Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling
Abstract
1. Introduction
2. Results
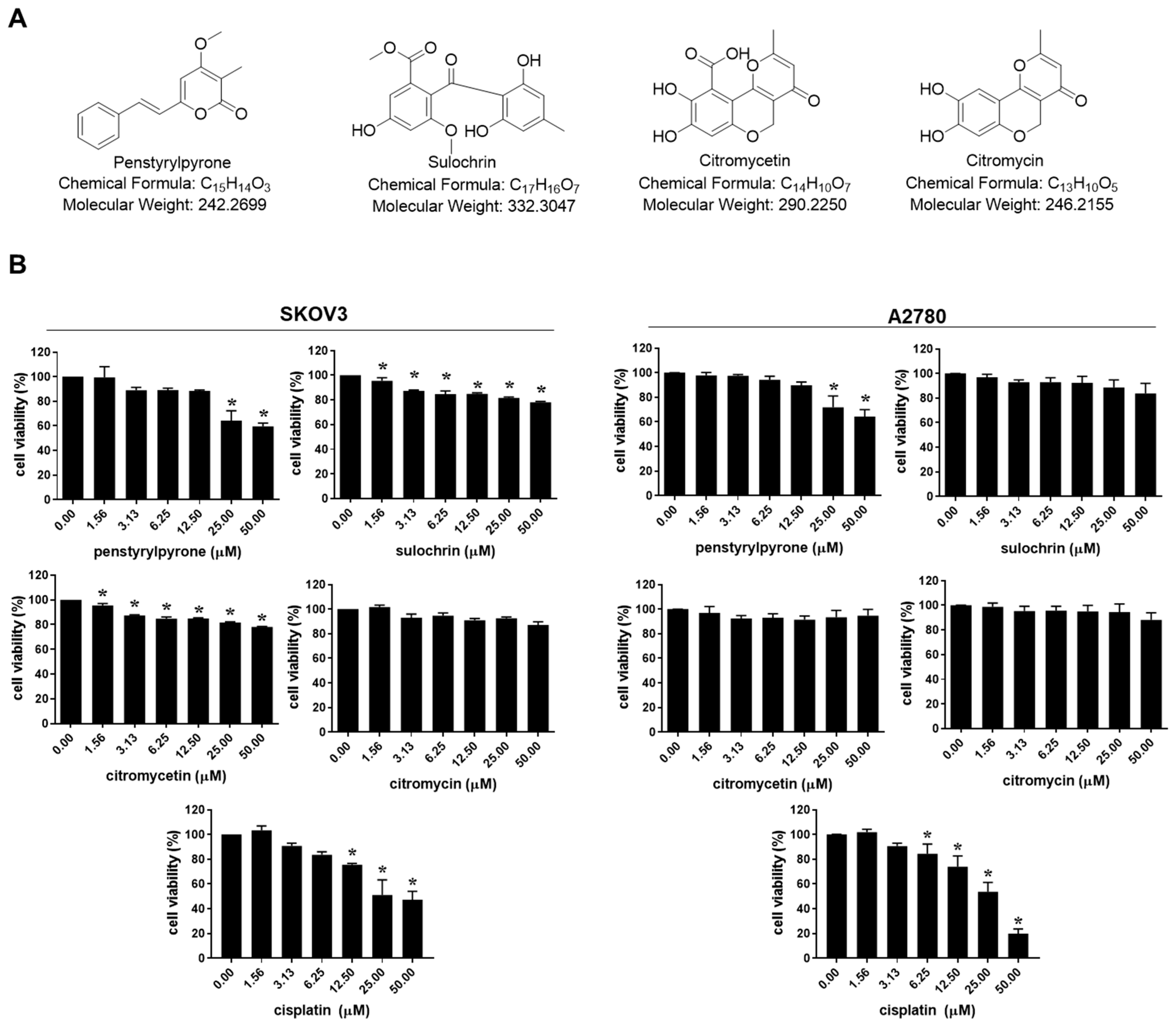
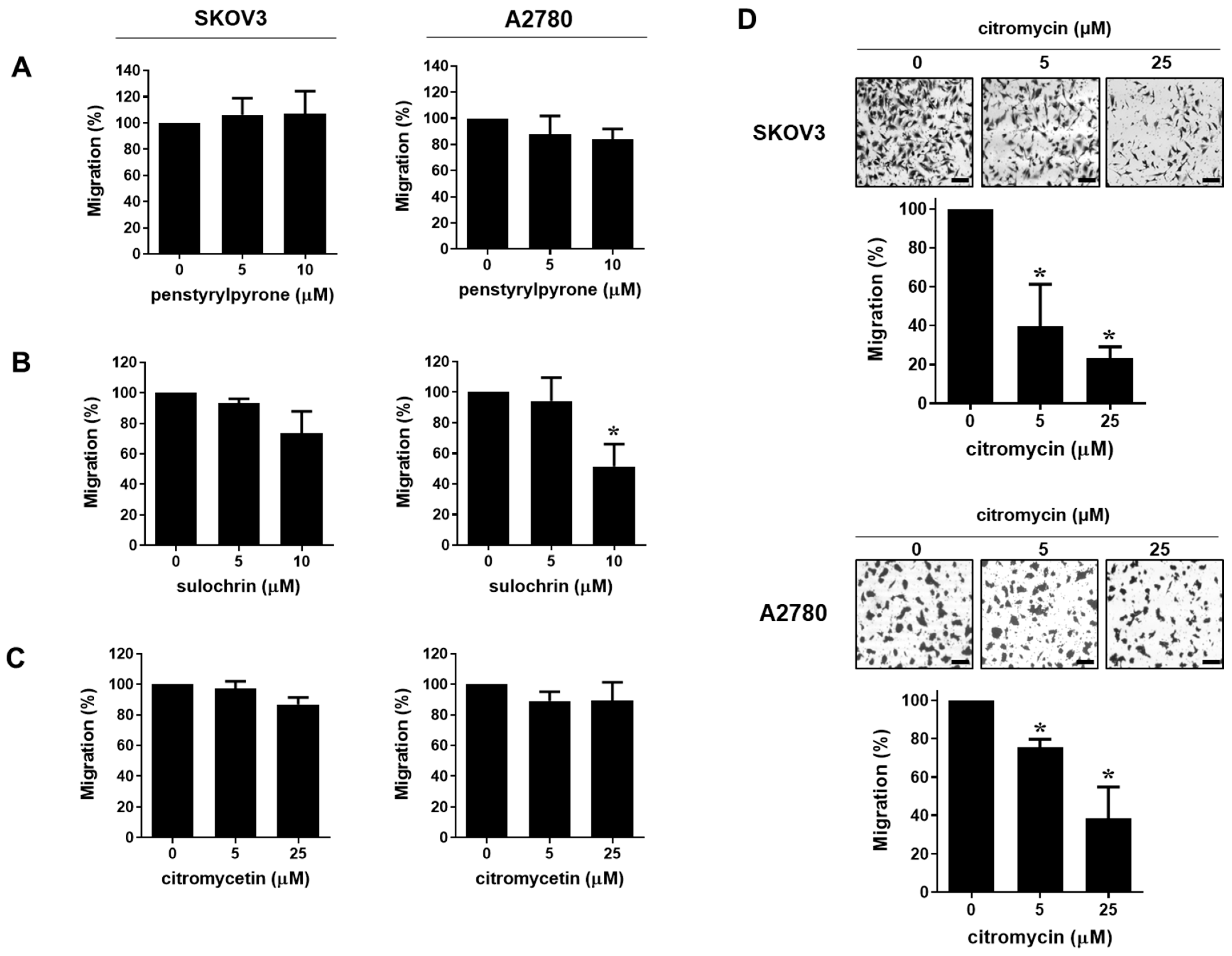
2.1. Citromycin Inhibits the Migration and Iinvasion of Human Ovarian Cancer Cells
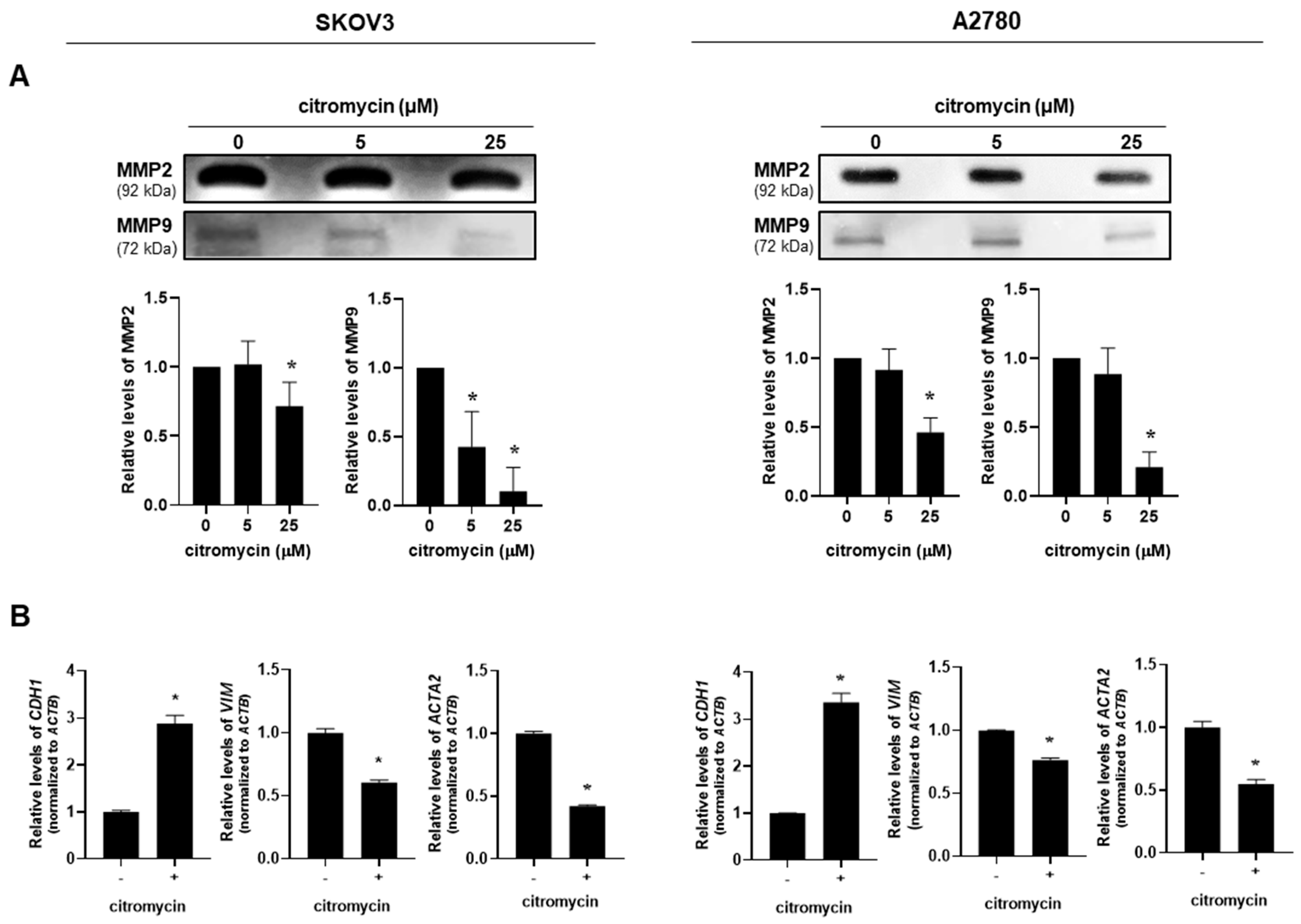
2.2. Citromycin Regulates MMP Activation and EMT-Related Gene Expression Levels in Human Ovarian Cancer Cells
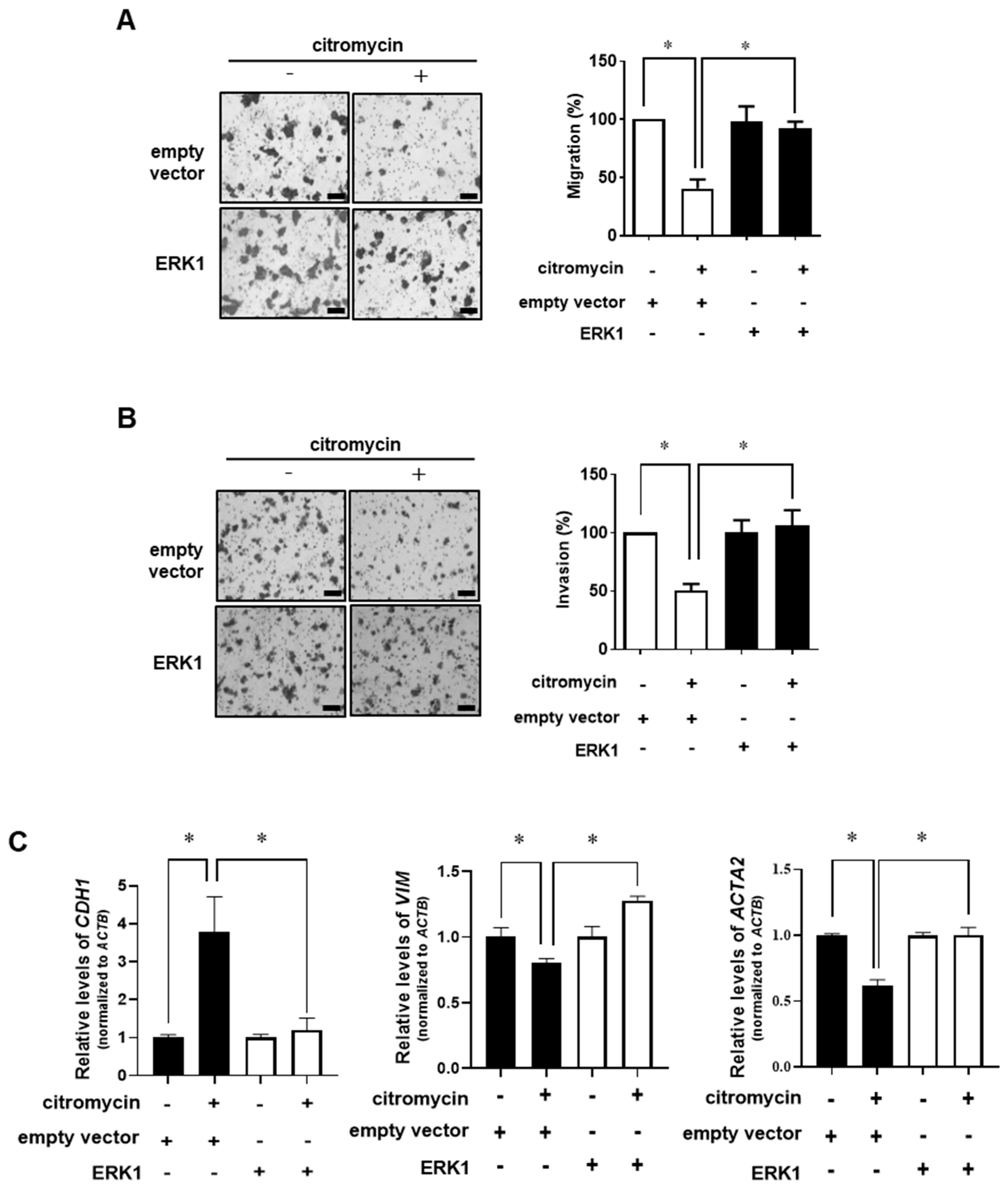
2.3. Extracellular Signal-Regulated Kinase (ERK)-1/2 Pathway Is Involved in Citromycin-Mediated Inhibition of Cell Migration and Invasion in Human Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Compounds
4.2. Cell Culture
4.3. MTT Assay
4.4. Western Blotting
4.5. Gelatin Zymography
4.6. Migration and Invasion Assay
4.7. Transfection
4.8. Real-Time Reverse Transcription PCR (RT-PCR)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Holschneider, C.H.; Berek, J.S. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin. Surg. Oncol. 2000, 19, 3–10. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Bhatt, P.; Vhora, I.; Patil, S.; Amrutiya, J.; Bhattacharya, C.; Misra, A.; Mashru, R. Role of antibodies in diagnosis and treatment of ovarian cancer: Basic approach and clinical status. J. Control Release 2016, 226, 148–167. [Google Scholar] [CrossRef]
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44–46, 1–6. [Google Scholar] [CrossRef]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by mmp-2 cleavage of vitronectin and fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Huang, S.; Van Arsdall, M.; Tedjarati, S.; McCarty, M.; Wu, W.; Langley, R.; Fidler, I.J. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J. Natl. Cancer Inst. 2002, 94, 1134–1142. [Google Scholar] [CrossRef]
- Wang, L.; Jin, X.; Lin, D.; Liu, Z.; Zhang, X.; Lu, Y.; Liu, Y.; Wang, M.; Yang, M.; Li, J.; et al. Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases -2 expression in ovarian carcinoma. Diagn. Pathol. 2013, 8, 190. [Google Scholar] [CrossRef]
- Li, L.N.; Zhou, X.; Gu, Y.; Yan, J. Prognostic value of mmp-9 in ovarian cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 4107–4113. [Google Scholar] [CrossRef]
- Nieman, M.T.; Prudoff, R.S.; Johnson, K.R.; Wheelock, M.J. N-cadherin promotes motility in human breast cancer cells regardless of their e-cadherin expression. J. Cell Biol. 1999, 147, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, M.; Kubota, S.; Kawase, A.; Kohyama, N.; Kobayashi, Y.; Yamamoto, T. Involvement of epithelial-mesenchymal transition in methotrexate-induced pulmonary fibrosis. J. Toxicol. Sci. 2014, 39, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. Emt, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for e-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, B.; Jiang, X.; Cao, J.; Yu, J.; Wang, Y.; Wang, X.; Liu, H. Identification and validation of a stromal emt related lncrna signature as a potential marker to predict bladder cancer outcome. Front. Oncol. 2021, 11, 620674. [Google Scholar] [CrossRef]
- Veatch, A.L.; Carson, L.F.; Ramakrishnan, S. Differential expression of the cell-cell adhesion molecule e-cadherin in ascites and solid human ovarian tumor cells. Int. J. Cancer 1994, 58, 393–399. [Google Scholar] [CrossRef]
- Cho, E.Y.; Choi, Y.; Chae, S.W.; Sohn, J.H.; Ahn, G.H. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol. Int. 2006, 56, 62–70. [Google Scholar] [CrossRef]
- Ojasalu, K.; Brehm, C.; Hartung, K.; Nischak, M.; Finkernagel, F.; Rexin, P.; Nist, A.; Pavlakis, E.; Stiewe, T.; Jansen, J.M.; et al. Upregulation of mesothelial genes in ovarian carcinoma cells is associated with an unfavorable clinical outcome and the promotion of cancer cell adhesion. Mol. Oncol. 2020, 14, 2142–2162. [Google Scholar] [CrossRef]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef]
- Webster, N.S.; Negri, A.P.; Munro, M.M.; Battershill, C.N. Diverse microbial communities inhabit antarctic sponges. Environ. Microbiol. 2004, 6, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Yim, J.H.; Lee, D.H. Metabolites from antarctic marine-derived sporothrix sp. Sf-7266. Life Sci. Nat. Resour. Res. 2020, 28, 45–49. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi phellinus and inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Lee, D.S.; Jang, J.H.; Ko, W.; Kim, K.S.; Sohn, J.H.; Kang, M.S.; Ahn, J.S.; Kim, Y.C.; Oh, H. Ptp1b inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. Jf-55. Mar. Drugs 2013, 11, 1409–1426. [Google Scholar] [CrossRef]
- Ohashi, H.; Ishikawa, M.; Ito, J.; Ueno, A.; Gleich, G.J.; Kita, H.; Kawai, H.; Fukamachi, H. Sulochrin inhibits eosinophil degranulation. J. Antibiot. 1997, 50, 972–974. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Zhang, W.; Zhou, J.; Tang, B.; Li, L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet. 2012, 286, 1537–1543. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural products from marine fungi--still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine fungi: A source of potential anticancer compounds. Front. Microbiol. 2017, 8, 2536. [Google Scholar] [CrossRef]
- Xu, J.L.; Liu, H.X.; Chen, Y.C.; Tan, H.B.; Guo, H.; Xu, L.Q.; Li, S.N.; Huang, Z.L.; Li, H.H.; Gao, X.X.; et al. Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-sea derived fungus Phomopsis lithocarpus fs508. Mar. Drugs 2018, 16, 329. [Google Scholar] [CrossRef]
- Inamori, Y.; Kato, Y.; Kubo, M.; Kamiki, T.; Takemoto, T.; Nomoto, K. Studies on metabolites produced by Aspergillus terreus var. aureus. I. Chemical structures and antimicrobial activities of metabolites isolated from culture broth. Chem. Pharm. Bull. 1983, 31, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of polyketides in streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains a-c: New aromatic polyketides and diketopiperazines from australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Jouda, J.B.; Tamokou, J.D.; Mbazoa, C.D.; Sarkar, P.; Bag, P.K.; Wandji, J. Anticancer and antibacterial secondary metabolites from the endophytic fungus Penicillium sp. Cam64 against multi-drug resistant gram-negative bacteria. Afr. Health Sci. 2016, 16, 734–743. [Google Scholar] [CrossRef]
- Jouda, J.B.; Mawabo, I.K.; Notedji, A.; Mbazoa, C.D.; Nkenfou, J.; Wandji, J.; Nkenfou, C.N. Anti-mycobacterial activity of polyketides from penicillium sp. Endophyte isolated from garcinia nobilis against mycobacteriumsmegmatis. Int. J. Mycobacteriol. 2016, 5, 192–196. [Google Scholar] [CrossRef] [PubMed]
- George, T.K.; Devadasan, D.; Jisha, M.S. Chemotaxonomic profiling of penicillium setosum using high-resolution mass spectrometry (lc-q-tof-ms). Heliyon 2019, 5, e02484. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, Y.; Yamauchi, Y.; Nagatsu, C.; Abe, H.; Akasaki, K. Citromycin, a new antibiotic. I. Isolation and characterization. J. Antibiot. 1969, 22, 112–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumla, D.; Pereira, J.A.; Dethoup, T.; Gales, L.; Freitas-Silva, J.; Costa, P.M.; Lee, M.; Silva, A.M.S.; Sekeroglu, N.; Pinto, M.M.M.; et al. Chromone derivatives and other constituents from cultures of the marine sponge-associated fungus Penicillium erubescens kufa0220 and their antibacterial activity. Mar. Drugs 2018, 16, 289. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Krohn, K.; Ludewig, K.; Aust, H.J.; Draeger, S.; Schulz, B. Biologically active metabolites from fungi. 3. Sporothriolide, discosiolide, and 4-epi-ethisolide--new furofurandiones from Sporothrix sp., Discosia sp., and pezicula livida. J. Antibiot. 1994, 47, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kim, Y.P.; Takamatsu, S.; Preeprame, S.; Komiya, T.; Masuma, R.; Tanaka, H.; Komiyama, K.; Omura, S. Chlovalicin, a new cytocidal antibiotic produced by Sporothrix sp. Fo-4649. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1996, 49, 631–634. [Google Scholar] [CrossRef][Green Version]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.L.; Vrijmoed, L.L.; Jones, E.B.; Lin, Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the south china sea. J. Org. Chem. 2009, 74, 1093–1098. [Google Scholar] [PubMed]
- Choudhury, S.R.; Traquair, J.A.; Jarvis, W.R. 4-methyl-7,11-heptadecadienal and 4-methyl-7,11-heptadecadienoic acid: New antibiotics from Sporothrix flocculosa and Sporothrix rugulosa. J. Nat. Prod. 1994, 57, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jang, D.S.; Choi, J.H. Lancemaside a isolated from the root of codonopsis lanceolata inhibits ovarian cancer cell invasion via the reactive oxygen species (ros)-mediated p38 pathway. Am. J. Chin. Med. 2020, 48, 1021–1034. [Google Scholar] [CrossRef]
- Yamauchi, K.; Afroze, S.H.; Mitsunaga, T.; McCormick, T.C.; Kuehl, T.J.; Zawieja, D.C.; Uddin, M.N. 3,4′,7-o-trimethylquercetin inhibits invasion and migration of ovarian cancer cells. Anticancer Res. 2017, 37, 2823–2829. [Google Scholar]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med. 1999, 31, 34–45. [Google Scholar] [CrossRef]
- Lakka, S.S.; Jasti, S.L.; Gondi, C.; Boyd, D.; Chandrasekar, N.; Dinh, D.H.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene 2002, 21, 5601–5608. [Google Scholar] [CrossRef]
- Yang, C.Q.; Li, W.; Li, S.Q.; Li, J.; Li, Y.W.; Kong, S.X.; Liu, R.M.; Wang, S.M.; Lv, W.M. MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol. Biochem. 2014, 34, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, L.; Chen, Y.; Liu, T.; Liu, R.; Wang, Z.; Mou, K.; Xu, J.; Li, B.; Song, H. Nitidine chloride inhibits ovarian cancer cell migration and invasion by suppressing mmp-2/9 production via the erk signaling pathway. Mol. Med. Rep. 2016, 13, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Moulik, S.; Pal, S.; Biswas, J.; Chatterjee, A. Role of ERK in modulating MMP 2 and MMP 9 with respect to tumour invasiveness in human cancer cell line MCF-7 and MDA-MB-231. J. Tumor 2014, 2, 87–98. [Google Scholar]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.Y.; Ahn, J.-H.; Kwon, H.; Yim, J.H.; Lee, D.; Choi, J.-H. Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling. Mar. Drugs 2022, 20, 275. https://doi.org/10.3390/md20050275
Choi HY, Ahn J-H, Kwon H, Yim JH, Lee D, Choi J-H. Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling. Marine Drugs. 2022; 20(5):275. https://doi.org/10.3390/md20050275
Chicago/Turabian StyleChoi, He Yun, Ji-Hye Ahn, Haeun Kwon, Joung Han Yim, Dongho Lee, and Jung-Hye Choi. 2022. "Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling" Marine Drugs 20, no. 5: 275. https://doi.org/10.3390/md20050275
APA StyleChoi, H. Y., Ahn, J.-H., Kwon, H., Yim, J. H., Lee, D., & Choi, J.-H. (2022). Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling. Marine Drugs, 20(5), 275. https://doi.org/10.3390/md20050275






