by the Marine Sponge Isolate Streptomyces sp. SM17 When Compared to Its Terrestrial Relative S. albidoflavus J1074. Microorganisms 2019, 7, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Zhang, G.; Wang, B.; Li, X.; Yue, S.; Chen, J.; Zhang, H.; Wang, H. Production and Identification of Inthomycin B Produced by a Deep-Sea Sediment-Derived Streptomyces sp. YB104 Based on Cultivation-Dependent Approach. Curr. Microbiol. 2018, 75, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, Q.-Q.; Zhu, W.-M.; Cui, C.-B.; Fan, G.-T. Trichodermamide A and Aspergillazine A, Two Cytotoxic Modified Dipeptides from a Marine-Derived Fungus Spicaria elegans. Arch. Pharm. Res. 2005, 28, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Zuck, K.M.; Shipley, S.; Newman, D.J. Induced Production of N-Formyl Alkaloids from Aspergillus fumigatus by Co-Culture with Streptomyces peucetius. J. Nat. Prod. 2011, 74, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Hallyburton, I.; Houssen, W.E.; Bull, A.T.; Goodfellow, M.; Santhanam, R.; Jaspars, M.; Ebel, R. Induction of Diverse Secondary Metabolites in Aspergillus fumigatus by Microbial Co-Culture. RSC Adv. 2013, 3, 14444–14450. [Google Scholar] [CrossRef] [Green Version]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated Indole Alkaloids from Co-Culture of Marine-Derived Fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef]
- Xu, C.; Sun, X.; Jin, M.; Zhang, X. A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells. Mar. Drugs 2017, 15, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.-H.; Xu, M.-Y.; Li, H.-J.; Li, J.-Q.; Chen, Y.-X.; Ma, W.-Z.; Li, Y.-P.; Xu, J.; Yang, D.-P.; Lan, W.-J. Amino Acid-Directed Strategy for Inducing the Marine-Derived Fungus Scedosporium apiospermum F41-1 to Maximize Alkaloid Diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.-H. Antiviral Peptides from Marine Gorgonian-Derived Fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in Cytostatic Constituents of a Sponge-Derived Gymnascella dankaliensis by Manipulating the Carbon Source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, J.; Lin, X.; Zhou, X.; Zhang, Y.; Liu, Y. New Prenylated Indole Alkaloids from Fungus Penicillium sp. Derived of Mangrove Soil Sample. Tetrahedron 2014, 70, 3859–3863. [Google Scholar] [CrossRef]
- Tao, H.; Wei, X.; Lin, X.; Zhou, X.; Dong, J.; Yang, B. Penixanthones A and B, Two New Xanthone Derivatives from Fungus Penicillium sp. SYFz-1 Derived of Mangrove Soil Sample. Nat. Prod. Res. 2017, 31, 2218–2222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Wang, B.-G. N-Formyllapatin A, a New N-Formylspiroquinazoline Derivative from the Marine-Derived Fungus Penicillium adametzioides AS-53. Phytochem. Lett. 2014, 10, 145–148. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Jiang, W.-L.; Xu, G.-M.; Huang, C.-G.; Wang, B.-G. Bisthiodiketopiperazines and Acorane Sesquiterpenes Produced by the Marine-Derived Fungus Penicillium adametzioides AS-53 on Different Culture Media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, O.; Murakami, K.; Namikoshi, M. Induced Production of a New Unprecedented Epitrithiodiketopiperazine, Chlorotrithiobrevamide, by a Culture of the Marine-Derived Trichoderma cf. brevicompactum with Dimethyl Sulfoxide. Tetrahedron Lett. 2015, 56, 6262–6265. [Google Scholar] [CrossRef]
- Sureram, S.; Wiyakrutta, S.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Depsidones, Aromatase Inhibitors and Radical Scavenging Agents from the Marine-Derived Fungus Aspergillus unguis CRI282-03. Planta Med. 2012, 78, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ding, P.; Liang, Z.; Song, Y.; Liu, Y.; Chen, G.; Li, J.L. Activated Production of Silent Metabolites from Marine-Derived Fungus Penicillium citrinum. Fitoterapia 2018, 127, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, X.; Auckloo, B.N.; Chen, C.T.A.; Ye, Y.; Wang, K.; Wu, B. An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt. Molecules 2016, 21, 105. [Google Scholar] [CrossRef] [Green Version]
- Gulder, T.A.M.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.F.; Gross, H. Isolation, Structure Elucidation and Total Synthesis of Lajollamide A from the Marine Fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.-L.; Zhang, D.; Xia, J.-M.; Hu, C.-C.; Lin, T.; Lin, Y.-K.; Wang, G.-H.; Tian, W.-J.; Li, Z.-P.; Zhang, X.-K.; et al. Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Wang, N.; Xie, C.-L.; Fan, Z.; Luo, Z.; Chen, H.F.; Yang, X.-W. Roquefortine J, a Novel Roquefortine Alkaloid, from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018, 71, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.B.; Yim, G.; Tsui, W.; McClure, J.A.; Surette, M.G.; Davies, J. Transcriptional Modulation of Bacterial Gene Expression by Subinhibitory Concentrations of Antibiotics. Proc. Natl. Acad. Sci. USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; She, J.; Yang, X.; Liu, J.; Zhou, X.; Yang, B. A New Macrodiolide and Two New Polycyclic Chromones from the Fungus Penicillium sp. SCSIO041218. Molecules 2019, 24, 1686. [Google Scholar] [CrossRef] [Green Version]
- Christian, O.E.; Compton, J.; Christian, K.R.; Mooberry, S.L.; Valeriote, F.A.; Crews, P. Using Jasplakinolide to Turn on Pathways That Enable the Isolation of New Chaetoglobosins from Phomospis asparagi. J. Nat. Prod. 2005, 68, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Fisch, K.M.; Gillaspy, A.F.; Gipson, M.; Henrikson, J.C.; Hoover, A.R.; Jackson, L.; Najar, F.Z.; Wägele, H.; Cichewicz, R.H. Chemical Induction of Silent Biosynthetic Pathway Transcription in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 2009, 36, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Bradshaw, E.; Seipke, R.F.; Hutchings, M.I.; McArthur, M. Use and Discovery of Chemical Elicitors that Stimulate Biosynthetic Gene Clusters in Streptomyces Bacteria. In Natural Product Biosynthesis by Microorganisms and Plants Part C, 1st ed.; Hopwood, D.A., Ed.; Elsevier Inc.: San Diego, CA, USA, 2012; Volume 517, pp. 367–385. ISBN 9780124046344. [Google Scholar]
- Kamauchi, H.; Kinoshita, K.; Sugita, T.; Koyama, K. Conditional Changes Enhanced Production of Bioactive Metabolites of Marine Derived Fungus Eurotium rubrum. Bioorg. Med. Chem. Lett. 2016, 26, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, L.; Zhang, X.; Liang, Y.; Anjum, K.; Chen, L.; Lian, X.-Y. Bioactive Bafilomycins and a New N-Arylpyrazinone Derivative from Marine-Derived Streptomyces sp. HZP-2216E. Planta Med. 2017, 83, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, L.; Chai, W.; Lian, X.-Y.; Zhang, Z. A Unique Indolizinium Alkaloid Streptopertusacin A and Bioactive afilomycins from Marine-Derived Streptomyces sp. HZP-2216E. Phytochemistry 2017, 144, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Lv, C.-T.; Huang, C.-G.; Wang, B.-G. Brocazines A–F, Cytotoxic Bisthiodiketopiperazine Derivatives from Penicillium brocae MA-231, an Endophytic Fungus Derived from the Marine Mangrove Plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Zhang, P.; Li, X.-M.; Wang, B.-G. Penicibrocazines A–E, Five New Sulfide Diketopiperazines from the Marine-Derived Endophytic Fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Xu, G.-M.; Wang, B.-G. Antimicrobial Alkaloids Produced by the Mangrove Endophyte Penicillium brocae MA-231 Using the OSMAC Approach. RSC Adv. 2017, 7, 55026–55033. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Tao, H.; Lin, X.; Wang, J.; Liao, S.; Dong, J.; Zhou, X.; Liu, Y. Prenylated Indole Alkaloids and Chromone Derivatives from the Fungus Penicillium sp. SCSIO041218. Tetrahedron 2018, 74, 77–82. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Zhu, T.; Zhu, W. Pyrazinone Derivatives from the Coral-Derived Aspergillus ochraceus LCJ11-102 under High Iodide Salt. Arch. Pharm. Res. 2018, 41, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Tang, M.; Lin, S.; Chen, G.; Feng, Q.; Wang, Y.; Hua, H.; Bai, J.; Wang, H.; Pei, Y. Cytotoxic Cytochalasans from Aspergillus flavipes PJ03-11 by OSMAC Method. Tetrahedron Lett. 2018, 59, 1767–1771. [Google Scholar] [CrossRef]
- Yamazaki, H.; Rotinsulu, H.; Narita, R.; Takahashi, R.; Namikoshi, M. Induced Production of Halogenated Epidithiodiketopiperazines by a Marine-Derived Trichoderma cf. brevicompactum with Sodium Halides. J. Nat. Prod. 2015, 78, 2319–2321. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G.; Fang, Y.; Zhu, T.; Liu, H. 10-Phenyl-[12]-cytochalasins Z7, Z8, and Z9 from the Marine-Derived Fungus Spicaria elegans. J. Nat. Prod. 2006, 69, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lin, Z.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Novel Open-Chain Cytochalsins from the Marine-Derived Fungus Spicaria elegans. J. Nat. Prod. 2008, 71, 1127–1132. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, T.; Wei, H.; Zhang, G.; Wang, H.; Gu, Q. Spicochalasin A and New Aspochalasins from the Marine-Derived Fungus Spicaria elegans. Eur. J. Org. Chem. 2009, 18, 3045–3051. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhu, T.J.; Chen, L.; Gu, Q.Q. Three New Aspochalasin Derivatives from the Marine-Derived Fungus Spicaria elegans. Chin. Chem. Lett. 2010, 21, 824–826. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Zhu, T.-J.; Zhang, G.-J.; Wei, H.-J.; Gu, Q.-Q. Deoxy-cytochalasins from a Marine-Derived Fungus Spicaria elegans. Can. J. Chem. 2009, 87, 486–489. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Wei, H.-J.; Zhu, T.-J.; Li, D.-H.; Lin, Z.-J.; Gu, Q.-Q. Three New Cytochalasins from the Marine-Derived Fungus Spicaria elegans KLA03 by Supplementing the Cultures with l- and d-Tryptophan. Chem. Biodivers. 2011, 8, 887–894. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Xu, M.-Y.; Li, H.-J.; Zeng, K.-J.; Ma, W.-Z.; Tian, G.-B.; Xu, J.; Yang, D.-P.; Lan, W.-J. Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1. Mar. Drugs 2017, 15, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.-L.; Li, H.-J.; Smith, D.R.; Jaratsittisin, J.; Xia-Ke-Er, X.-F.; Ma, W.-Z.; Guo, Y.-W.; Dong, J.; Shen, J.; Yang, D.-P.; et al. Polyketides and Alkaloids from the Marine-Derived Fungus Dichotomomyces cejpii F31-1 and the Antiviral Activity of Scequinadoline A against Dengue Virus. Mar. Drugs 2018, 16, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.-X.; Qiu, Y.; Ran, Y.-Q.; Feng, G.-K.; Deng, R.; Zhu, X.-F.; Lan, W.-J.; Li, H.-J. Exploration of Indole Alkaloids from Marine Fungus Pseudallescheria boydii F44-1 Using an Amino Acid-Directed Strategy. Mar. Drugs 2019, 17, 77. [Google Scholar] [CrossRef] [Green Version]
- Shaker, S.; Fan, R.-Z.; Li, H.-J.; Lan, W.-J. A Pair of Novel Bisindole Alkaloid Enantiomers from Marine Fungus Fusarium sp. XBB-9. Nat. Prod. Res. 2021, 35, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, X.; Huang, Z.-H.; Qi, S.-H. New Alkaloids and Isocoumarins from the Marine Gorgonian-Derived Fungus Aspergillus sp. SCSIO 41501. Nat. Prod. Res. 2020, 34, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, C.; Auckloo, B.N.; Chen, X.; Chen, C.-T.A.; Wang, K.; Wu, X.; Ye, Y.; Wu, B. Stress-Driven Discovery of a Cryptic Antibiotic Produced by Streptomyces sp. WU20 from Kueishantao Hydrothermal Vent with an Integrated Metabolomics Strategy. Appl. Microbiol. Biotechnol. 2017, 101, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Lang, G.; Wiese, J.; Imhoff, J.F. Subinhibitory Concentrations of Antibiotics Induce Phenazine Production in a Marine Streptomyces sp. J. Nat. Prod. 2008, 71, 824–827. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, X.; Guaciaro, M.; Molino, B.F.; Mocek, U.; Reategui, R.; Rhea, J.; Morley, T. The Revised Structure, Total Synthesis, and Absolute Configuration of Streptophenazine A. Org. Lett. 2011, 13, 5436–5439. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, L.; Wang, B.; Xu, Y.; Zhu, G.; Lan, M.; Zhu, W.; Sun, K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-S.; Li, X.-M.; Gao, S.-S.; Lu, Y.-H.; Wang, B.-G. Cytotoxic Anthranilic Acid Derivatives from Deep Sea Sediment-Derived Fungus Penicillium paneum SD-44. Mar. Drugs 2013, 11, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-N.; Gao, H.-Q.; Cai, S.-X.; Zhu, T.-J.; Gu, Q.-Q.; Li, D.-. H Two New Cyclic Pentapeptides from the Marine-Derived Fungus Aspergillus versicolor. Helv. Chim. Acta 2011, 94, 1065–1070. [Google Scholar] [CrossRef]
- Peng, J.; Gao, H.; Zhang, X.; Wang, S.; Wu, C.; Gu, Q.; Guo, P.; Zhu, T.; Li, D. Psychrophilins E-H and Versicotide C, Cyclic Peptides from the Marine-Derived Fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tanrıkul, T.T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of New Metabolites from Sponge-Associated Fungus Aspergillus carneus by OSMAC Approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-M.; Ju, G.-L.; Xiao, L.; Zhang, X.-F.; Du, F.-Y. Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions. Mar. Drugs 2018, 16, 519. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhu, H.; Hong, K.; Wang, Y.; Liu, P.; Wang, X.; Peng, X.; Zhu, W. Novel Cyclic Hexapeptides from Marine-Derived Fungus, Aspergillus sclerotiorum PT06-1. Org. Lett. 2009, 11, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, Z.; Wang, Y.; Hong, K.; Liu, P.; Zhu, W. Cyclic Tripeptides from the Halotolerant Fungus Aspergillus sclerotiorum PT06-1. J. Nat. Prod. 2010, 73, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Shen, L.; Jiang, W.; Ye, Y.; Chen, C.-T.A.; Wu, X.; Wang, K.; Wu, B. Zn-Driven Discovery of a Hydrothermal Vent Fungal Metabolite Clavatustide C, and an Experimental Study of the Anti-Cancer Mechanism of Clavatustide B. Mar. Drugs 2014, 12, 3203–3217. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Zhang, X.-Y.; Xu, X.-Y.; He, F.; Nong, X.-H.; Qi, S.-H. New Cyclic Tetrapeptides and Asteltoxins from Gorgonian-Derived Fungus Aspergillus sp. SCSGAF 0076. Tetrahedron 2013, 69, 2113–2117. [Google Scholar] [CrossRef]
- Vervoort, H.C.; Drašković, M.; Crews, P. Histone Deacetylase Inhibitors as a Tool to Up-Regulate New Fungal Biosynthetic Products: Isolation of EGM-556, a Cyclodepsipeptide, from Microascus sp. Org. Lett. 2011, 13, 410–413. [Google Scholar] [CrossRef] [Green Version]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-Driven Discovery of New Angucycline-Type Antibiotics from a Marine Streptomyces pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; He, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r. Mar. Drugs 2018, 16, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Three New Sorbicillin Trimers, Trisorbicillinones B, C, and D, from a Deep Ocean Sediment Derived Fungus, Phialocephala sp. FL3Or. Tetrahedron 2010, 66, 5101–5106. [Google Scholar] [CrossRef]
- Guo, W.; Peng, J.; Zhu, T.; Gu, Q.; Keyzers, R.A.; Li, D. Sorbicillamines A-E, Nitrogen-Containing Sorbicillinoids from the Deep-Sea-Derived Fungus Penicillium sp. F23-2. J. Nat. Prod. 2013, 76, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, Z.; Zhu, T.; Gu, Q.; Li, D. Penicyclones A–E, Antibacterial Polyketides from the Deep-Sea-Derived Fungus Penicillium sp. F23-2. J. Nat. Prod. 2015, 78, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Adpressa, D.A.; Loesgen, S. Bioprospecting Chemical Diversity and Bioactivity in a Marine Derived Aspergillus terreus. Chem. Biodivers. 2016, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.W.; Chiang, Y.R.; Pang, K.L.; Kuo, Y.H.; Shih, T.Y.; Lee, T.H. Highly Oxygenated Constituents from a Marine Alga-Derived Fungus Aspergillus giganteus NTU967. Mar. Drugs 2020, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, L.; Cai, S.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Diorcinols B–E, New Prenylated Diphenyl Ethers from the Marine-Derived Fungus Aspergillus versicolor ZLN-60. J. Antibiot. 2013, 66, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sun, X.; Yu, G.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Cladosins A–E, Hybrid Polyketides from a Deep-Sea-Derived Fungus, Cladosporium sphaerospermum. J. Nat. Prod. 2014, 77, 270–275. [Google Scholar] [CrossRef]
- Yu, G.H.; Wu, G.W.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Cladosins F and G, Two New Hybrid Polyketides from the Deep-Sea-Derived Cladosporium sphaerospermum 2005-01-E3. J. Asian Nat. Prod. Res. 2015, 17, 120–124. [Google Scholar] [CrossRef]
- Dong, Y.J.; Hou, G.M.; Lin, B.; Li, D.Y.; Li, Z.L. Three New Polyketides from Ascotricha sp. ZJ-M-5 by the OSMAC Strategy. J. Asian Nat. Prod. Res. 2019, 21, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.G.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and Sesquiterpene Derivatives from a Marine-Derived Fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Singab, A.; Lin, W.; Liu, Z.; Proksch, P. Two New Triterpenoids and a New Naphthoquinone Derivative Isolated from a Hard Coral-Derived Fungus Scopulariopsis sp. Fitoterapia 2017, 116, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Wei, H.; Zhang, Z.; Che, Q.; Liu, Y.; Zhu, T.; Mándi, A.; Kurtán, T.; Gu, Q.; Li, D. Eleganketal a, a Highly Oxygenated Dibenzospiroketal from the Marine-Derived Fungus Spicaria elegans KLA03. J. Nat. Prod. 2014, 77, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; He, W.; Lin, X.; Zhou, X.; Liu, Y. One Strain-Many Compounds Method for Production of Polyketide Metabolites Using the Sponge-Derived Fungus Arthrinium arundinis ZSDS1-F3. Chem. Nat. Compd. 2017, 53, 373–374. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Qin, Y.; Karthik, L.; Zhu, G.; Hou, C.; Jiang, L.; Liu, M.; Ye, X.; Liu, M.; et al. A New Abyssomicin Polyketide with Anti-Influenza A Virus Activity from a Marine-Derived Verrucosispora sp. MS100137. Appl. Microbiol. Biotechnol. 2020, 104, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R.A. Dereplication Strategies for Targeted Isolation of New Antitrypanosomal Actinosporins A and B from a Marine Sponge Associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Wang, J.N.; Zhang, D.S.; Ma, Z.J. Derivatives of Holomycin and Cyclopropaneacetic Acid from Streptomyces sp. DT-A37. Chem. Biodivers. 2017, 14, e1700140. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.J.; Liu, W.; Liang, W.L.; Xu, Z.; Le, X.; Xu, J.; Lam, C.K.; Yang, D.P.; Li, H.J.; Wang, L.Y. Pseudaboydins A and B: Novel Isobenzofuranone Derivatives from Marine Fungus Pseudallescheria boydii Associated with Starfish Acanthaster planci. Mar. Drugs 2014, 12, 4188–4199. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.F.; Lan, W.J.; Wang, K.T.; Huang, L.; Jiang, C.W.; Li, H.J. Two Chlorinated Benzofuran Derivatives from the Marine Fungus Pseudallescheria boydii. Nat. Prod. Commun. 2015, 10, 621–622. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.S.; Rong, X.G.; Kang, H.H.; Ma, L.Y.; Hamann, M.T.; Liu, W.Z. Raistrickiones A–E from a Highly Productive Strain of Penicillium raistrickii Generated through Thermo Change. Mar. Drugs 2018, 16, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auckloo, B.N.; Pan, C.; Akhter, N.; Wu, B.; Wu, X.; He, S. Stress-Driven Discovery of Novel Cryptic Antibiotics from a Marine Fungus Penicillium sp. BB1122. Front. Microbiol. 2017, 8, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureram, S.; Kesornpun, C.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Directed Biosynthesis through Biohalogenation of Secondary Metabolites of the Marine-Derived Fungus Aspergillus unguis. RSC Adv. 2013, 3, 1781–1788. [Google Scholar] [CrossRef]
- Huang, H.; Wang, F.; Luo, M.; Chen, Y.; Song, Y.; Zhang, W.; Zhang, S.; Ju, J. Halogenated Anthraquinones from the Marine-Derived Fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012, 75, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Dyshlovoy, S.A.; Von Amsberg, G.; Yurchenko, E.A.; Afiyatullov, S.S. Unique Prostate Cancer-Toxic Polyketides from Marine Sediment-Derived Fungus Isaria felina. J. Antibiot. 2017, 70, 856–858. [Google Scholar] [CrossRef]
- Yin, Y.; Fu, Q.; Wu, W.; Cai, M.; Zhou, X.; Zhang, Y. Producing Novel Fibrinolytic Isoindolinone Derivatives in Marine Fungus Stachybotrys longispora FG216 by the Rational Supply of Amino Compounds According to Its Biosynthesis Pathway. Mar. Drugs 2017, 15, 214. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, D.; Ding, N.; Chen, S.; Song, C.; Luo, Y.; Fu, X.; Bi, X.; Niu, H. New Cytotoxic Natural Products from the Marine Sponge-Derived Fungus: Pestalotiopsis sp. by Epigenetic Modification. RSC Adv. 2020, 10, 37982–37988. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA Methyltransferase Inhibitor Induced Fungal Biosynthetic Products: Diethylene Glycol Phthalate Ester Oligomers from the Marine-Derived Fungus Cochliobolus lunatus. Mar. Biotechnol. 2016, 18, 409–417. [Google Scholar] [CrossRef]
- He, X.; Zhang, Z.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Varilactones and Wortmannilactones Produced by Penicillium variabile Cultured with Histone Deacetylase Inhibitor. Arch. Pharm. Res. 2018, 41, 57–63. [Google Scholar] [CrossRef]
- Igboeli, H.A.; Marchbank, D.H.; Correa, H.; Overy, D.; Kerr, R.G. Discovery of Primarolides A and B from Marine Fungus Asteromyces cruciatus Using Osmotic Stress and Treatment with Suberoylanilide Hydroxamic Acid. Mar. Drugs 2019, 17, 435. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Shi, X.H.; Zhang, Y.H.; Shao, C.L.; Fu, X.M.; Li, X.; Yao, G.S.; Wang, C.Y. Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules 2020, 25, 3927. [Google Scholar] [CrossRef]
- Li, X.; Xia, Z.; Tang, J.; Wu, J.; Tong, J.; Li, M.; Ju, J.; Chen, H.; Wang, L. Identification and Biological Evaluation of Secondary Metabolites from Marine Derived Fungi Aspergillus sp. SCSIOW3, Cultivated in the Presence of Epigenetic Modifying Agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, X.; Wu, G.; Liu, C.; Lu, C.; Gu, Q.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Aniline-Tetramic Acids from the Deep-Sea-Derived Fungus Cladosporium sphaerospermum L3P3 Cultured with the HDAC Inhibitor SAHA. J. Nat. Prod. 2018, 81, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; Van Olphen, A.; Baker, B.J. Epigenetic Tailoring for the Production of Anti-Infective Cytosporones from the Marine Fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Henrikson, J.C.; Hoover, A.R.; Lee, A.E.; Cichewicz, R.H. Epigenetic Remodeling of the Fungal Secondary Metabolome. Org. Biomol. Chem. 2008, 6, 1895–1897. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, C.; Chen, M.; Liu, Q.; Wang, C. Brominated Resorcylic Acid Lactones from the Marine-Derived Fungus Cochliobolus lunatus Induced by Histone Deacetylase Inhibitors. Tetrahedron Lett. 2014, 55, 4888–4891. [Google Scholar] [CrossRef]
- Gao, S.S.; Shang, Z.; Li, X.M.; Li, C.S.; Cui, C.M.; Wang, B.G. Secondary Metabolites Produced by Solid Fermentation of the Marine-Derived Fungus Penicillium commune QSD-17. Biosci. Biotechnol. Biochem. 2012, 76, 358–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.J.; Li, D.Y.; Li, Y.C.; Hua, H.M.; Ma, E.L.; Li, Z.L. Caryophyllene Sesquiterpenes from the Marine-Derived Fungus Ascotricha sp. ZJ-M-5 by the One Strain-Many Compounds Strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef]
- Xie, L.R.; Li, D.Y.; Li, Z.L.; Hua, H.M.; Le Wang, P.; Wu, X. A New Cyclonerol Derivative from a Marine-Derived Fungus Ascotricha sp. ZJ-M-5. Nat. Prod. Res. 2013, 27, 847–850. [Google Scholar] [CrossRef]
- Xie, L.R.; Li, D.Y.; Wang, P.L.; Hua, H.M.; Wu, X.; Li, Z.L. A New 3, 4-seco-lanostane Triterpenoid from a Marine-Derived Fungus Ascotricha sp. ZJ-M-5. Acta Pharm. Sin. 2013, 48, 89–93. [Google Scholar]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the Coral-Derived Fungus Trichoderma harzianum (XS-20090075) Induced by Chemical Epigenetic Manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eremophilane-Type Sesquiterpenoids in a Deep-Sea Fungus Eutypella sp. Activated by Chemical Epigenetic Manipulation. Tetrahedron 2018, 74, 7310–7325. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Tang, J.; Li, X. Eremophilane Sesquiterpenes from a Deep Marine-Derived Fungus, Aspergillus sp. SCSIOW2, Cultivated in the Presence of Epigenetic Modifying Agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Zhu, G.; Hao, J.; Wang, Y.; Zhu, W. Chemical-Epigenetic Method to Enhance the Chemodiversity of the Marine Algicolous Fungus, Aspergillus terreus OUCMDZ-2739. Tetrahedron 2018, 74, 83–87. [Google Scholar] [CrossRef]
- Miao, F.P.; Liang, X.R.; Liu, X.H.; Ji, N.Y. Aspewentins A–C, Norditerpenes from a Cryptic Pathway in an Algicolous Strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef]
- Chung, Y.M.; Wei, C.K.; Chuang, D.W.; El-Shazly, M.; Hsieh, C.T.; Asai, T.; Oshima, Y.; Hsieh, T.J.; Hwang, T.L.; Wu, Y.C.; et al. An Epigenetic Modifier Enhances the Production of Anti-Diabetic and Anti-Inflammatory Sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef]
- Wu, J.S.; Yao, G.S.; Shi, X.H.; Rehman, S.U.; Xu, Y.; Fu, X.M.; Zhang, X.L.; Liu, Y.; Wang, C.Y. Epigenetic Agents Trigger the Production of Bioactive Nucleoside Derivatives and Bisabolane Sesquiterpenes from the Marine-Derived Fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersen, V.; Teppo, R.M.; Isaksson, J.; Andersen, J.H.; Gerwick, W.H.; Hansen, E. Characterization of Rhamnolipids Produced by an Arctic Marine Bacterium from the Pseudomonas fluorescence Group. Mar. Drugs 2018, 16, 163. [Google Scholar] [CrossRef] [Green Version]
- Uchoa, P.K.S.; Pimenta, A.T.A.; Braz-Filho, R.; de Oliveira, M.D.C.F.; Saraiva, N.N.; Rodrigues, B.S.F.; Pfenning, L.H.; Abreu, L.M.; Wilke, D.V.; Florêncio, K.G.D.; et al. New Cytotoxic Furan from the Marine Sediment-Derived Fungi Aspergillus niger. Nat. Prod. Res. 2017, 31, 2599–2603. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.H.; Liang, X.; Qi, S.H. Eight New Cyclopentenone and Cyclohexenone Derivatives from the Marine-Derived Fungus Aspergillus sp. SCSIO 41501 by OSMAC Strategy. Nat. Prod. Res. 2020, 35, 3810–3819. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Sun, K.; Zhu, W. Effects of High Salt Stress on Secondary Metabolite Production in the Marine-Derived Fungus Spicaria elegans. Mar. Drugs 2011, 9, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.X.; Ding, L.; He, S. Discovery of a New Biphenyl Derivative by Epigenetic Manipulation of Marine-Derived Fungus Aspergillus versicolor. Nat. Prod. Res. 2019, 33, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Z.; Chen, Y.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Varitatin A, a Highly Modified Fatty Acid Amide from Penicillium variabile Cultured with a DNA Methyltransferase Inhibitor. J. Nat. Prod. 2015, 78, 2841–2845. [Google Scholar] [CrossRef]
- Liu, M.; Grkovic, T.; Liu, X.; Han, J.; Zhang, L.; Quinn, R.J. A Systems Approach Using OSMAC, Log P and NMR Fingerprinting: An approach to novelty. Synth. Syst. Biotechnol. 2017, 2, 276–286. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinedo-Rivilla, C.; Aleu, J.; Durán-Patrón, R. Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches. Mar. Drugs 2022, 20, 84. https://doi.org/10.3390/md20020084
Pinedo-Rivilla C, Aleu J, Durán-Patrón R. Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches. Marine Drugs. 2022; 20(2):84. https://doi.org/10.3390/md20020084
Chicago/Turabian StylePinedo-Rivilla, Cristina, Josefina Aleu, and Rosa Durán-Patrón. 2022. "Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches" Marine Drugs 20, no. 2: 84. https://doi.org/10.3390/md20020084
APA StylePinedo-Rivilla, C., Aleu, J., & Durán-Patrón, R. (2022). Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches. Marine Drugs, 20(2), 84. https://doi.org/10.3390/md20020084







