BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections
Abstract
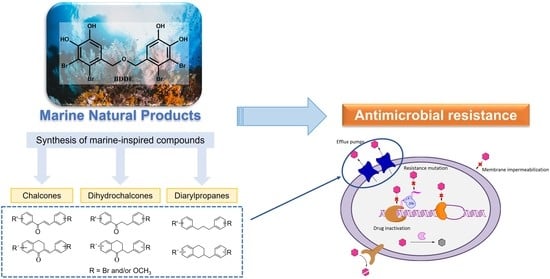
:1. Introduction
2. Results and Discussion
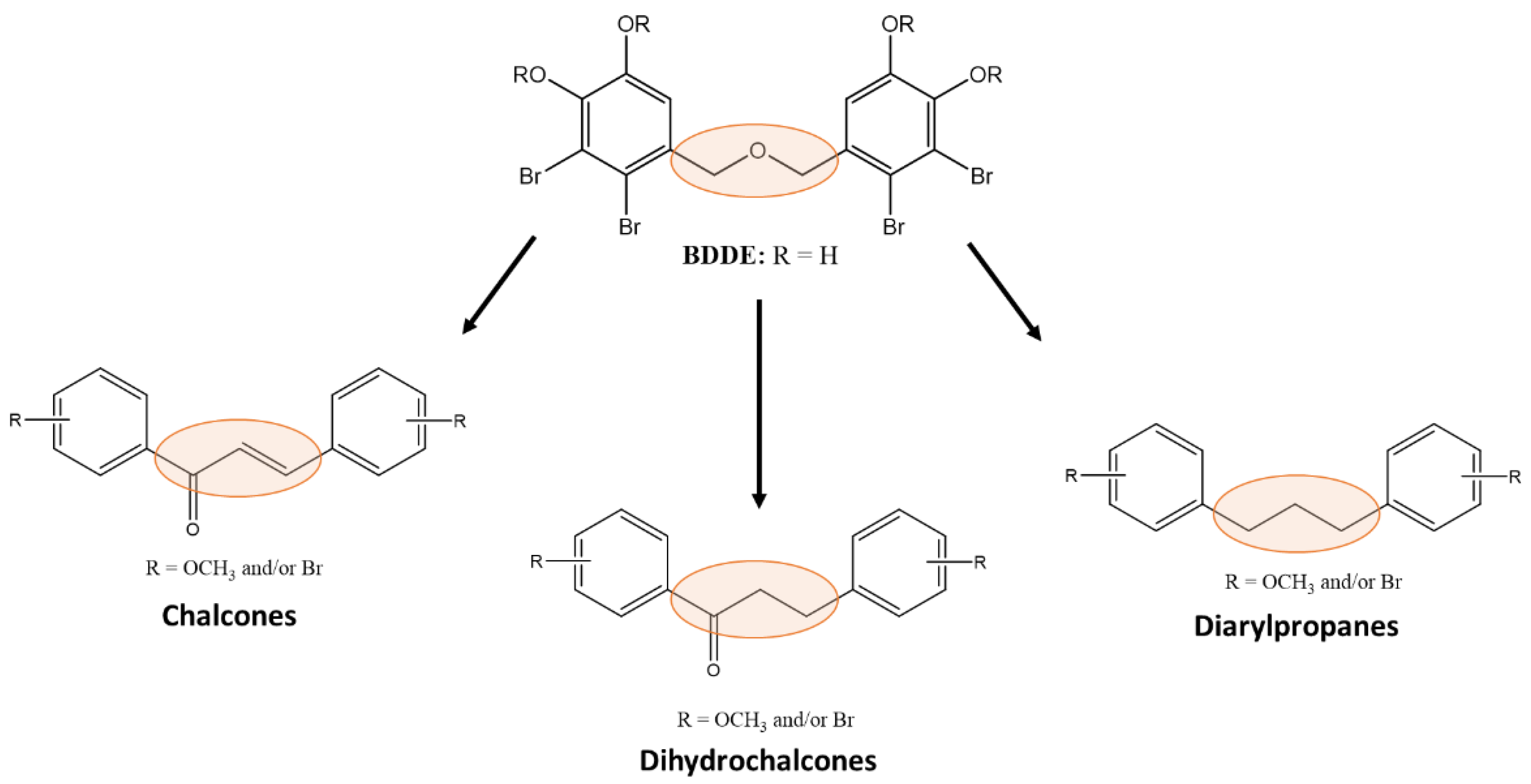
2.1. Synthesis
2.2. Antibacterial Activity and Potentiation of Antimicrobials
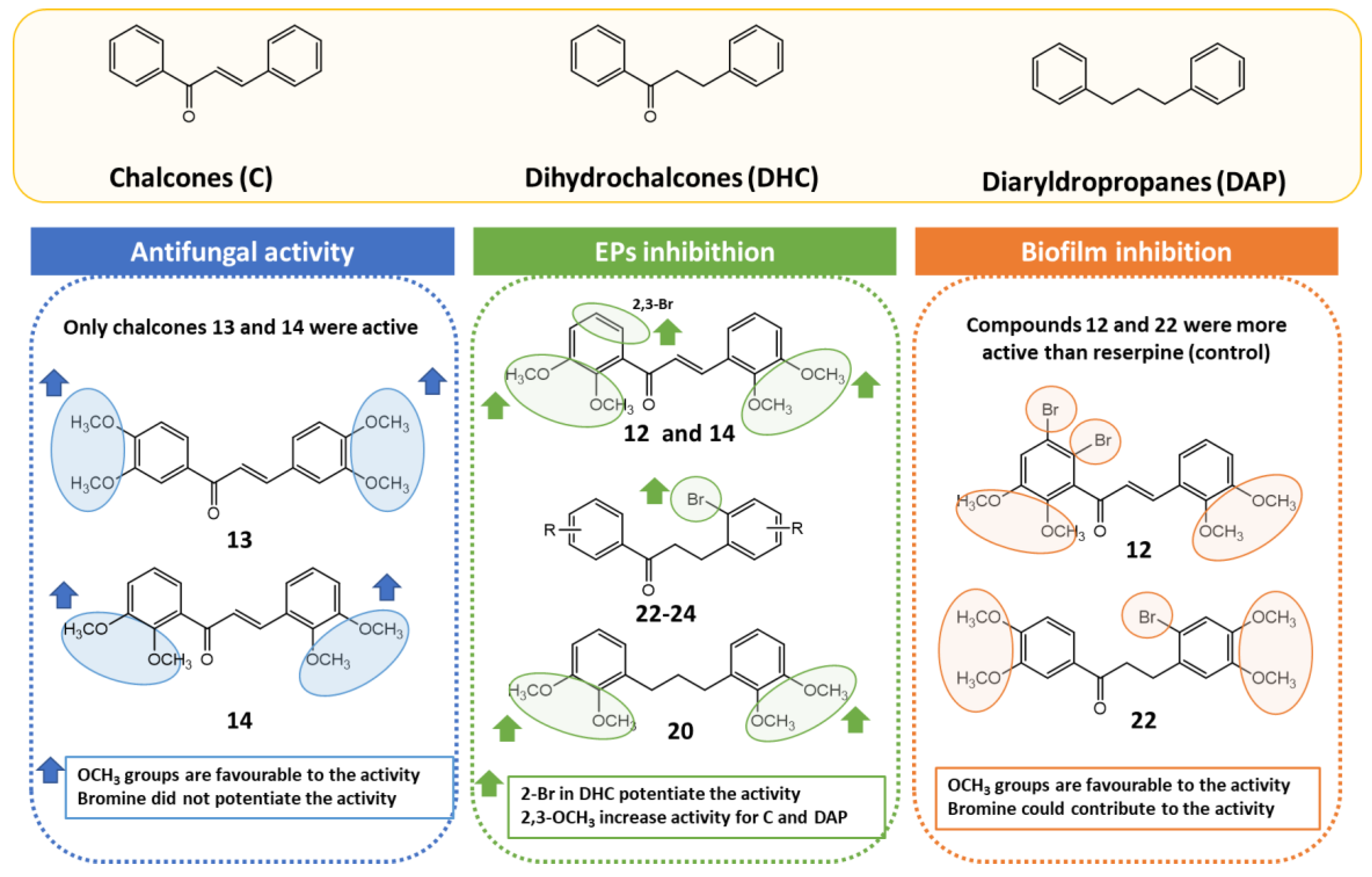
2.3. Antifungal Activity
2.4. Efflux Pump Inhibition Assay
2.5. Inhibition of Biofilm Formation
2.6. Cytotoxicity Assay
3. Materials and Methods
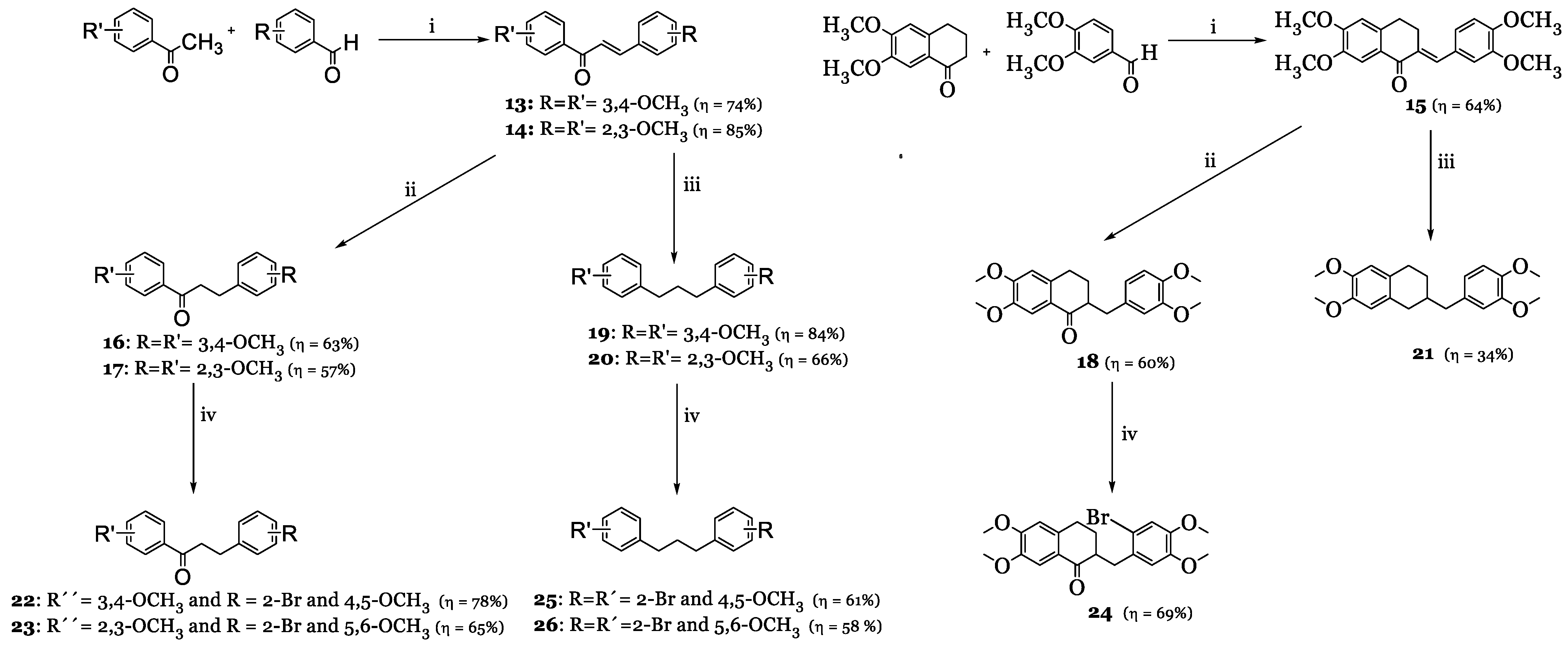
3.1. Synthesis
3.1.1. Synthesis of Brominated Benzaldehydes 1–3 and Acetophenones 4–6
3.1.2. Synthesis of Chalcones 9–15
3.1.3. Synthesis of Dihydrochalcones 16–18
3.1.4. Synthesis of Brominated Dihydrochalcones 22–24
3.1.5. Synthesis of Diarylpropanes 19–21
3.1.6. Synthesis of Brominated Diarylpropanes 25 and 26
3.2. Biological Activity
3.2.1. Culture Media and Chemicals
3.2.2. Microorganisms
3.2.3. Antibacterial Assay and Potentiation of Antibiotics
3.2.4. Antifungal Assay
3.2.5. Efflux Pump Inhibition Assays
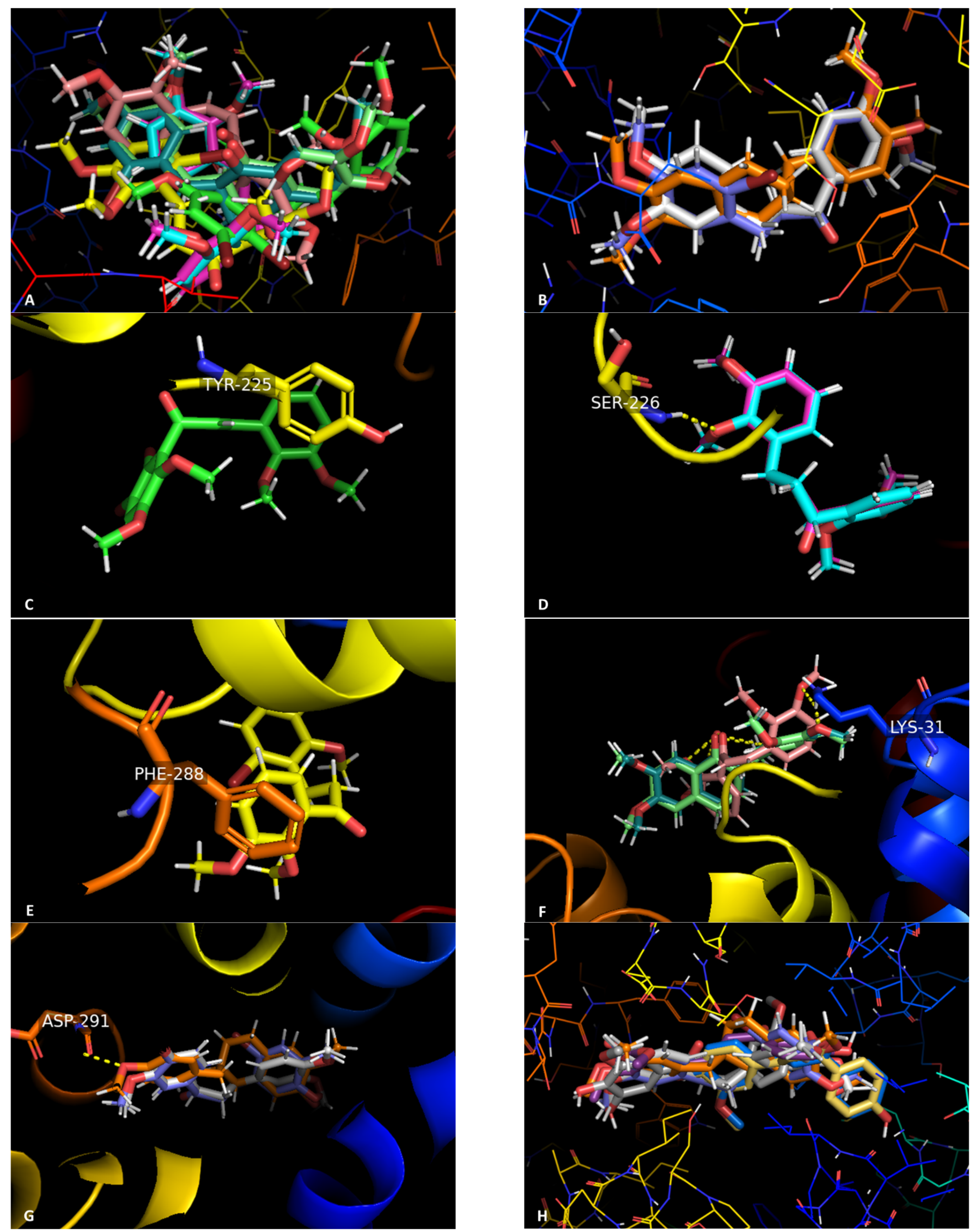
3.2.6. Docking Studies
3.2.7. Inhibition of Biofilm Formation
3.2.8. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resende, D.I.S.P.; Pereira-Terra, P.; Moreira, J.; Freitas-Silva, J.; Lemos, A.; Gales, L.; Pinto, E.; de Sousa, M.E.; da Costa, P.M.; Pinto, M.M.M. Synthesis of a Small Library of Nature-Inspired Xanthones and Study of Their Antimicrobial Activity. Molecules 2020, 25, 2405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Dai, M.; Ahmed, S.; Hao, H.; Wang, X.; Yuan, Z. Antimicrobial Drugs in Fighting against Antimicrobial Resistance. Front. Microbiol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durães, F.; Pinto, M.; Sousa, E. Medicinal Chemistry Updates on Bacterial Efflux Pump Modulators. Curr. Med. Chem. 2018, 25, 6030–6069. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Micro. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Sabatini, S.; Piccioni, M.; Felicetti, T.; De Marco, S.; Manfroni, G.; Pagiotti, R.; Nocchetti, M.; Cecchetti, V.; Pietrella, D. Investigation on the effect of known potent S. aureus NorA efflux pump inhibitors on the staphylococcal biofilm formation. RSC Adv. 2017, 7, 37007–37014. [Google Scholar] [CrossRef] [Green Version]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Sharma, P.; Joshi, P.; Saini, K.; Sharma, A.; Puri, V.; Chander, J.; Singh, T.G.; Arora, S. Chalcones: A privileged scaffold with diverse biological activities. Plant Arch. 2020, 20, 3812–3819. [Google Scholar]
- Dan, W.; Dai, J. Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem. 2020, 187, 111980. [Google Scholar] [CrossRef]
- Rezende-Junior, L.M.; Andrade, L.M.S.; Leal, A.; Mesquita, A.B.S.; Santos, A.; Neto, J.S.L.; Siqueira-Junior, J.P.; Nogueira, C.E.S.; Kaatz, G.W.; Coutinho, H.D.M.; et al. Chalcones Isolated from Arrabidaea brachypoda Flowers as Inhibitors of NorA and MepA Multidrug Efflux Pumps of Staphylococcus aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef]
- Moreira, J.; Durães, F.; Freitas-Silva, J.; Szemerédi, N.; Resende, D.I.S.P.; Pinto, E.; da Costa, P.M.; Pinto, M.; Spengler, G.; Cidade, H.; et al. New Diarylpentanoids and Chalcones as Potential Antimicrobial Adjuvants. Bioorg. Med. Chem. Lett. 2022, 67, 128743. [Google Scholar] [CrossRef]
- Hasan, S.A.; Nasser, N.H.; Hussein, A.K.; Abdulsada, A.H.; Jasim, Z.M.; Shaalan, S.H.; Musai, H.K. Synthesis and Antibacterial Evaluation of new Ofloxacin-Chalcone derivatives Conjugates as Possible Mutual Prodrugs. J. Pharm. Sci. Res. 2018, 10, 3061–3065. [Google Scholar]
- Liu, Y.-T.; Sun, X.-M.; Yin, D.-W.; Yuan, F. Syntheses and biological activity of chalcones-imidazole derivatives. Res. Chem. Intermed. 2012, 39, 1037–1048. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. Alpha-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two New Bromophenols from the Red Alga Odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, N.-J.; Shi, J.-G. Bromophenols from the Red Alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl Bromophenols with Diverse Dimerization Patterns from the Brown Alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Biediger, R.J.B.; Michele, A.; Lindsay Bonner, H.; Vincent, A.B.; Robert, V.M.; Thomas, P.T.; Brandon, M.Y. Preparation of Propionic Acid Derivatives for Inhibiting the Binding of an Integrin. U.S. Patent 20180312523, 1 November 2018. [Google Scholar]
- Cao, J.; Chen, L.; Sun, F.-N.; Sun, Y.-L.; Jiang, K.-Z.; Yang, K.-F.; Xu, Z.; Xu, L.-W. Pd-Catalyzed Enantioselective Ring Opening/Cross-Coupling and Cyclopropanation of Cyclobutanones. Angew. Chem. Int. Ed. 2019, 58, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.Q.N.L.; Sikorski, J.A.; Hoong, L.K. Preparation of 1,3-bis-(substituted-phenyl)-2-propen-1-ones as VCAM-1 Inhibitors for Treatment of Inflammatory Disorders. U.S. Patent WO 2001098291, 27 December 2001. [Google Scholar]
- Pinto, P.; Machado, C.M.; Moreira, J.; Almeida, J.D.P.; Silva, P.M.A.; Henriques, A.C.; Soares, J.X.; Salvador, J.A.R.; Afonso, C.; Pinto, M.; et al. Chalcone derivatives targeting mitosis: Synthesis, evaluation of antitumor activity and lipophilicity. Eur. J. Med. Chem. 2019, 184, 111752. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Paulson, M.C. The preparation of chalcones from hydroxy and methoxy aldehydes and ketones. J. Am. Chem. Soc. 1954, 76, 4486–4487. [Google Scholar] [CrossRef]
- Shih, H.; Deng, L.; Carrera, C.J.; Adachi, S.; Cottam, H.B.; Carson, D.A. Rational design, synthesis and structure-activity relationships of antitumor (E)-2-benzylidene-1-tetralones and (E)-2-benzylidene-1-indanones. Bioorg. Med. Chem. Lett. 2000, 10, 487–490. [Google Scholar] [CrossRef]
- Vijaya Bhaskar Reddy, M.; Hung, H.Y.; Kuo, P.C.; Huang, G.J.; Chan, Y.Y.; Huang, S.C.; Wu, S.J.; Morris-Natschke, S.L.; Lee, K.H.; Wu, T.S. Synthesis and biological evaluation of chalcone, dihydrochalcone, and 1,3-diarylpropane analogs as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2017, 27, 1547–1550. [Google Scholar] [CrossRef]
- Henin, J.; Gardent, J. Hexahydro-5,6,6a,7,12,14 isoquino[2,3-b]benzazépine-2. Nouvelle voie d’accès. J. Heterocycl. Chem. 1986, 23, 975–979. [Google Scholar] [CrossRef]
- Bessa, L.J.; Barbosa-Vasconcelos, A.; Mendes, A.; Vaz-Pires, P.; da Costa, P.M. High prevalence of multidrug-resistant Escherichia coli and Enterococcus spp. in river water, upstream and downstream of a wastewater treatment plant. J. Water Health 2014, 12, 426–435. [Google Scholar] [CrossRef]
- Durães, F.; Resende, D.I.S.P.; Palmeira, A.; Szemerédi, N.; Pinto, M.M.M.; Spengler, G.; Sousa, E. Xanthones Active against Multidrug Resistance and Virulence Mechanisms of Bacteria. Antibiotics 2021, 10, 600. [Google Scholar] [CrossRef]
- Kincses, A.; Varga, B.; Csonka, Á.; Sancha, S.; Mulhovo, S.; Madureira, A.M.; Ferreira, M.U.; Spengler, G. Bioactive compounds from the African medicinal plant Cleistochlamys kirkii as resistance modifiers in bacteria. Phytother. Res. 2018, 32, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef]
- Costa, S.S.; Sobkowiak, B.; Parreira, R.; Edgeworth, J.D.; Viveiros, M.; Clark, T.G.; Couto, I. Genetic Diversity of norA, Coding for a Main Efflux Pump of Staphylococcus aureus. Front. Genet. 2019, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.K. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; M07, C.S., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved standard—CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition, CLSI document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Spengler, G.; Takács, D.; Horváth, A.; Szabó, A.M.; Riedl, Z.; Hajós, G.; Molnár, J.; Burián, K. Efflux pump inhibiting properties of racemic phenothiazine derivatives and their enantiomers on the bacterial AcrAB-TolC system. In Vivo 2014, 28, 1071–1075. [Google Scholar]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [CrossRef] [Green Version]
- Zárate, S.G.; Morales, P.; Świderek, K.; Bolanos-Garcia, V.M.; Bastida, A. A Molecular Modeling Approach to Identify Novel Inhibitors of the Major Facilitator Superfamily of Efflux Pump Transporters. Antibiotics 2019, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, F.J.; Fluit, A.C.; Luckefahr, M.; Engler, B.; Hofmann, B.; Verhoef, J.; Heinz, H.P.; Hadding, U.; Jones, M.E. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 807–810. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kincses, A.; Szabó, S.; Rácz, B.; Szemerédi, N.; Watanabe, G.; Saijo, R.; Sekiya, H.; Tamai, E.; Molnár, J.; Kawase, M.; et al. Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria. Antibiotics 2020, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Kincses, A.; Gajdács, M.; Spengler, G.; dos Santos, D.J.V.A.; Molnár, J.; Ferreira, M.-J.U. Terpenoids from Euphorbia pedroi as Multidrug-Resistance Reversers. J. Nat. Prod. 2018, 81, 2032–2040. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Antibacterial Activity and Potentiation of Antimicrobials (CTX: Cefotaxime; VAN: Vancomycin) | EP Inhibition Assay | Inhibition of Biofilm Formation (%) | ||
|---|---|---|---|---|---|
| E. coli SA/2 MIC CTX = 562 µM | E. faecalis B3/101 MIC VAN = 707 µM | Relative Fluorescence Index (RFI) ± SD | |||
| MIC Reduction | MIC Reduction | S. aureus 272123 | S. aureus ATCC 25923 | S. aureus 272123 | |
| 9 | 2-fold | 4-fold | 0.31 ± 0.03 | - | - |
| 10 | No effect | 2-fold | 0.29 ± 0.09 | - | - |
| 11 | No effect | 2-fold | 0.37 ± 0.08 | - | - |
| 12 | 2-fold | 2-fold | 0.66 ± 0.09 | 3.05 ± 1.41 | 7.76 ± 2.14 |
| 13 | 2-fold | 2-fold | 0.32 ± 0.04 | - | - |
| 14 | 2-fold | 4-fold | 0.81 ± 0.06 | 0 | 87.28 ± 3.84 |
| 15 | 2-fold | 2-fold | 0.37 ± 0.03 | - | - |
| 16 | 2-fold | 2-fold | 0.23 ± 0.01 | - | - |
| 17 | 2-fold | 2-fold | 0.75 ± 0.04 | 0 | 6.89 ± 2.41 |
| 18 | 2-fold | 2-fold | 0.43 ± 0.02 | 0 | 8.15 ± 0.64 |
| 19 | 2-fold | 2-fold | 0.53 ± 0.04 | 0 | 49.59 ± 0.39 |
| 20 | 2-fold | 2-fold | 1.30 ± 0.08 | 0 | 23.80 ± 0.13 |
| 21 | 2-fold | 2-fold | 0.70 ± 0.07 | 0 | 71.33 ± 1.09 |
| 22 | 2-fold | 2-fold | 0.88 ± 0.05 | 13.28 ± 6.67 | 7.95 ± 0.65 |
| 23 | 2-fold | 2-fold | 1.20 ± 0.01 | 0 | 7.08 ± 2.72 |
| 24 | No effect | 4-fold | 0.67 ± 0.04 | 0 | 10.65 ± 0.76 |
| 25 | 2-fold | 2-fold | 0.18 ± 0.02 | - | - |
| 26 | No effect | 2-fold | 0.22 ± 0.05 | - | - |
| Reserpine (control) | - | - | 0.41 ± 0.01 | 2.49 ± 1.38 | 77.62 ± 4.08 |
| Name | IC50 (µM) ± SD |
|---|---|
| Doxorubicin | 12.05 ± 0.81 |
| 12 | 28.31 ± 0.25 |
| 14 | 30.16 ± 1.04 |
| 17 | >100 |
| 18 | >100 |
| 22 | >100 |
| 23 | >100 |
| 24 | >100 |
| 19 | >100 |
| 20 | >100 |
| 21 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, A.; Durães, F.; Szemerédi, N.; Freitas-Silva, J.; da Costa, P.M.; Pinto, E.; Pinto, M.; Spengler, G.; Sousa, E.; Cidade, H. BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections. Mar. Drugs 2022, 20, 315. https://doi.org/10.3390/md20050315
Jesus A, Durães F, Szemerédi N, Freitas-Silva J, da Costa PM, Pinto E, Pinto M, Spengler G, Sousa E, Cidade H. BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections. Marine Drugs. 2022; 20(5):315. https://doi.org/10.3390/md20050315
Chicago/Turabian StyleJesus, Ana, Fernando Durães, Nikoletta Szemerédi, Joana Freitas-Silva, Paulo Martins da Costa, Eugénia Pinto, Madalena Pinto, Gabriella Spengler, Emília Sousa, and Honorina Cidade. 2022. "BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections" Marine Drugs 20, no. 5: 315. https://doi.org/10.3390/md20050315
APA StyleJesus, A., Durães, F., Szemerédi, N., Freitas-Silva, J., da Costa, P. M., Pinto, E., Pinto, M., Spengler, G., Sousa, E., & Cidade, H. (2022). BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections. Marine Drugs, 20(5), 315. https://doi.org/10.3390/md20050315