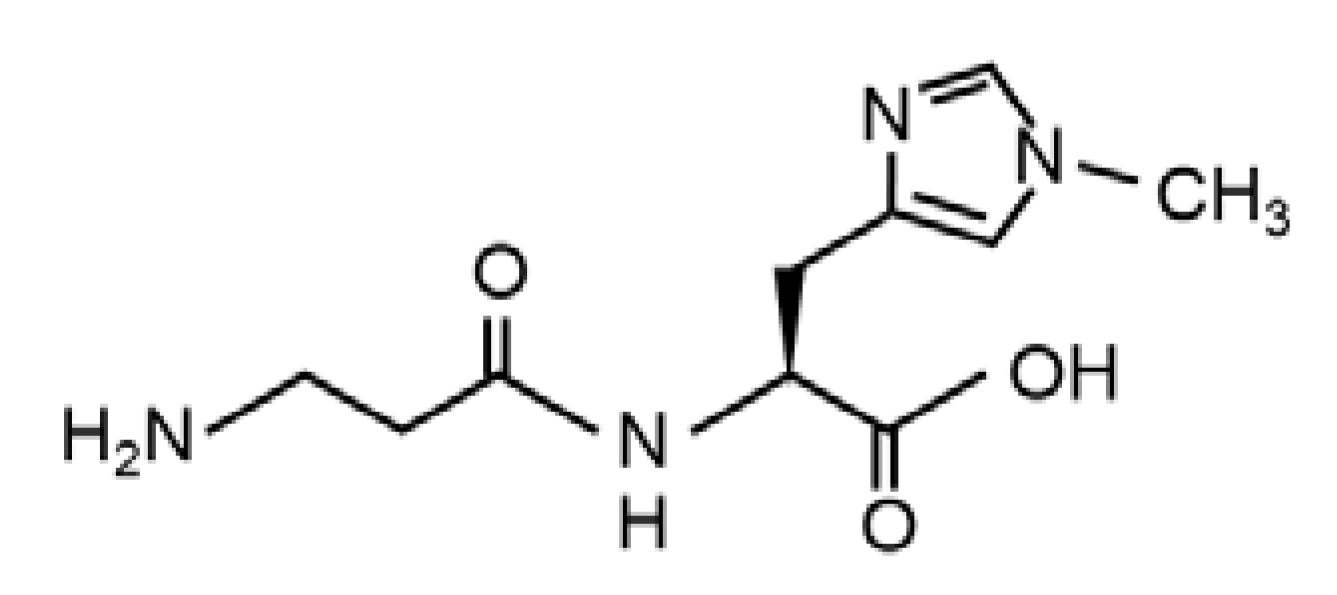
Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells
Abstract
1. Introduction
2. Results
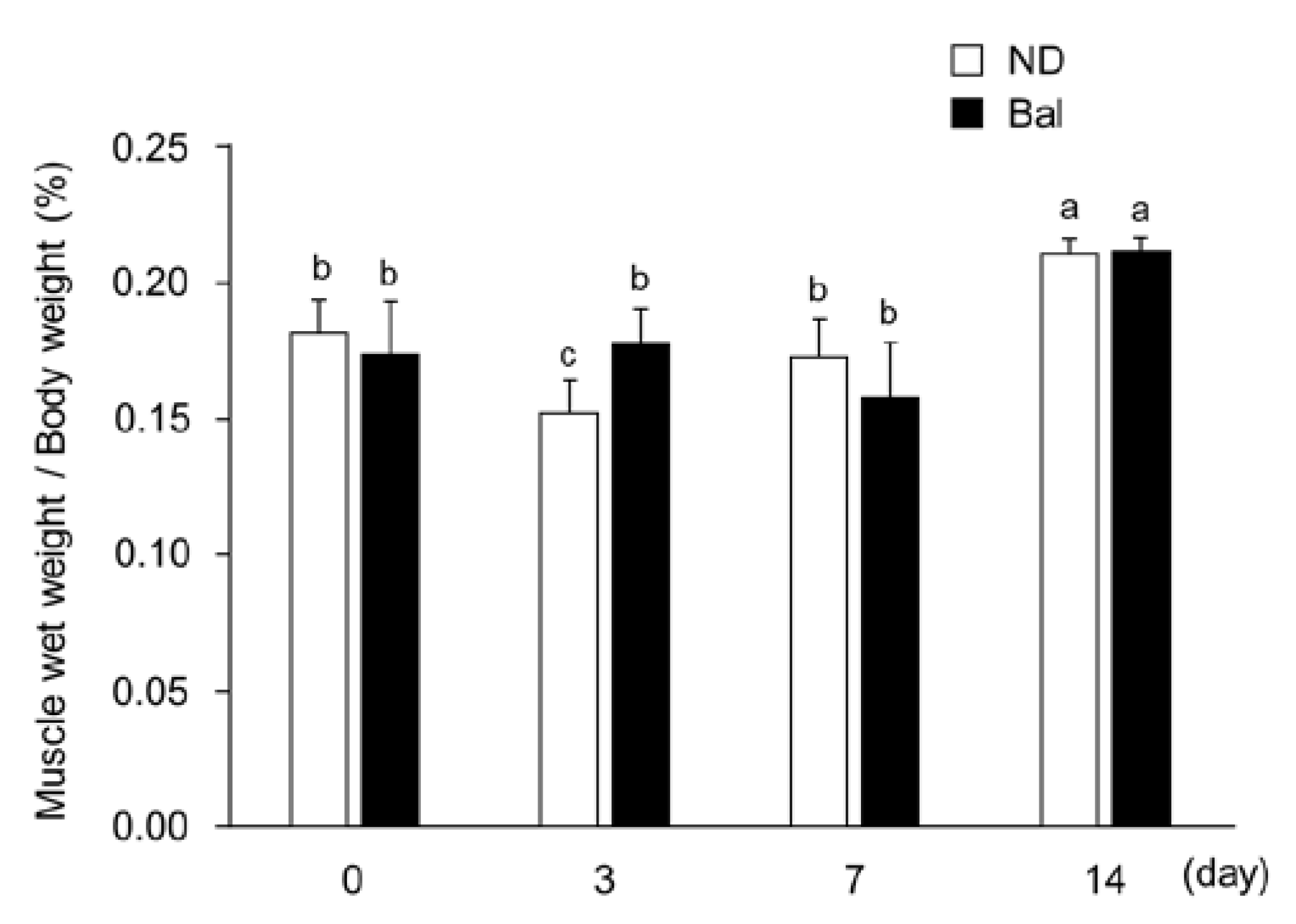
2.1. Effect of Dietary Balenine-Enriched Extract on Wet Weight and Morphological Change in Muscle in a CTX-Indued Muscle Degeneration/Regeneration Model
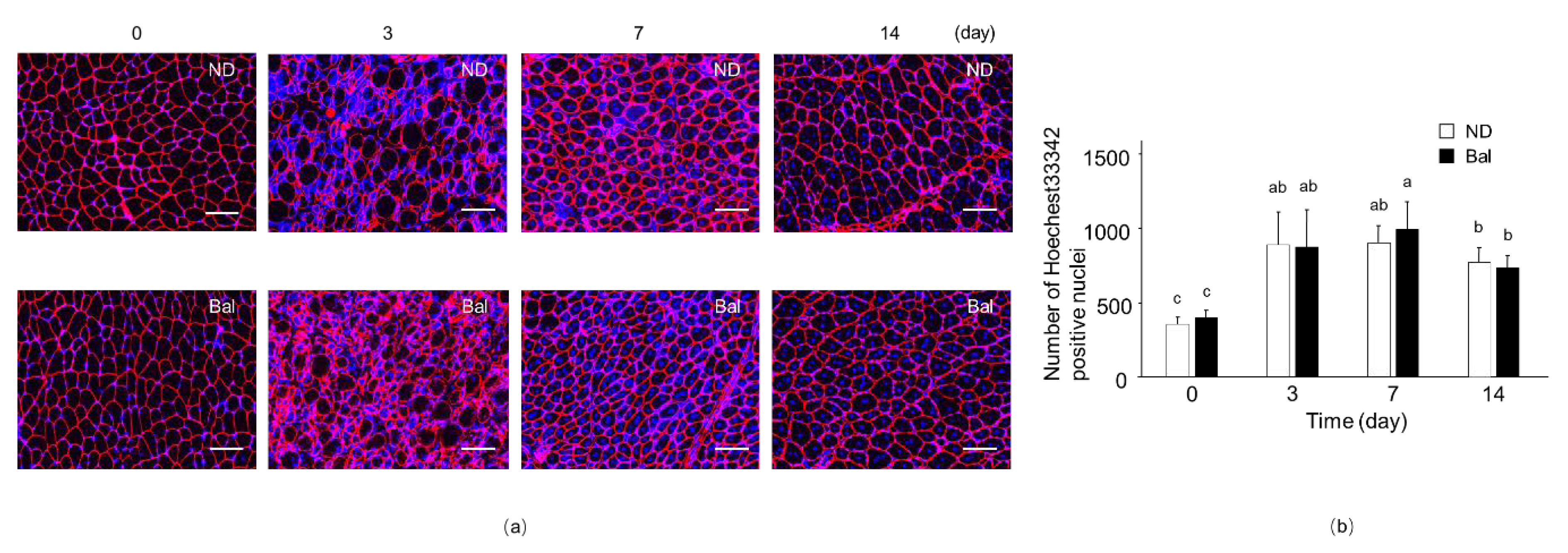
2.2. Effect of Dietary Balenine-Enriched Extract on Regeneration Stage in CTX-Indued Muscle
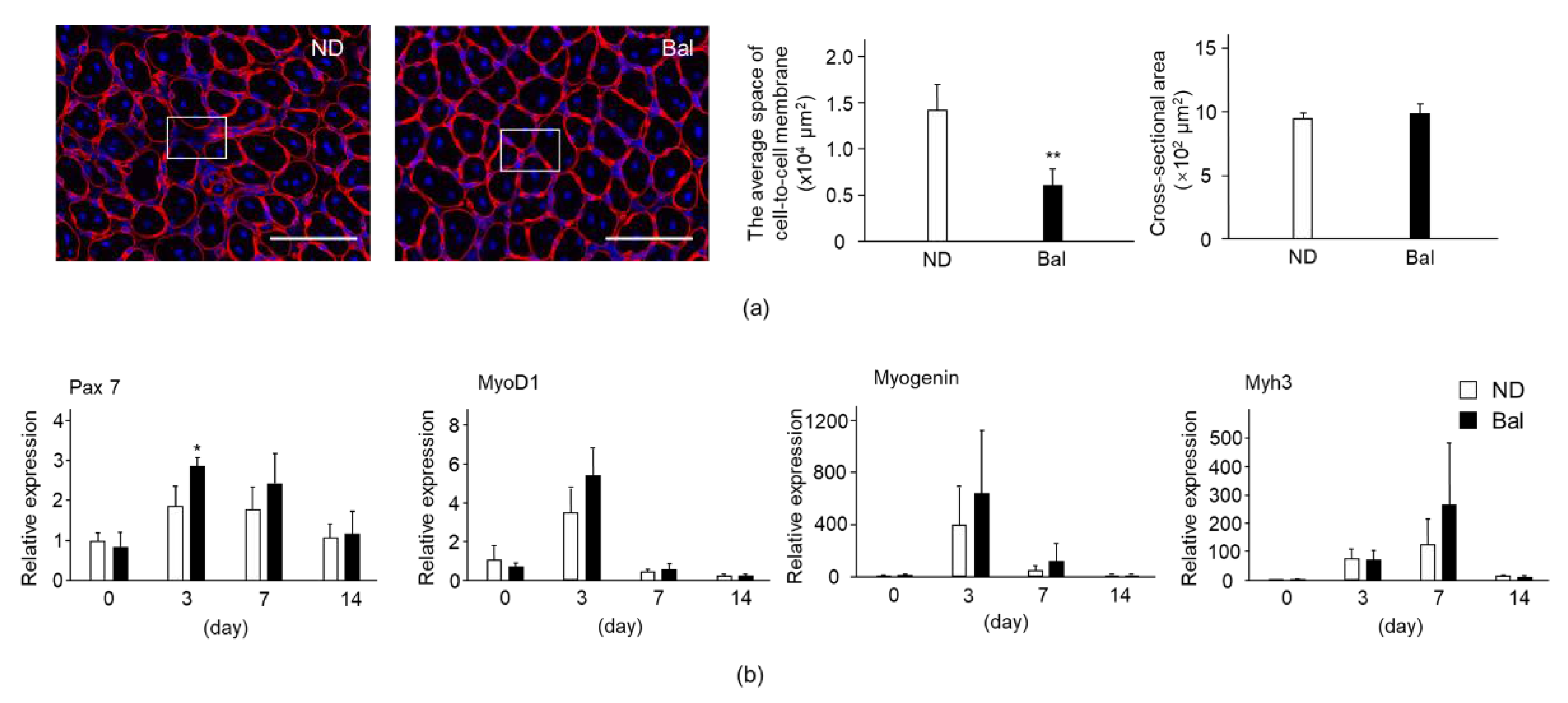
2.3. Effect of Dietary Balenine-Enriched Extract on Degeneration Stage in CTX-Indued Muscle
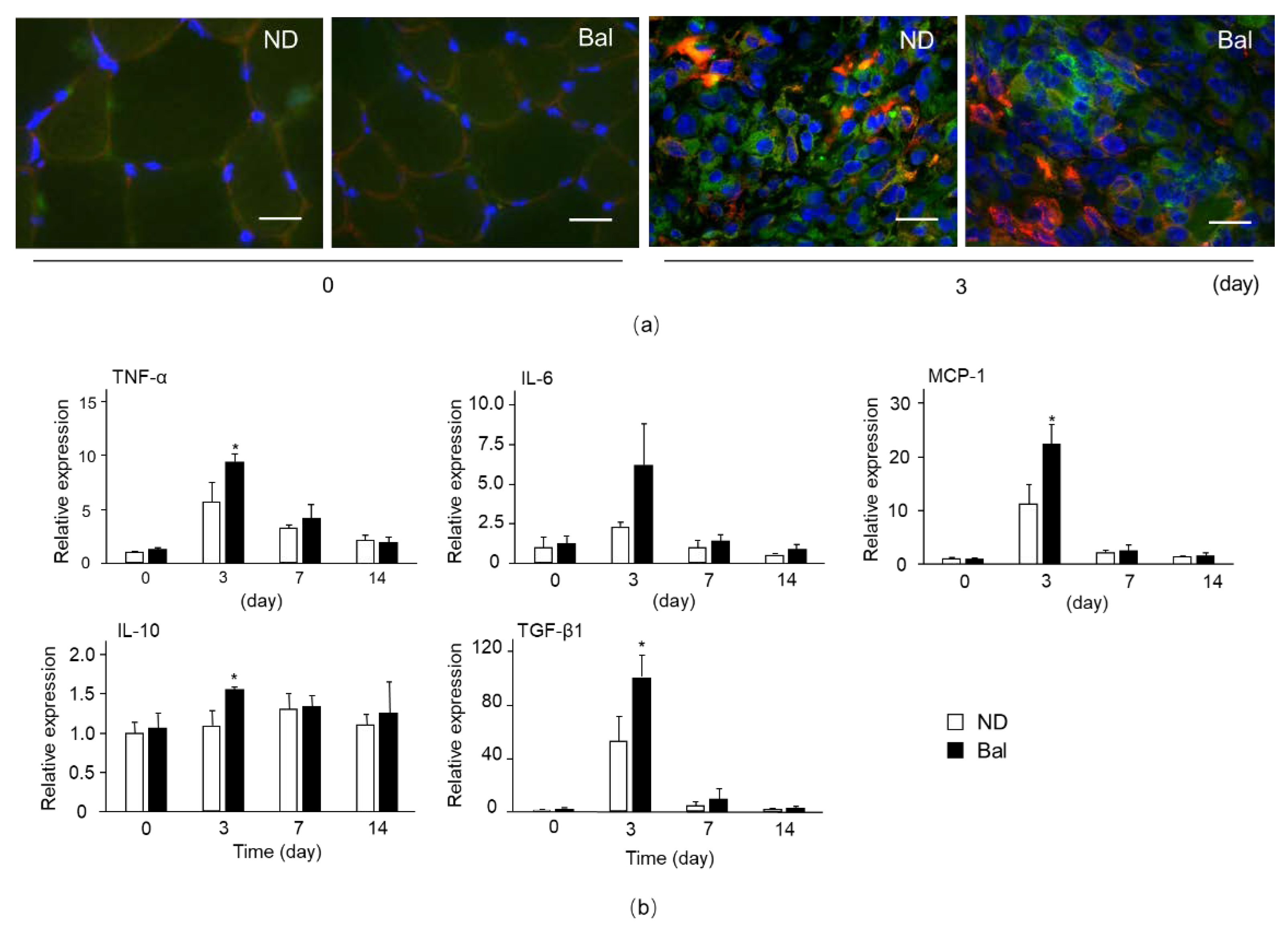
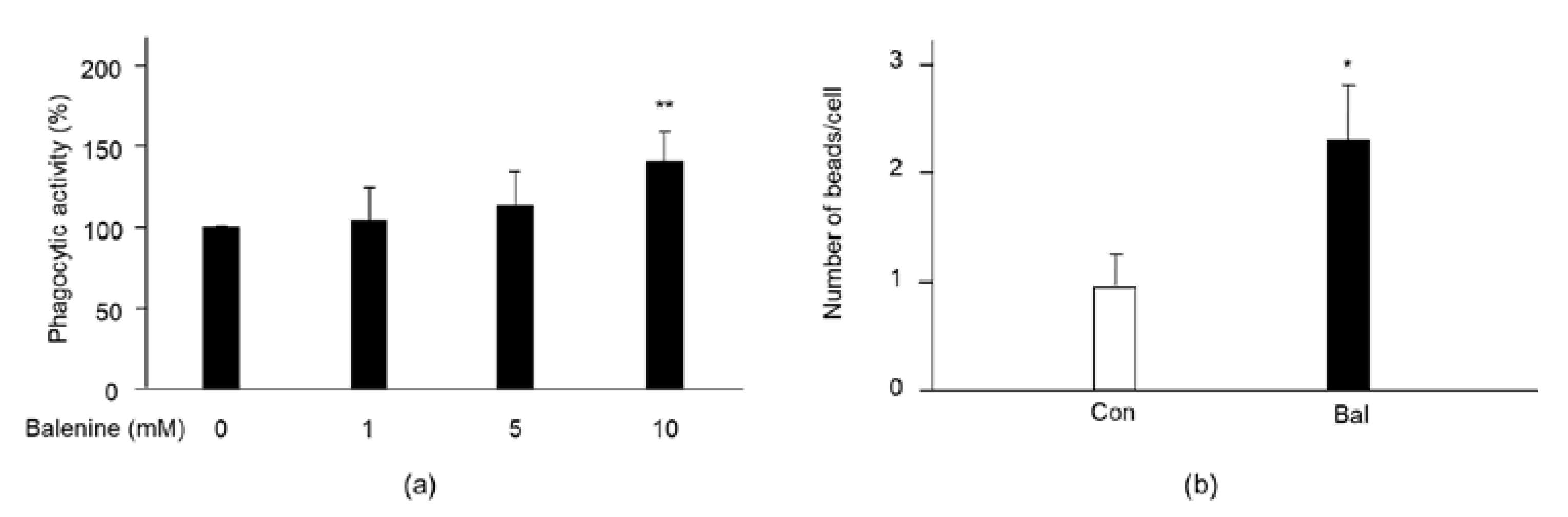
2.4. Effect of Balenine on Phagocytic Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Muscle Injury Model
4.2. Immunofluorescence Staining
4.3. Quantitative Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
4.4. Phagocytic Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Undrum, T.; Lunde, H.; Gjessing, L.R. Determination of ophidine in human urine. Chromatogr. B Biomed. Sci. Appl. 1982, 227, 53–59. [Google Scholar] [CrossRef]
- Christman, A.A. Factors affecting anserine and carnosine levels in skeletal muscles of various animals. Int. J. Biochem. 1976, 7, 519–527. [Google Scholar] [CrossRef][Green Version]
- Bonfanti, L.; Peretto, P.; Marchis, S.; Fasolo, A. Carnosine-related dipeptides in the mammalian brain. Prog. Neurobiol. 1999, 59, 333–353. [Google Scholar] [CrossRef]
- Omura, Y.; Kimiya, T.; Matsuda, T.; Kuniyoshi, M.; Maegawa, T.; Kawabata, Y.; Adachi, S.; Amitani, Y. Analysis of balenine in muscle extract of opah Lampris guttatus with automatic amino acid analyzer. Nippon Suisan Gakkaishi 2018, 84, 1025–1033. [Google Scholar] [CrossRef]
- Carnegie, P.R.; Hee, K.P.; Bell, A.W. Ophidine (beta-alanyl-L-3-methylhistidine, ‘balenine’) and other histidine dipeptides in pig muscles and tinned hams. J. Sci. Food Agric. 1982, 33, 795–801. [Google Scholar] [CrossRef]
- Dennis, P.O.; Lorkin, P.A. Isolation and synthesis of balenine, a dipeptide occurring in whale-meat extract. J. Chem. Soc. 1965, 9, 4968–4972. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Kawabata, Y.; Maegawa, T.; Taniyama, S.; Tachibana, K.; Hirasaka, K. Balenine, imidazole dipeptide, induces activation of superoxide dismutase in myotubes. Fish. Sci. 2021, 87, 403–409. [Google Scholar] [CrossRef]
- Ishihara, K.; Watanabe, R.; Kato, T.; Seko, T.; Matsuda, T.; Omura, Y.; Shigemura, Y.; Kawabata, Y.; Maegawa, T. Isolation of balenine from opah (Lampris megalopsis) muscle and comparison of antioxidant and iron-chelating activities with other major imidazole dipeptides. Food Chem. 2021, 364, 130343. [Google Scholar] [CrossRef]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Fu, H.; Katsumura, Y.; Lin, M.; Muroya, Y.; Hata, K.; Fujii, K.; Yokoya, A.; Hatano, Y. Free radical scavenging and radioprotective effects of carnosine and anserine. Radiat. Phys. Chem. 2009, 78, 1192–1197. [Google Scholar] [CrossRef]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef]
- Mirzakhani, N.; Farshid, A.A.; Tamaddonfard, E.; Imani, M.; Erfanparast, A.; Noroozinia, F. Carnosine improves functional recovery and structural regeneration after sciatic nerve crush injury in rats. Life Sci. 2018, 215, 22–30. [Google Scholar] [CrossRef]
- Guardiola, O.; Andolfi, G.; Tirone, M.; Iavarone, F.; Brunelli, S.; Minchiotti, G. Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. J. Vis. Exp. 2017, 119, 54515. [Google Scholar] [CrossRef]
- Piper, A.K.; Sophocleous, R.A.; Ross, S.E.; Evesson, F.J.; Cooper, S.T. Loss of calpains-1 and -2 prevents repair of plasma membrane scrape injuries, but not small pores, and induces a severe muscular dystrophy. Am. J. Physiol. Cell Physiol. 2020, 318, C1226–C1237. [Google Scholar] [CrossRef]
- Garry, G.A.; Antony, M.L.; Garry, D.J. Cardiotoxin Induced Injury and Skeletal Muscle Regeneration. Methods Mol. Biol. 2016, 1460, 61–71. [Google Scholar]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Musarò, A. The basis of muscle regeneration. Adv. Biol. 2014, 2014, 612471. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Gao, S.; Zhao, J.; Xu, N. Carnosine stimulates macrophage-mediated clearance of senescent skin cells through activation of the AKT2 signaling pathway by CD36 and RAGE. Front. Pharmacol. 2020, 11, 593832. [Google Scholar] [CrossRef]
- Shelar, S.B.; Narasimhan, M.; Shanmugam, G.; Litovsky, S.H.; Gounder, S.S.; Karan, G.; Arulvasu, C.; Kensler, T.W.; Hoidal, J.R.; Darley-Usmar, V.M.; et al. Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: A mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle. FASEB J. 2016, 30, 1865–1879. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, L.; Garcia, G.E.; Wang, X.H.; Han, S.; Du, J.; Mitch, W.E. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am. J. Pathol. 2009, 175, 2518–2527. [Google Scholar] [CrossRef]
- Juban, G. Transcriptional control of macrophage inflammatory shift during skeletal muscle regeneration. Semin. Cell Dev. Biol. 2021, 119, 82–88. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar]
- Neves, J.d.C.; Rizzato, V.R.; Fappi, A.; Garcia, M.M.; Chadi, G.; Vlekkert, D.; d’Azzo, A.; Zanoteli, E. Neuraminidase-1 mediates skeletal muscle regeneration. Biochim. Biophys. Acta 2015, 9, 1755–1764. [Google Scholar] [CrossRef]
- Alarcin, E.; Bal-Öztürk, A.; Avci, H.; Ghorbanpoor, H.; Guzel, D.F.; Akpek, A.; Yesiltas, G.; Canak-Ipek, T.; Avci-Adali, M. Current Strategies for the Regeneration of Skeletal Muscle Tissue. Int. J. Mol. Sci. 2021, 22, 5929. [Google Scholar] [CrossRef]
- Kitajima, Y.; Suzuki, N.; Nunomiya, A.; Osana, S.; Yoshioka, K.; Tashiro, Y.; Takahashi, R.; Ono, Y.; Aoki, M.; Nagatomi, R. The Ubiquitin-Proteasome System Is Indispensable for the Maintenance of Muscle Stem Cells. Stem Cell Rep. 2018, 11, 1523–1538. [Google Scholar] [CrossRef]
- Yaden, B.C.; Croy, J.E.; Wang, Y.; Wilson, J.M.; Datta-Mannan, A.; Shetler, P.; Milner, A.; Bryant, H.U.; Andrews, J.; Dai, G.; et al. Follistatin: A novel therapeutic for the improvement of muscle regeneration. J. Pharmacol. Exp. Ther. 2014, 349, 355–371. [Google Scholar] [CrossRef]
- Chen, B.; Shan, T. The role of satellite and other functional cell types in muscle repair and regeneration. J. Muscle Res. Cell Motil. 2019, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smigiel, K.S.; Parks, W.C. Macrophages, wound healing, and fibrosis: Recent insights. Curr. Rheumatol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, H.; Zhang, Z.; Liu, S.; Yang, J.; Chen, X.; Fan, M.; Wang, X. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell Mol. Neurobiol. 2008, 28, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Bosurgi, L.; Corna, G.; Vezzoli, M.; Touvier, T.; Cossu, G.; Manfredi, A.A.; Brunelli, S.; Rovere-Querini, P. Transplanted mesoangioblasts require macrophage IL-10 for survival in a mouse model of muscle injury. J. Immunol. 2012, 188, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.K.; Lee, S.T.; Fiore, D.; Zhang, R.H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M. Nilotinib reduces muscle fbrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fbro/adipogenic progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Budai, Z.; Al-Zaeed, N.; Szentesi, P.; Halász, H.; Csernoch, L.; Szondy, Z.; Sarang, Z. Impaired Skeletal Muscle Development and Regeneration in Transglutaminase 2 Knockout Mice. Cells 2021, 10, 3089. [Google Scholar] [CrossRef]
- Juban, G.; Chazaud, B. Efferocytosis during Skeletal Muscle Regeneration. Cells 2021, 10, 3267. [Google Scholar] [CrossRef]
- Shigemura, Y.; Iwasaki, Y.; Sato, Y.; Kato, T.; Seko, T.; Ishihara, K. Detection of Balenine in Mouse Plasma after Administration of Opah-Derived Balenine by HPLC with PITC Pre-Column Derivatization. Foods 2022, 11, 590. [Google Scholar] [CrossRef]
- Everaert, I.; Stegen, S.; Vanheel, B.; Taes, Y.; Derave, W. Effect of beta-alanine and carnosine supplementation on muscle contractility in mice. Med. Sci. Sports Exerc. 2013, 45, 43–51. [Google Scholar] [CrossRef]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef]
- Tidball, J.G.; Flores, I.; Welc, S.S.; Wehling-Henricks, M.; Ochi, E. Aging of the immune system and impaired muscle regeneration: A failure of immunomodulation of adult myogenesis. Exp. Gerontol. 2021, 145, 111200. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Vasson, M.P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Jiang, T.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Taniyama, S.; Tachibana, K.; Hirasaka, K. Safety evaluation and physiological function of dietary balenine derived from opah lampris guttatus on skeletal muscle of mice. Int. J. Pept. Res. Ther. 2021, 27, 2083–2089. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Sun, L.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Nikawa, T.; Hirasaka, K. Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Mar. Drugs 2022, 20, 313. https://doi.org/10.3390/md20050313
Yang M, Sun L, Kawabata Y, Murayama F, Maegawa T, Nikawa T, Hirasaka K. Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Marine Drugs. 2022; 20(5):313. https://doi.org/10.3390/md20050313
Chicago/Turabian StyleYang, Min, Luchuanyang Sun, Yasunosuke Kawabata, Fumihito Murayama, Takahiro Maegawa, Takeshi Nikawa, and Katsuya Hirasaka. 2022. "Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells" Marine Drugs 20, no. 5: 313. https://doi.org/10.3390/md20050313
APA StyleYang, M., Sun, L., Kawabata, Y., Murayama, F., Maegawa, T., Nikawa, T., & Hirasaka, K. (2022). Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Marine Drugs, 20(5), 313. https://doi.org/10.3390/md20050313





