Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity of Heated Crude MAAs
2.2. ABTS Radical Scavenging Activity of Heated MAAs
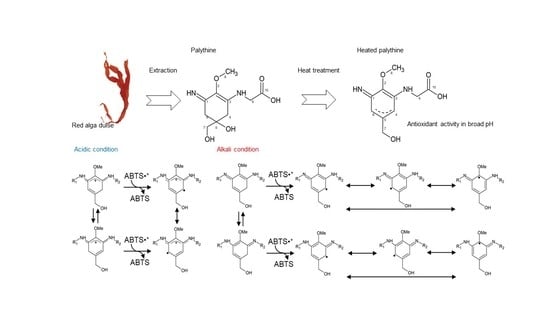
2.3. Structural Changes of Heated MAAs
2.4. NMR Analyses of Heated Palythine
2.5. Presumed Stabilization Mechanisms of Heated MAAs
2.6. Efficient Production of Heated MAAs
2.7. Character of Heated Usujirene
3. Materials and Methods
3.1. Algal Material
3.2. Preparation of Crude MAAs
3.3. Preparation of Purified MAAs from Dulse
3.4. Heat Treatment of MAAs
3.5. Assay of Antioxidant Activity
3.6. Spectrophotometric Analysis of Heat-Treated MAAs
3.7. ESI-MS Analysis of Heat-Treated MAAs
3.8. 1H- and 13C-NMR Analyses of Heat-Treated Palythine
3.9. Efficient Production of Heated MAAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef] [PubMed]
- Code, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Biol. 2016, 164, 289–295. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [Green Version]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Kumagai, Y.; Tsubouchi, R.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. Complete sequence of mitochondrial DNA of red alga dulse Palmaria palmata (Linnaeus) Weber & Mohr in Japan. Mitochondrial DNA Part B 2019, 4, 3177–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyabe, Y.; Furuta, T.; Takeda, T.; Kanno, G.; Shimizu, T.; Tanaka, Y.; Gai, Z.; Yasui, H.; Kishimura, H. Structural Properties of Phycoerythrin from Dulse Palmaria palmata. J. Food Biochem. 2017, 41, e12301. [Google Scholar] [CrossRef] [Green Version]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, Y.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. In Silico Analysis of Relationship between Proteins from Plastid Genome of Red Alga Palmaria sp. (Japan) and Angiotensin I Converting Enzyme Inhibitory Peptides. Mar. Drugs 2019, 17, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kumagai, Y.; Yamamoto, Y.; Yasui, H.; Kishimura, H. Identification of a Key Enzyme for the Hydrolysis of β-(1→3)-Xylosyl Linkage in Red Alga Dulse Xylooligosaccharide from Bifidobacterium adolescentis. Mar. Drugs 2020, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Kobayashi, M.; Miyabe, Y.; Kishimura, H.; Hatanaka, T.; Kumagai, Y. Preparation of β(1→3)/β(1→4) xylooligosaccharides from red alga dulse by two xylanases from Streptomyces thermogriseus. Bioresour. Bioprocess. 2021, 8, 38. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kishimura, H.; Kinoshita, Y.; Saburi, W.; Kumagai, Y.; Yasui, H.; Ojima, T. Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem. 2019, 82, 117–122. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Yoshiki, M.; Tsuge, K.; Tsuruta, Y.; Yoshimura, T.; Koganemaru, K.; Sumi, T.; Matsui, T.; Matsumoto, K. Production of new antioxidant compound from mycosporine-like amino acid, porphyra-334 by heat treatment. Food Chem. 2009, 113, 1127–1132. [Google Scholar] [CrossRef]
- Nakano, T.; Watanabe, M.; Sato, M.; Takeuchi, M. Characterization of catalase from the seaweed Porphyra yezoensis. Plant Sci. 1995, 104, 127–133. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; López-Hernández, J. An Overview on Effects of Processing on the Nutritional Content and Bioactive Compounds in Seaweeds. Foods 2021, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.C.; Wright, J.L.C.; Simpson, F.J. Review of chemical constituents of the red alga Palmaria palmata (dulse). Econ. Bot. 1980, 34, 27–50. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the Major Antioxidant in Hijikia fusiformis, a Common Edible Seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine Natural Products as Novel Antioxidant Prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Xiaojun, Y.; Xiancui, L.; Chengxu, Z.; Xiao, F. Prevention of Fish Oil Rancidity by Phlorotannins from Sargassum kjellmanianum. J. Appl. Phycol. 1996, 8, 201–203. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant Activity of Fresh and Processed Edible Seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Yoshie, Y.; Wang, W.E.I.; Petillo, D.; Suzuki, T. Distribution of Catechins in Japanese Seaweeds. Fish. Sci. 2000, 66, 998–1000. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The Cyanobacterial UV-Absorbing Pigment Scytonemin Displays Radical-Scavenging Activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Burritt, D.J.; Larkindale, J.; Hurd, C.L. Antioxidant Metabolism in the Intertidal Red Seaweed Stictosiphonia arbuscula Following Desiccation. Planta 2002, 215, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-Screening Compounds, Mycosporine-Like Amino Acids, and Scytonemin in the Cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial Antioxidants and Thermal Stability Evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Nakayama, R.; Tamura, Y.; Kikuzaki, H.; Nakatani, N. Antioxidant effect of the constituents of susabinori (Porphyra yezoensis). J. Am. Oil Chem. Soc. 1999, 76, 649. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and Structure of Two New Amino Acids, Palythinol and Palythene, from the Zoanthid Palythoa tubercolosa. Tetrahedron Lett. 1978, 19, 4909–4912. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Daleo, G.; Marco, S.G.D. Occurrence of Mycosporine-Like Amino Acids in the Red-Tide Dinoflagellate Alexandrium excavatum: UV-Photoprotective Compounds? J. Plankton Res. 1990, 12, 909–921. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T. Changes of Radical-Scavenging Capacity and Ferrous Reducing Power in Chub Mackerel Scomber japonicus and Pacific Saury Cololabis saira During 4 °C Storage and Retorting. LWT-Food Sci. Technol. 2009, 42, 1070–1075. [Google Scholar] [CrossRef]







| Compounds | pH | |||
|---|---|---|---|---|
| 5.8 | 6.6 | 7.4 | 8.0 | |
| (µM) | ||||
| Palythine * | >72.0 | >72.0 | 23.4 | 12.0 |
| Heated palythine | 11.4 | 8.7 | 8.7 | 6.7 |
| Porphyra-334 * | >72.0 | >72.0 | 27.5 | 20.8 |
| Heated porphyra-334 | 12.8 | 13.4 | 12.4 | 6.3 |
| Ascorbic acid * | 19.1 | 19.4 | 12.4 | 8.9 |
| Position | Palythine * | Heated Palythine | ||
|---|---|---|---|---|
| δ1H (ppm) | δ13C (ppm) | δ1H (ppm) | δ13C (ppm) | |
| 1 | - | 161.5 | - | 165.9 |
| 2 | - | 125.9 | - | 127 |
| 3 | - | 158.7 | - | 160.9 |
| 4 | 2.60 (d) | 33.5 | 4.58 (s) or 4.66 (s) | 144.4 |
| 2.75 (d) | ||||
| 5 | - | 71.2 | - | 141.2 |
| 6 | 2.58 (d) | 35.7 | 4.58 (s) or 4.66 (s) | 29 |
| 2.86 (d) | ||||
| 7 | 3.48 (s) | 67.3 | 3.81 (s) | 65.8 |
| 8 | 3.56 (s) | 58.9 | 3.87 (s) | 63.5 |
| 9 | 3.95 (s) | 47 | 4.13 (s) | 47.4 |
| 10 | - | 174.6 | - | 178.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2022, 20, 184. https://doi.org/10.3390/md20030184
Nishida Y, Saburi W, Miyabe Y, Kishimura H, Kumagai Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Marine Drugs. 2022; 20(3):184. https://doi.org/10.3390/md20030184
Chicago/Turabian StyleNishida, Yuki, Wataru Saburi, Yoshikatsu Miyabe, Hideki Kishimura, and Yuya Kumagai. 2022. "Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan" Marine Drugs 20, no. 3: 184. https://doi.org/10.3390/md20030184
APA StyleNishida, Y., Saburi, W., Miyabe, Y., Kishimura, H., & Kumagai, Y. (2022). Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Marine Drugs, 20(3), 184. https://doi.org/10.3390/md20030184