Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects
Abstract
:1. Introduction
2. Results
2.1. Demographic, Baseline Characteristics, and Compliance
2.2. Food Intake and Physical Activity
2.3. Anthropometric Measurement, Body Composition, Blood Pressure, and Heart Rate
2.4. Fasting Glycemic, Lipid, and Hepatic Biomarkers in the Fasting State
2.5. Glucose, Insulin, and C-Peptide during OGTT
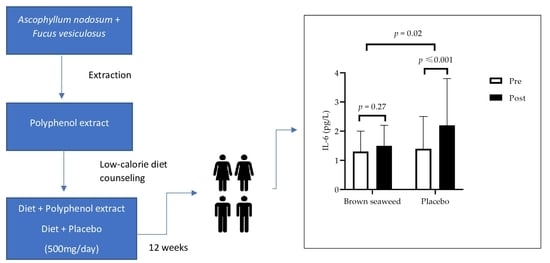
2.6. Inflammatory Status, Oxidative Stress Status, and Gut Integrity
2.7. Adverse Effects
3. Discussion
3.1. Weight Loss
3.2. Glucose, Insulin, and C-Peptide
3.3. Lipid Profile, Inflammation, and Heart Rate
3.4. Integrity of Gut Barrier
4. Materials and Methods
4.1. Study Design
4.2. Population
4.3. Intervention
4.4. Anthropometric and Body Composition Measurements
4.5. Blood Pressure and Heart Rate Measurements
4.6. Oral Glucose Tolerance Test (OGTT)
4.7. Blood Collection and Storage
4.8. Glycemia, Insulin, C-Peptide, and Glycated Hemoglobin A1C Measurements
4.9. Lipid Profile Biomarkers Measurements
4.10. Hepatic Enzymes Measurements
4.11. Inflammatory, Oxidative Stress and Gut Integrity Biomarkers
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF) (Ed.) IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- International Diabetes Federation (IDF) (Ed.) IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013. [Google Scholar]
- Algoblan, A.; Alalfi, M.; Khan, M. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.; Punthakee, Z. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2013, 37, S8–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Chen, C.; Li, S.; Kong, F.; Shan, P.; Huang, W. Serum markers of endothelial dysfunction and inflammation increase in hypertension with prediabetes mellitus. Genet. Test. Mol. Biomark. 2016, 20, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Maschirow, L.; Khalaf, K.; Al-Aubaidy, H.; Jelinek, H. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes—Biomarkers as a possible tool for early disease detection for rural screening. Clin. Biochem. 2015, 48, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allin, K.H.; The IMI-DIRECT Consortium; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2017, 40, S57–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovic, T.P.; Jenkins, A.B.; Campbell, L.V.; Furler, S.M.; Kraegen, E.W.; Chisholm, D.J. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 1998, 21, 687–694. [Google Scholar] [CrossRef]
- Wing, R.R.; Blair, E.H.; Bononi, P.; Marcus, M.D.; Watanabe, R.; Bergman, R.N. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994, 17, 30–36. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Lau, D.C.W.; Douketis, J.D.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; for the Obesity Canada Clinical Practice Guidelines Steering Committee and Expert Panel. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. Can. Med. Assoc. J. 2007, 176, 1103–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards New pharmacological interventions for diabetes mellitus type 2. Molecules 2017, 22, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.-C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Gabbia, D.; Dall’Acqua, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; Giron, M.C.; et al. The phytocomplex from Fucus vesiculosus and Ascophyllum nodosum controls postprandial plasma glucose levels: An in vitro and in vivo study in a mouse model of NASH. Mar. Drugs 2017, 15, 41. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Shan, X.D.; Liu, X.; Hao, J.J.; Cai, C.; Fan, F.; Dun, Y.L.; Zhao, X.L.; Liu, X.X.; Li, C.X.; Yu, G.L. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef]
- Lee, T.; Dugoua, J.-J. Nutritional supplements and their effect on glucose control. Curr. Diabetes Rep. 2011, 11, 142–148. [Google Scholar] [CrossRef]
- Khaodhiar, L.; Cummings, S.; Apovian, C.M. Treating diabetes and prediabetes by focusing on obesity management. Curr. Diabetes Rep. 2009, 9, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Padwal, R.S.; Sharma, A.M. Prevention of cardiovascular disease: Obesity, diabetes and the metabolic syndrome. Can. J. Cardiol. 2010, 26, 18C–20C. [Google Scholar] [CrossRef] [Green Version]
- Lau, D.C. Current and emerging pharmacotherapies for type 2 diabetes. Can. J. Diabetes 2015, 39, S127–S128. [Google Scholar] [CrossRef] [Green Version]
- De Martin, S.; Gabbia, D.; Carrara, M.; Ferri, N. The brown algae Fucus vesiculosus and Ascophyllum nodosum reduce metabolic syndrome risk factors: A clinical study. Nat. Prod. Commun. 2018, 13, 1934578X1801301228. [Google Scholar] [CrossRef] [Green Version]
- DeRosa, G.; Cicero, A.F.; D’Angelo, A.; Maffioli, P. Ascophyllum nodosum and Fucus vesiculosus on glycemic status and on endothelial damage markers in dysglicemic patients. Phytother. Res. 2019, 33, 791–797. [Google Scholar] [CrossRef]
- Kowall, B.; Rathmann, W.; Heier, M.; Holle, R.; Peters, A.; Thorand, B.; Herder, C.; Strassburger, K.; Giani, G.; Meisinger, C. Impact of weight and weight change on normalization of prediabetes and on persistence of normal glucose tolerance in an older population: The KORA S4/F4 study. Int. J. Obes. 2012, 36, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, O. Le Peptide-C. Immunoanal. Biol. Spec. 2000, 15, 94–95. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, B.; Hu, F.; Su, Q. OGTT 1 h serum C-peptide to plasma glucose concentration ratio is more related to beta cell function and diabetes mellitus. Oncotarget 2017, 8, 51786–51791. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Boyko, E.J.; Sato, K.K.; McNeely, M.J.; Leonetti, D.L.; Kahn, S.E.; Fujimoto, W.Y. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care 2013, 36, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; Jenkinson, C.P.; Richardson, D.K.; Tripathy, D.; DeFronzo, R.A. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: Results from the veterans administration genetic epidemiology study. Diabetes 2006, 55, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult treatment panel III). JAMA 2012, 285, 2486–2497. [Google Scholar]
- American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care 2004, 27, s68–s71. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean Individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Iacoviello, L.; Zito, F.; Rago, L.; Di Castelnuovo, A.; De Curtis, A.; Zappacosta, B.; de Gaetano, G.; Donati, M.B.; Cerletti, C. Prolonged administration of Ascophyllum nodosum to healthy human volunteers and cardiovascular risk. Nutrafoods 2013, 12, 137–144. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Hayes, M.; Sweeney, T. Extracts of brown seaweeds can attenuate the bacterial lipopolysaccharide-induced pro-inflammatory response in the porcine colon ex vivo. J. Anim. Sci. 2012, 90, 46–48. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Moradi, F.; Maleki, V.; Saleh-Ghadimi, S.; Kooshki, F.; Gargari, B.P. Potential roles of chromium on inflammatory biomarkers in diabetes: A systematic. Clin. Exp. Pharmacol. Physiol. 2019, 46, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Park, W.-C.; Seo, I.; Kim, S.-H.; Lee, Y.-J.; Ahn, S.V. Association between resting heart rate and inflammatory markers (white blood cell count and high-sensitivity C-reactive protein) in healthy Korean people. Korean J. Fam. Med. 2017, 38, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Whelton, S.P.; Narla, V.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Jenny, N.S.; Al-Mallah, M.; Michos, E. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the multi-ethnic study of atherosclerosis). Am. J. Cardiol. 2014, 113, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-K.; Heo, S.-J.; Jeon, Y.-J.; Lee, C.-M.; Park, Y.-M.; Byun, H.-G.; Choi, Y.H.; Park, S.-G.; Choi, I.-W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; Macdonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.W.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Charoensiddhi, S.; Conlon, M.A.; Methacanon, P.; Franco, C.; Su, P.; Zhang, W. Gut health benefits of brown seaweed Ecklonia radiata and its polysaccharides demonstrated in vivo in a rat model. J. Funct. Foods 2017, 37, 676–684. [Google Scholar] [CrossRef]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed components as potential modulators of the gut microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef] [Green Version]
- Flack, K.D.; Siders, W.A.; Johnson, L.; Roemmich, J.N. Cross-validation of resting metabolic rate prediction equations. J. Acad. Nutr. Diet. 2016, 116, 1413–1422. [Google Scholar] [CrossRef] [Green Version]
- Diabetes-Québec. Meal Planning for People with Diabetes. 2017. Available online: https://publications.msss.gouv.qc.ca/msss/fichiers/2016/16-215-01A.pdf (accessed on 20 February 2020).
- Harvard Medical School. The Healthy Eating Plate. 2011. Available online: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/30/2012/09/HEPJan2015.jpg (accessed on 6 September 2019).
- Labonté, M.; Cyr, A.; Baril-Gravel, L.; Royer, M.-M.; Lamarche, B. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur. J. Clin. Nutr. 2012, 66, 166–173. [Google Scholar] [CrossRef] [Green Version]
- IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). Short and Long Forms. 2005. Available online: http://www.ipaq.ki.se/scoring.pdf (accessed on 15 March 2010).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Pullar, T.; Kumar, S.; Feely, M. Compliance in clinical trials. Ann. Rheum. Dis. 1989, 48, 871–875. [Google Scholar] [CrossRef]
- Kunst, A.; Draeger, B.; Ziegenhorn, J. UV methods with hexokinase and glucose-6-phosphate dehydrogenase. In Methods of Enzymatic Analysis; Bergemeyer, H.Y., Ed.; Verlag Chemie: Deerfield, Germany, 1984; Volume VI, pp. 163–172. [Google Scholar]
- Pacini, G.; Mari, A. Methods for clinical assessment of insulin sensitivity and β-cell function. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 305–322. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Scheibe, P.; Wahlefeld, A.W. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin. Chem. 1978, 24, 58–73. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Bisson, M.; Larose, J.; Pronovost, E.; Brien, M.; Greffard, K.; Marc, I. Physical fitness is associated with prostaglandin F2alpha isomers during pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 2019, 145, 7–14. [Google Scholar] [CrossRef]



| Brown Seaweed Extract (n = 27) | Placebo (n = 29) | pIxG3 | |||||
|---|---|---|---|---|---|---|---|
| Pre 1 | Post 1 | pI2 | Pre 1 | Post 1 | pI2 | ||
| Weight (kg) | 91 ± 13 | 89 ± 13 | <0.001 | 91 ± 14 | 89 ± 14 | 0.003 | 0.51 |
| BMI (kg/m2) | 33 ± 4 | 32 ± 4 | <0.001 | 33 ± 5 | 32 ± 4 | 0.003 | 0.55 |
| Waist (cm) | 109 ± 10 | 108 ± 10 | 0.003 | 108 ± 10 | 107 ± 10 | 0.007 | 0.76 |
| Total fat mass (kg) † | 38 ± 9 | 37 ± 9 | 0.001 | 39 ± 10 | 38 ± 10 | 0.001 | 0.93 |
| Total lean mass (kg) | 51 ± 8 | 50 ± 8 | 0.03 | 50 ± 10 | 50 ± 10 | 0.65 | 0.20 |
| Visceral fat mass (kg) | 1.9 ± 0.6 | 1.7 ± 0.6 | 0.04 | 1.6 ± 0.6 | 1.6 ± 0.5 | 0.23 | 0.52 |
| SBP (mmHg) | 119 ± 11 | 121 ± 12 | 0.27 | 118 ± 11 | 118 ± 12 | 0.93 | 0.39 |
| DBP (mmHg) | 75 ± 9 | 74 ± 9 | 0.51 | 76 ± 7 | 74 ± 8 | 0.14 | 0.58 |
| Brown Seaweed Extract (n = 27) | Placebo (n = 29) | pIxG3 | |||||
|---|---|---|---|---|---|---|---|
| Pre 1 | Post 1 | pI2 | Pre 1 | Post 1 | pI2 | ||
| Fasting glucose (mmol/L) | 5.8 ± 0.5 | 5.7 ± 0.4 | 0.14 | 5.7 ± 0.6 | 5.6 ± 0.5 | 0.45 | 0.60 |
| Fasting insulin (pmol/L) † | 129 ± 49 | 123 ± 48 | 0.22 | 123 ± 63 | 114 ± 73 | 0.49 | 0.76 |
| Fasting C-peptide (pmol/L) | 1009 ± 298 | 957 ± 284 | 0.06 | 945 ± 307 | 916 ± 306 | 0.26 | 0.60 |
| Hemoglobin A1c (%) | 5.6 ± 0.3 | 5.6 ± 0.3 | 0.83 | 5.6 ± 0.2 | 5.6 ± 0.2 | 0.46 | 0.72 |
| Total chol (mmol/L) | 5.5 ± 0.8 | 5.2 ± 0.8 | 0.07 | 5.1 ± 0.8 | 5.1 ± 0.7 | 0.48 | 0.07 |
| Triglycerides (mmol/L) | 1.5 ± 0.7 | 1.7 ± 0.8 | 0.20 | 1.5 ± 0.5 | 1.5 ± 0.4 | 0.55 | 0.60 |
| LDLc (mmol/L) | 3.1 ± 0.7 | 2.9 ± 0.8 | 0.06 | 3.0 ± 0.7 | 3.0 ± 0.6 | 0.36 | 0.05 |
| HDLc (mmol/L) | 1.4 ± 0.3 | 1.3 ± 0.2 | 0.001 | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.25 | 0.09 |
| Chol/HDLc | 4.4 ± 0.8 | 4.4 ± 0.9 | 0.12 | 4.4 ± 0.9 | 4.4 ± 0.8 | 0.17 | 0.86 |
| HOMA-IR | 4.8 ± 1.9 | 4.5 ± 1.8 | 0.18 | 4.5 ± 2.5 | 4.0 ± 2.8 | 0.65 | 0.57 |
| AST (U/L) | 23 ± 8 | 20 ± 5 | 0.02 | 25 ± 11 | 20 ± 8 | 0.004 | 0.69 |
| ALT (U/L) | 24 ± 8 | 24 ± 8 | 0.99 | 28 ± 20 | 31 ± 18 | 0.04 | 0.15 |
| AST/ALT | 1.1 ± 0.5 | 0.9 ± 0.2 | 0.07 | 1.1 ± 0.4 | 0.8 ± 0.3 | 0.001 | 0.23 |
| Brown Seaweed Extract (n = 27) | Placebo (n = 29) | pIxG3 | |||||
|---|---|---|---|---|---|---|---|
| Pre 1 | Post 1 | pI2 | Pre 1 | Post 1 | pI2 | ||
| hsCRP (mg/L) † | 3.0 ± 1.9 | 3.6 ± 2.7 | 0.67 | 2.7 ± 1.6 | 3.4 ± 2.6 | 0.88 | 0.84 |
| F2-isoprostane (8-iso PGF2α) × 10−1 ng/mL | 2.2 ± 0.8 | 2.4 ± 0.8 | 0.65 | 2.5 ± 1.9 | 3.0 ± 2.3 | 0.53 | 0.76 |
| LBP × 103 (ng/mL) | 6.4 ± 1.9 | 8.3 ± 1.8 | 0.002 | 6.8 ± 1.3 | 7.7 ± 1.9 | 0.17 | 0.20 |
| Zonulin (ng/mL) | 1.2 ± 0.6 | 1.2 ± 1.0 | 0.60 | 1.7 ± 1.0 | 1.9 ± 1.1 | 0.53 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.-F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174. https://doi.org/10.3390/md20030174
Vodouhè M, Marois J, Guay V, Leblanc N, Weisnagel SJ, Bilodeau J-F, Jacques H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Marine Drugs. 2022; 20(3):174. https://doi.org/10.3390/md20030174
Chicago/Turabian StyleVodouhè, Marlène, Julie Marois, Valérie Guay, Nadine Leblanc, Stanley John Weisnagel, Jean-François Bilodeau, and Hélène Jacques. 2022. "Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects" Marine Drugs 20, no. 3: 174. https://doi.org/10.3390/md20030174
APA StyleVodouhè, M., Marois, J., Guay, V., Leblanc, N., Weisnagel, S. J., Bilodeau, J.-F., & Jacques, H. (2022). Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Marine Drugs, 20(3), 174. https://doi.org/10.3390/md20030174