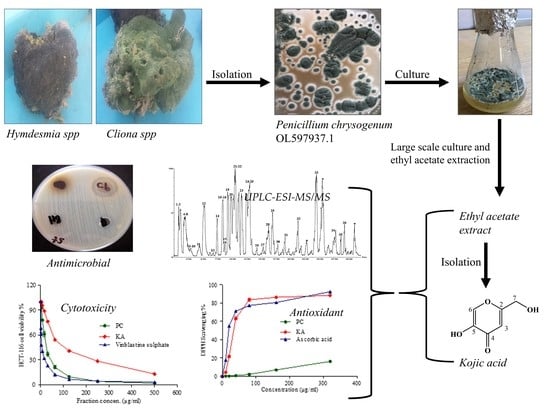
Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt)
Abstract
:1. Introduction
2. Results and Discussion
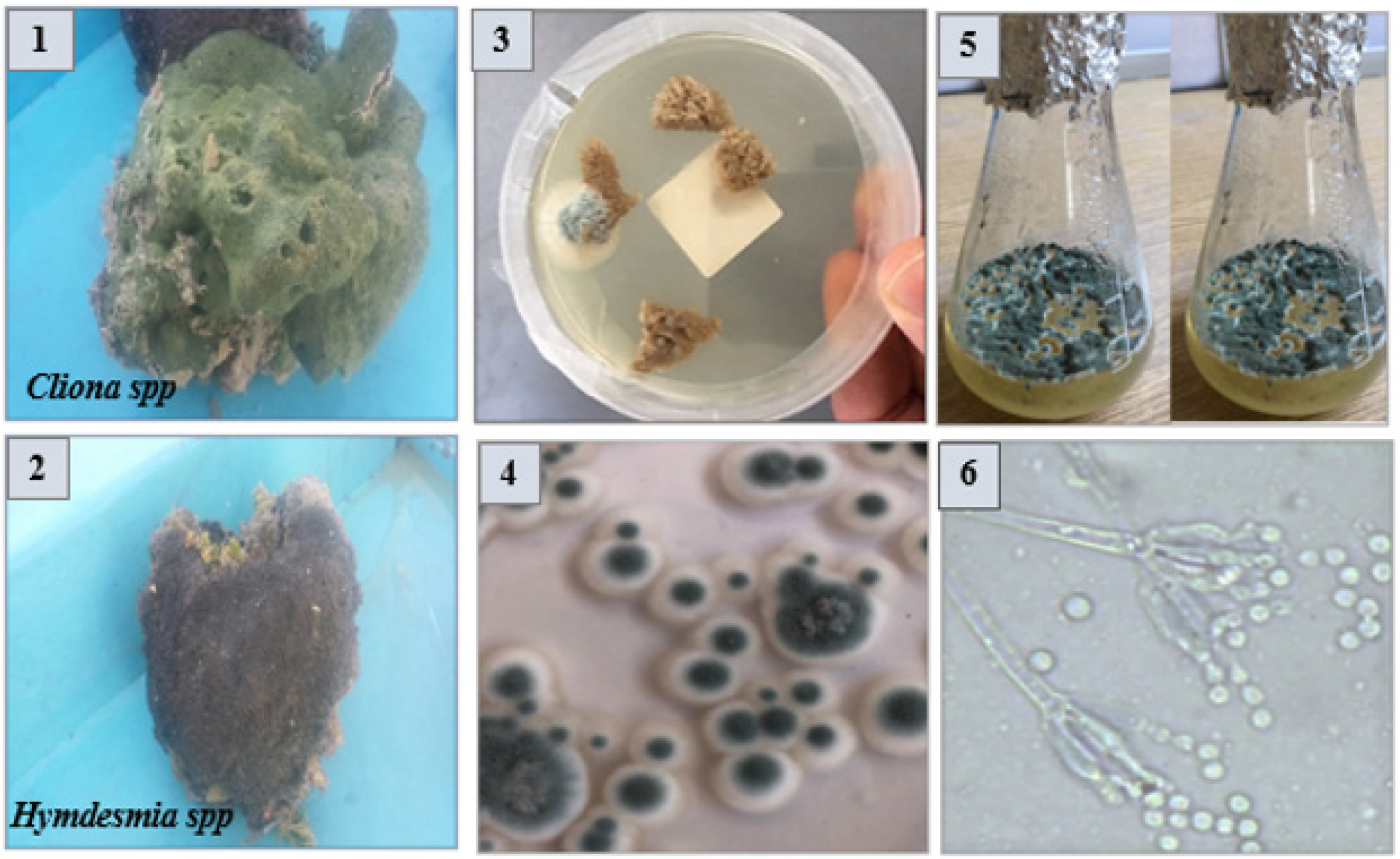
2.1. Isolation and Screening of Endozoic Fungi with Potent Antimicrobial Activity from the Medicinal Marine Sponge
2.2. Characterization of Penicillium Chrysogenum Ethyl Acetate Extract (PC) and the Isolated Kojic Acid (KA)
2.2.1. Spectroscopic Analyses of the Isolated Kojic Acid
2.2.2. Structural Identification of Constituents of P. chrysogenum Ethyl Acetate Extract by UPLC-ESI-MS/MS
| No. | Compound Name | Rt (min.) | Parent ion (m/z) | MS2 Fragments (m/z) | Area% Total | Reference |
|---|---|---|---|---|---|---|
| 1 | Amphetamine * | 0.79 | 136.0710 [M + H]+ | 119, 92, 54 | 1.51 | [24,25] |
| 2 | Pyrroline carboxylic acid | 0.88 | 115.0515 [M + 2H]+ | 96, 69, 41 | 0.50 | [26] |
| 3 | 3-hydroxy kojic acid * | 1.03 | 159.1239 [M + H]+ | 143, 125, 113, 96, 69 | 0.18 | [28] |
| 4 | Kojic acid | 1.13 | 143.0706 [M + H]+ | 126, 113, 97, 69 | 2.87 | [21,22] |
| 5 | Aspyrone * | 1.20 | 185.1388 [M + H]+ | 167, 139, 125 | 0.50 | [21] |
| 6 | Flufuran * | 1.35 | 143.0601 [M + H]+ | 125, 113, 96, 69 | 0.59 | [7] |
| 7 | Pyrroline carboxylic acid isomer | 1.84 | 115.0537 [M + 2H]+ | 96, 69, 41 | 0.50 | [26] |
| 8 | N-methyl-benzodioxazoylbutanamine (MBDB) | 2.15 | 208.0558 [M + H]+ | 177, 136, 85 | 0.59 | [24] |
| 9 | Asperlactone * | 3.35 | 185.1051 [M + H]+ | 158, 141, 128, 113, 98 | 0.77 | [21] |
| 10 | Acetyl Kojic acid * | 3.53 | 185.0992 [M + H]+ | 143, 125, 113, 97, 68 | 0.50 | [21] |
| 11 | Kojic acid aldehyde * | 4.83 | 141.0650 [M + H]+ | 123, 95, 67, 43 | 5.92 | [33] |
| 12 | Penicillin G | 6.11 | 356.1262 [M + Na]+ | 217, 176 | 0.46 | [34] |
| 13 | Anserine * | 6.48 | 241.1167 [M + H]+ | 212, 171, 69 | 1.75 | [26,36] |
| 14 | Penicillin G isomer | 7.87 | 356.9433 [M + Na]+ | 217, 176 | 3.34 | [34] |
| 15 | N-acetyl methionine | 8.02 | 193.1551 [M + H]+ | 160, 139, 115, 104 | 4.05 | [37] |
| 16 | Sorbicillin | 8.03 | 233.1105 [M + H]+ | 215, 200, 173, 145, 119 | 0.24 | [13] |
| 17 | Penillic acid | 8.55 | 335.1497 [M + H]+ | 155 | 0.24 | [39] |
| 18 | L-saccharopine * | 8.82 | 277.0930 [M + H]+ | 259, 231, 215, 203, 147, 84 | 4.05 | [26] |
| 19 | Quinolactacide * | 9.09 | 237.1015 [M + H]+ | 209, 192, 181, 169, 154 | 0.24 | [40] |
| 20 | L-saccharopine isomer | 10.29 | 277.2620 [M + H]+ | 259, 231, 215, 203, 147, 84 | 0.15 | [26] |
| 21 | Camptothecin * | 10.38 | 349.1757 [M + H]+ | 305, 277, 249, 221 | 3.77 | [41,42] |
| 22 | Camptothecin isomer | 10.98 | 349.2006 [M + H]+ | 305, 277, 249, 221 | 3.38 | [41,42] |
| 23 | Dihydrosorbicillin | 12.37 | 235.2265 [M + H]+ | 217, 199, 188, 174, 160, 147, 133, 106, 94, 69 (100%). | 4.51 | [13] |
| 24 | Sohirnone B | 12.81 | 271.1547 [M + Na]+ | 247, 229, 207, 181, 153, 94 | 0.31 | [38] |
| 25 | Kynurenine * | 12.97 | 209.2372 [M + H]+ | 164, 148, 136, 118, 94 | 7.95 | [26,46] |
| 26 | Penilloic acid * | 14.42 | 309.1964 [M + H]+ | 217, 189, 159, 148. | 0.36 | [47] |
| 27 | Sohirnone B derivative | 15.17 | 475.2501 [M + H]+ | 248, 207, 180 | 0.27 | [38] |
| 28 | Sorrentanone | 16.08 | 269.1486 [M + Na]+ | 250, 208, 180, 155 | 3.72 | [44] |
| 29 | Sorrentanone isomer | 17.02 | 269.1831 [M + Na]+ | 250, 208, 180, 155 | 3.11 | [44] |
| 30 | Fulvic acid | 18.79 | 309.1964 [M + H]+ | 231, 198, 181 | 2.82 | [21] |
| 31 | Citrinin | 19.72 | 273.2188 [M + Na]+ | 255, 227, 119, 115, 91 | 0.61 | [50,51] |
| 32 | Zearalenone | 21.98 | 319.2695 [M + H]+ | 283, 98, 83, 59 | 0.55 | [55] |
| 33 | Dehydro ergosterol | 24.21 | 377.2721 [M + H-H2O]+ | 267, 252, 189, 156, 134 | 0.44 | [57] |
| 34 | Stigmasterol | 27.47 | 395.3597 [M + H-H2O]+ | 311, 255, 215 | 1.09 | [60,61] |
| 35 | Ergosta-4,6,8(14),22 tetraen-3one | 27.70 | 393.3486 [M + H]+ | 335, 268, 250, 173 | 1.11 | [64] |
| 36 | Brassicasterol palmitate * | 29.91 | 639.6279 [M + H-H2O]+ | 381 | 1.62 | [60] |
2.3. Biological Activities of Penicillium Chrysogenum Ethyl Acetate Extract and Kojic Acid (KA)
2.3.1. Antimicrobial Activity and MIC
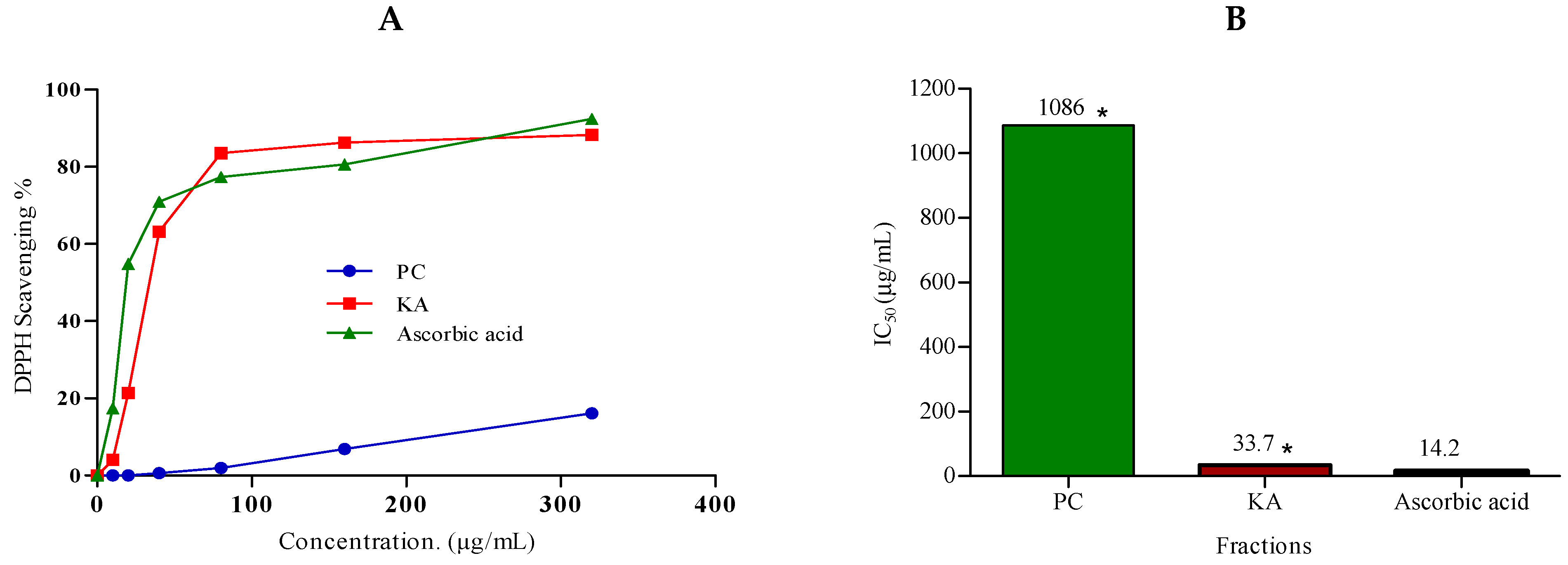
2.3.2. Antioxidant Activity
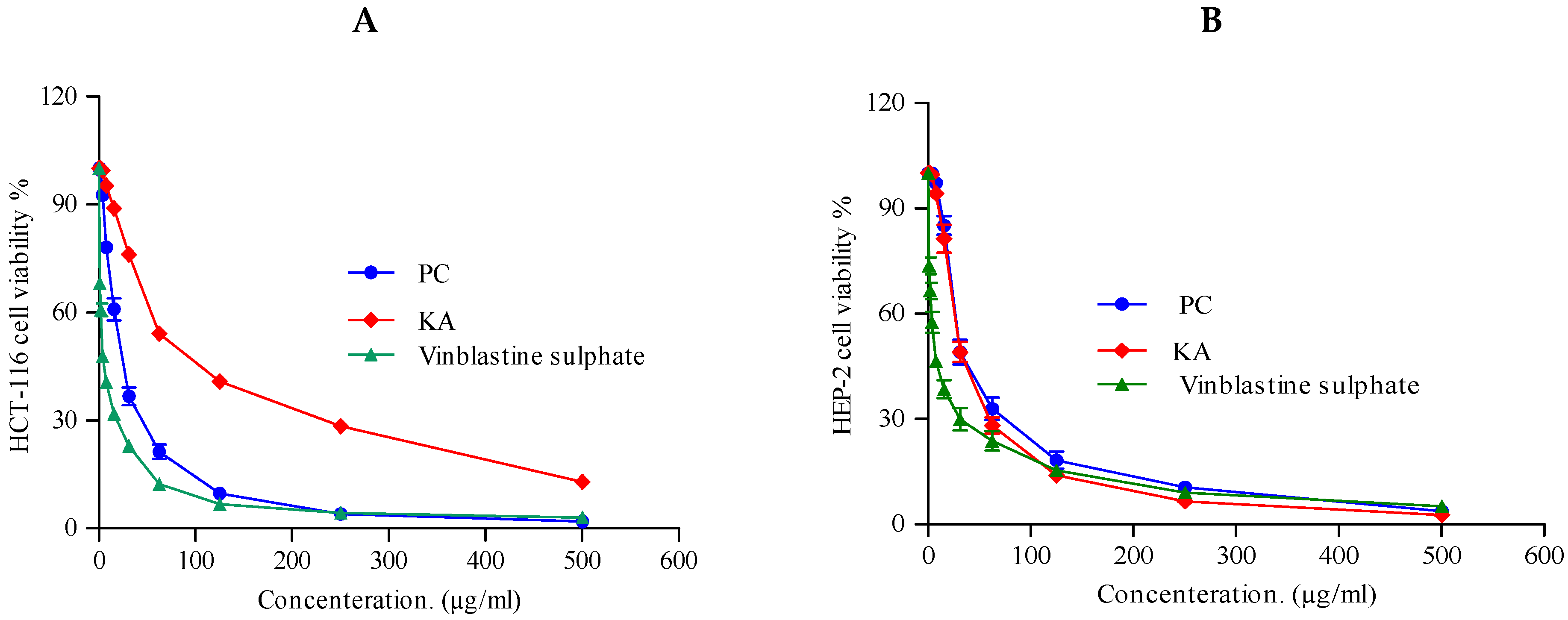
2.3.3. Cytotoxic Activity
3. Materials and Methods
3.1. General Materials and Methods
3.2. Collection of Marine Sponge Samples, Isolation, and Morphological Identification of Endophytic Fungi
3.3. Preparation of P. chrysogenum Crude Ethyl Acetate Extract
3.4. Isolation of Kojic Acid from the Ethyl Acetate Extract of P. chrysogenum
3.5. UPLC-ESI-MS/MS Profiling for Fungal Strain P. chrysogenum
3.6. Antioxidant Activity
DPPH Radical Scavenging Activity
3.7. Cytotoxic Activity
3.8. Antimicrobial Activity (Well Diffusion Method)
Determination of Minimum Inhibitory Concentration (MIC)
3.9. Fungal Deposition
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.J.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, U.; Usher, K.M.; Taylor, M.W. Marine sponges as microbial fermenters. FEMS Microbiol. Ecol. 2006, 55, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Qu, P.; Zhou, J.; Wang, Y.; Wang, L.; Zhu, W. p-Terphenyl alcohols from a marine sponge-derived fungus, Aspergillus candidus OUCMDZ-1051. Mar. Life Sci. Technol. 2020, 2, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Bovio, E.; Garzoli, L.; Poli, A.; Prigione, V.; Firsova, D.; McCormack, G.; Varese, G. The culturable mycobiota associated with three Atlantic sponges, including two new species: Thelebolus balaustiformis and T. spongiae. Fungal Syst. Evol. 2018, 1, 141–167. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.-M.; Tang, X.-L.; Sun, Y.-T.; Song, D.-Y.; Cheng, Y.-J.; Liu, H.; Li, P.-L.; Li, G.-Q. Biological and Chemical Diversity of Marine Sponge-Derived Microorganisms over the Last Two Decades from 1998 to 2017. Molecules 2020, 25, 853. [Google Scholar] [CrossRef] [Green Version]
- DellaGreca, M.; De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; Becchimanzi, A.; Iuliano, M.; Andolfi, A. The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus. Molecules 2019, 24, 1709. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-L.; Yuan, X.-L.; Du, Y.-M.; Zhang, Z.-F.; Zhang, P. Benzophenone derivatives from an algal-endophytic isolate of Penicillium chrysogenum and their cytotoxicity. Molecules 2018, 23, 3378. [Google Scholar] [CrossRef] [Green Version]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 2013, 66, 571–591. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A.; D’Elios, M.M. New therapeutic approaches by using microorganism-derived compounds. Curr. Med. Chem. 2012, 19, 3822–3840. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Trincone, A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.-S.; Li, X.-M.; Du, F.-Y.; Li, C.-S.; Proksch, P.; Wang, B.-G. Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Mar. Drugs 2011, 9, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Academic Press: London, UK, 1980; Volume 1, ISBN 0125577507. [Google Scholar]
- Pitt, J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press Inc.: London, UK, 1979; ISBN 0125577507. [Google Scholar]
- Raper, K.B.; Fennell, D.I. The genus Aspergillus; Williams and Wilkins Co.: Baltimore, MD, USA, 1965; pp. 1–686. [Google Scholar]
- De Oliveira, A.M.; Mesquita, M.d.S.; da Silva, G.C.; de Oliveira Lima, E.; de Medeiros, P.L.; Paiva, P.M.G.; Souza, I.A.d.; Napoleão, T.H. Evaluation of toxicity and antimicrobial activity of an ethanolic extract from leaves of Morus alba L.(Moraceae). Evid.-Based Complement. Altern. Med. 2015, 2015, 513978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Oh, S.-Y.; Fong, J.J.; Houbraken, J.; Lim, Y.W. The diversity and ecological roles of Penicillium in intertidal zones. Sci. Rep. 2019, 9, 13540. [Google Scholar] [CrossRef] [Green Version]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-) quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef] [Green Version]
- Nurunnabi, T.R.; Al-Majmaie, S.; Nakouti, I.; Nahar, L.; Rahman, M.; Sohrab, H.; Billah, M.; Ismail, F.M.; Sharples, G.P.; Sarker, S.D. Antimicrobial activity of kojic acid from endophytic fungus Colletotrichum gloeosporioides isolated from Sonneratia apetala, a mangrove plant of the Sundarbans. Asian Pac. J. Trop. Med. 2018, 11, 350–354. [Google Scholar]
- Li, T.-X.; Liang, J.-X.; Liu, L.-L.; Shi, F.-C.; Jia, X.-W.; Li, M.-H.; Xu, C.-P. Novel kojic acid derivatives with anti-inflammatory effects from Aspergillus versicolor. Fitoterapia 2021, 154, 105027. [Google Scholar] [CrossRef]
- Concheiro, M.; Simões, S.M.d.S.S.; Quintela, O.; de Castro, A.; Dias, M.J.R.; Cruz, A.; López-Rivadulla, M. Fast LC–MS/MS method for the determination of amphetamine, methamphetamine, MDA, MDMA, MDEA, MBDB and PMA in urine. Forensic Sci. Int. 2007, 171, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.F.; Enders, J.R.; Bell, D.S.; Cramer, H.M.; Wallace, F.N.; McIntire, G.L. Improved chiral separation of methamphetamine enantiomers using CSP-LC–MS-MS. J. Anal. Toxicol. 2016, 40, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piraud, M.; Vianey-Saban, C.; Petritis, K.; Elfakir, C.; Steghens, J.P.; Morla, A.; Bouchu, D. ESI-MS/MS analysis of underivatised amino acids: A new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom. 2003, 17, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Brötz-Oesterhelt, H.; Müller, W.E.; Wray, V.; Proksch, P. Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterapia 2013, 91, 100–106. [Google Scholar] [CrossRef]
- Terada, O.; Suzuki, S.; Kinoshita, S. 3-Oxykojic Acid, a New γ-Pyrone formed by Gluconobacter. Chem. Biol. Technol. Agric. 1961, 25, 802–803. [Google Scholar] [CrossRef]
- Saleh, R.M.; Kabli, S.A.; Al-Garni, S.M.; Mohamed, S.A. Screening and production of antibacterial compound from Trichoderma spp. against human-pathogenic bacteria. Afr. J. Microbiol. Res. 2011, 5, 1619–1628. [Google Scholar]
- Beélik, A. Kojic acid. In Advances in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1956; Volume 11, pp. 145–183. [Google Scholar]
- Koolen, H.; Soares, E.; Silva, F.; de Souza, A.; de Souza, A. Chemical constituents of Penicillium chrysogenum, an endophytic fungus from Strychnos toxifera. Chem. Nat. Compd. 2014, 49, 1164–1165. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakahara, S.; Fujioka, S. Aspyrone, a nematicidal compound isolated from the fungus, Aspergillus melleus. Biosci. Biotechnol. Biochem. 1996, 60, 1375–1376. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Murgia, S.; Arca, M.; Pintus, A.; Gans, P.; Niclos-Gutierrez, J.; Domínguez-Martín, A.; Castineiras, A. Kojic acid derivatives as powerful chelators for iron (III) and aluminium (III). Dalton Trans. 2011, 40, 5984–5998. [Google Scholar] [CrossRef]
- Suwanrumpha, S.; Flory, D.A.; Freas, R.B.; Vestal, M.L. Tandem mass spectrometric studies of the fragmentation of penicillins and their metabolites. Biomed. Environ. Mass Spectrom. 1988, 16, 381–386. [Google Scholar] [CrossRef]
- Devi, P.; Rodrigues, C.; Naik, C.; D’souza, L. Isolation and characterization of antibacterial compound from a mangrove-endophytic fungus, Penicillium chrysogenum MTCC 5108. Indian J. Microbiol. 2012, 52, 617–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, L.; Wei, H.; Zhou, W.; Zhou, W.; Li, F.; Lin, P.; Sheng, J.; Wang, Q.; Yan, C. A simultaneously quantitative profiling method for 40 endogenous amino acids and derivatives in cell lines using hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Talanta 2020, 207, 120256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-S.; Zhen-Fang, Z.; Xiao-Hong, Y.; Le-Fu, L.; Yu-Cheng, G.; Bo-Ping, Y.; Yue-Wei, G. Antibacterial sorbicillin and diketopiperazines from the endogenous fungus Penicillium sp. GD6 associated Chinese mangrove Bruguiera gymnorrhiza. Chin. J. Nat. Med. 2018, 16, 358–365. [Google Scholar] [CrossRef]
- Aldeek, F.; Canzani, D.; Standland, M.; Crosswhite, M.R.; Hammack, W.; Gerard, G.; Cook, J.M. Identification of Penicillin G Metabolites under Various Environmental Conditions Using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 6100–6107. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.; Yurchenko, A.; Ivanets, E.; Kirichuk, N.; Khudyakova, Y.V.; Yurchenko, E.; Afiyatullov, S. Metabolites of the Marine Fungus Penicillium citrinum Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2016, 52, 111–112. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Piacente, S.; Macchia, M.; Pizza, C. Metabolite fingerprinting of Camptotheca acuminata and the HPLC–ESI-MS/MS analysis of camptothecin and related alkaloids. J. Pharm. Biomed. Anal. 2010, 51, 405–415. [Google Scholar] [CrossRef]
- Srimany, A.; Ifa, D.R.; Naik, H.R.; Bhat, V.; Cooks, R.G.; Pradeep, T. Direct analysis of camptothecin from Nothapodytes nimmoniana by desorption electrospray ionization mass spectrometry (DESI-MS). Analyst 2011, 136, 3066–3068. [Google Scholar] [CrossRef]
- Guzmán-Chávez, F.; Salo, O.; Nygård, Y.; Lankhorst, P.P.; Bovenberg, R.A.; Driessen, A.J. Mechanism and regulation of sorbicillin biosynthesis by Penicillium chrysogenum. Microb. Biotechnol. 2017, 10, 958–968. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Xu, D.; Fu, X.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from fungi and their bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef]
- Maskey, R.P.; Grün-Wollny, I.; Laatsch, H. Sorbicillin analogues and related dimeric compounds from Penicillium n otatum. J. Nat. Prod. 2005, 68, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.; Truscott, R.J.; Richard, A.; Weimann, A.; Sheil, M.M. A study of kynurenine fragmentation using electrospray tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yang, M.; Hu, J.; Zhang, Y.; Chang, H.; Jin, F. Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ghosh, S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains A–C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.-Y.; Lin, C.-H. Simple and sensitive determination of citrinin in Monascus by GC-selected ion monitoring mass spectrometry. Anal. Sci. 2002, 18, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Vuković, G.L.; Bursić, V.P.; Aleksić, G.A.; Kuzmanović, S.T.; Cara, M.X.; Abd, E.-W.R.A. Data acquisition of triple quadrupole LC-MS for the citrinin determination. Zb. Matice Srp. Prir. Nauk. 2017, 133, 131–141. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological properties of citrinin. Arh. Hig. Rada Toksikol. 2009, 60, 457. [Google Scholar] [CrossRef] [Green Version]
- Meerpoel, C.; Vidal, A.; Andjelkovic, M.; De Boevre, M.; Tangni, E.K.; Huybrechts, B.; Devreese, M.; Croubels, S.; De Saeger, S. Dietary exposure assessment and risk characterization of citrinin and ochratoxin A in Belgium. Food Chem. Toxicol. 2021, 147, 111914. [Google Scholar] [CrossRef]
- Devi, P.; D’Souza, L.; Kamat, T.; Rodrigues, C.; Naik, C.G. Batch Culture Fermentation of Penicillium chrysogenum and a Report on the Isolation, Purification, Identification and Antibiotic Activity of Citrinin; CSIR: Pretoria, South Africa, 2009. [Google Scholar]
- Zhang, K.; Schaab, M.R.; Southwood, G.; Tor, E.R.; Aston, L.S.; Song, W.; Eitzer, B.; Majumdar, S.; Lapainis, T.; Mai, H. A collaborative study: Determination of mycotoxins in corn, peanut butter, and wheat flour using stable isotope dilution assay (SIDA) and liquid chromatography–tandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 2017, 65, 7138–7152. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Heald, S.L.; Jeffs, P.W.; Wheat, R.W. The identification of ergosterol and Δ9 (11)-dehydroergosterol from mycelia of Coccidioides immitis by reverse-phase high-performance liquid and gas chromatography and ultraviolet and mass spectrometry. Exp. Mycol. 1981, 5, 162–166. [Google Scholar] [CrossRef]
- Ano, Y.; Kutsukake, T.; Hoshi, A.; Yoshida, A.; Nakayama, H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE 2015, 10, e0116598. [Google Scholar] [CrossRef] [PubMed]
- Mioso, R.; Marante, F.J.; Laguna, I.H.; González, J.E.; Rodríguez, J.J. Biomolecules produced in liquid-state fermentation by a marine-derived fungus, Penicillium roqueforti. Química Nova 2014, 37, 260–267. [Google Scholar] [CrossRef]
- Rozenberg, R.; Ruibal-Mendieta, N.L.; Petitjean, G.; Cani, P.; Delacroix, D.L.; Delzenne, N.M.; Meurens, M.; Quetin-Leclercq, J.; Habib-Jiwan, J.-L. Phytosterol analysis and characterization in spelt (Triticum aestivum ssp. spelta L.) and wheat (T. aestivum L.) lipids by LC/APCI-MS. J. Cereal Sci. 2003, 38, 189–197. [Google Scholar] [CrossRef]
- Jiang, K.; Gachumi, G.; Poudel, A.; Shurmer, B.; Bashi, Z.; El-Aneed, A. The establishment of tandem mass spectrometric fingerprints of phytosterols and tocopherols and the development of targeted profiling strategies in vegetable oils. J. Am. Soc. Mass Spectrom 2019, 30, 1700–1712. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar]
- Ghanem, K.M.; Ghanem, N.B.; El-Refai, A.H. Ergosterol production under optimized conditions by Penicillium crustosum Thom. J. Islamic Acad. Sci. 1990, 3, 30–34. [Google Scholar]
- Ory, L.; Gentil, E.; Kumla, D.; Kijjoa, A.; Nazih, E.H.; Roullier, C. Detection of ergosterol using liquid chromatography/electrospray ionization mass spectrometry: Investigation of unusual in-source reactions. Rapid Commun. Mass Spectrom. 2020, 34, e8780. [Google Scholar] [CrossRef]
- Hamed, A.; El-Metwally, M.M.; Frese, M.; Ibrahim, T.M.; El-Haddad, A.F.; Sewald, N.; Shaaban, M. Diverse Bioactive Secondary Metabolites from Penicillium sp. 1P. J. At. Mol. 2017, 7, 1121–1132. [Google Scholar]
- Anjugam, M.; Bharathidasan, R.; Rani, A.S.; Ambikapathy, V. Evaluation of antimicrobial activities of endophytic fungal metabolites against clinical importance microbes. J. Pharmacogn. Phytochem. 2019, 8, 1004–1007. [Google Scholar]
- dela Cruz, T.E.E.; Notarte, K.I.R.; Apurillo, C.C.S.; Tarman, K.; Bungihan, M.E. Biomining fungal endophytes from tropical plants and seaweeds for drug discovery. In Biodiversity and Biomedicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–62. [Google Scholar]
- Kamat, S.; Kumari, M.; Taritla, S.; Jayabaskaran, C. Endophytic fungi of marine alga from Konkan coast, India—A rich source of bioactive material. Front. Mar. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Winarsi, H.; Yuniaty, A. Antioxidant exploration in cardamom rhizome potential as a functional food ingredient. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Moscow, Russian, 27 May–6 June 2019; IOP Publishing: Bristol, UK, 2019; p. 012019. [Google Scholar]
- Devi, K.B.D.; Vijayalakshmi, P.; Shilpa, V.; Kumar, B.V. Optimization of cultural parameters for cost effective production of kojic acid by fungal species isolated from soil. Microbiol. Res. J. Int. 2015, 7, 255–268. [Google Scholar] [CrossRef]
- Yan, S.; Tang, H.; Wang, S.; Xu, L.; Liu, H.; Guo, Y.; Yao, J. Improvement of kojic acid production in Aspergillus oryzae B008 mutant strain and its uses in fermentation of concentrated corn stalk hydrolysate. Bioprocess Biosyst. Eng. 2014, 37, 1095–1103. [Google Scholar] [CrossRef]
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Al-Abd, A.M.; Saber, F.R.; El-Halawany, A.M. Cytotoxic activity of acyl phloroglucinols isolated from the leaves of Eucalyptus cinerea F. Muell. ex Benth. cultivated in Egypt. Sci. Rep. 2014, 4, 5410. [Google Scholar] [CrossRef] [Green Version]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Women’s Health 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Novotný, L.; Rauko, P.; Abdel-Hamid, M.; Vachalkova, A. Kojic acid a new leading molecule for a preparation of compounds with an anti-neoplastic potential. Neoplasma 1999, 46, 89–92. [Google Scholar]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Proksch, P.; Ebel, R.; Edrada, R.; Riebe, F.; Liu, H.; Diesel, A.; Bayer, M.; Li, X.; Han Lin, W.; Grebenyuk, V.; et al. Sponge-associated fungi and their bioactive compounds: The Suberites case. Bot. Mar. 2008, 51, 209–218. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.S.A.; Akbar, A.; Iqrar, I.; Ali, R.; Norman, D.; Brennan, M.; Ali, G.S. A glucanolytic Pseudomonas sp. associated with Smilax bona-nox L. displays strong activity against Phytophthora parasitica. Microbiol. Res. 2018, 207, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.H.; Abdelaziz, S.; Al Yousef, H.M. Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioglu, O.I.; Oktay, M.; Büyükokuroglu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization, and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as anticancer agents. Monatsh. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hindler, J.; Howard, B.; Keiser, J. Antimicrobial agents and antimicrobial susceptibility testing. In Howard BJ Clinical and Pathogenic Microbiology, 2nd ed.; Mosby: St. Louis, MI, USA, 1994. [Google Scholar]
- Himratul-Aznita, W.H.; Mohd-Al-Faisal, N.; Fathilah, A. Determination of the percentage inhibition of diameter growth (PIDG) of Piper betle crude aqueous extract against oral Candida species. J. Med. Plants Res. 2011, 5, 878–884. [Google Scholar]
- Choudhary, M.I.; Thomsen, W.J. Bioassay Techniques for Drug Development; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wikler, M.A. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. CLSI (NCCLS) 2006, 26, M7-A7. [Google Scholar]
- Yoshida, T.; Jono, K.; Okonogi, K. Modified agar dilution susceptibility testing method for determining in vitro activities of antifungal agents, including azole compounds. Antimicrob. Agents Chemother. 1997, 41, 1349–1351. [Google Scholar] [CrossRef] [Green Version]
- Wayne, P. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. In CLSI Document M27-A2; CLSI: Malvern, PA, USA, 2002. [Google Scholar]




| Fungal Isolates | Gram Positive Bacteria | Gram Negative Bacteria | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 5368 | Escherichia coli ATCC 10536 | Pseudomonas aeruginosa ATCC 27853 | Candida albicans ATCC 10231 | |||||||
| IZ | MIC | IZ | MIC | IZ | MIC | IZ | MIC | |||
| Cliona sp. | 1 | Aspergillus orchaceous (C2) | 28 | 2000 | - | - | - | - | 14 | >3000 |
| 2 | Aspergillus terreus (C4) | 14 | 1000 | - | - | - | - | - | - | |
| 3 | Aspergillus niger (C5) | - | - | - | - | - | - | 14 (>3000) | >3000 | |
| 4 | Penicillium Chrysogenum (C6) | 23 | 250 | - | - | - | - | 40 | 93.75 | |
| Hymedesmia sp. | 1 | Aspergillus terreus (H2) | 19 | 250 | - | - | - | 20 | 187.5 | |
| 2 | Aspergillus awamori (H3) | - | - | - | - | - | - | - | - | |
| 3 | Aspergillus niger (H4) | - | - | - | - | - | - | - | - | |
| 4 | Aspergillus oryzae (H5) | 14 | >2000 | 14 | >2000 | 25 | 250 | 25 | 750 | |
| 5 | Alternaria alternata (H6) | 20 | >2000 | 15 | >2000 | 23 | >2000 | 25 | >3000 | |
| 9 | Trichoderma viridae (H7) | 21 | >2000 | 14 | >2000 | - | - | 18 | >1500 | |
| 7 | Penicillium lilacinum (H8) | - | - | - | - | - | - | 14 | >3000 | |
| 8 | Aspergillus astus (H9) | - | - | - | - | - | - | - | - | |
| Ciprofloxacin | - | 1.56 ± 1.2 | - | 3.125 ± 0.89 | - | 3.125 ± 0.24 | - | - | ||
| Fluconazole | - | - | - | - | - | - | 42 ± 0.58 | 50 ± 0.24 | ||
| DMSO (Negative control) | 10 | - | 10 | - | 19 | - | 12 | - | ||
| Extract/Compound | Inhibition Zone (IZ mm) Diameter (Mean ± SD)/Minimum Inhibitory Concentration (MIC µg/mL) | |||||
|---|---|---|---|---|---|---|
| Gram Positive Bacteria | Gram Negative Bacteria | |||||
| Staphylococcus aureus ATCC 5368 | Escherichia coli ATCC 10536 | Staphylococcus aureus ATCC 5368 | ||||
| IZ | MIC | IZ | MIC | IZ | MIC | |
| Solvent (DMSO) | 10 | 10 | 19 | |||
| PC Total extract | 23 ± 0.72 | 250 ± 0.82 | - | - | - | - |
| Kojic acid (KA) | 14 ± 0.82 | >2000 ± 1.4 | 14 ± 0.59 | >2000 ± 1.5 | 25 ±0.82 | 250 ± 0.82 |
| Ciprofloxacin | - | 1.56 ± 1.2 | - | 3.125 ± 0.89 | - | 3.125 ± 0.24 |
| Extract/ Compound | Inhibition Zone (IZ mm) Diameter (Mean ± SD)/Minimum Inhibitory Concentration (MIC µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | ||||||||||
| Candida albicans ATCC 10231 | Aspergillus fumigatus RCMB 002008 | Aspergillus flavus RCMB 002002 | Cryptococcus neoformans RCMB 0049001 | Fusarium oxysporum RCMB 001004 | ||||||
| IZ | MIC | IZ | MIC | IZ | MIC | IZ | MIC | IZ | MIC | |
| PC Total extract | 40 ± 0.45 | 93.75 ± 0.55 | 13 ± 0.63 | 625 ± 1.3 | 10 ± 0.73 | 1000 ± 1.3 | 22 ± 0.19 | 19.53 ± 0.48 | 9 ± 0.68 | 10000 ± 1.5 |
| Kojic acid (KA) | 25 ± 0.56 | 750 ± 0.38 | 13 ± 0.48 | 312.5 ± 0.47 | 9 ± 0.72 | 5000 ± 1.4 | 18 ± 0.58 | 39.06 ± 0.98 | 19 ± 0.93 | 39.06 ± 0.85 |
| Fluconazole | 42 ± 0.58 | 50 ± 0.24 | 18 ± 1.2 | 39.06 ± 0.72 | 17 ± 0.8 | 39.06 ± 0.48 | 25 ± 0.63 | 4.88 ± 0.32 | 19 ± 0.7 | 19.53 ± 0.82 |
| Cell Line | Tested Fractions | ||
|---|---|---|---|
| IC50 (µg/mL) | |||
| PC | KA | Vinblastine Sulphate | |
| HCT-116 (Colon carcinoma) | 22.6 ± 0.8 | 23.4 ± 1.4 | 2.34 ± 0.28 |
| HEP-2 (Human Larynx carcinoma) | 30.8 ± 1.3 | 30.8 ± 1.2 | 6.61 ± 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saleem, M.S.M.; Hassan, W.H.B.; El Sayed, Z.I.; Abdel-Aal, M.M.; Abdel-Mageed, W.M.; Abdelsalam, E.; Abdelaziz, S. Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt). Mar. Drugs 2022, 20, 326. https://doi.org/10.3390/md20050326
Al-Saleem MSM, Hassan WHB, El Sayed ZI, Abdel-Aal MM, Abdel-Mageed WM, Abdelsalam E, Abdelaziz S. Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt). Marine Drugs. 2022; 20(5):326. https://doi.org/10.3390/md20050326
Chicago/Turabian StyleAl-Saleem, Muneera S. M., Wafaa H. B. Hassan, Zeinab I. El Sayed, Mahmoud M. Abdel-Aal, Wael M. Abdel-Mageed, Eman Abdelsalam, and Sahar Abdelaziz. 2022. "Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt)" Marine Drugs 20, no. 5: 326. https://doi.org/10.3390/md20050326
APA StyleAl-Saleem, M. S. M., Hassan, W. H. B., El Sayed, Z. I., Abdel-Aal, M. M., Abdel-Mageed, W. M., Abdelsalam, E., & Abdelaziz, S. (2022). Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt). Marine Drugs, 20(5), 326. https://doi.org/10.3390/md20050326