Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review
Abstract
1. Introduction
2. Seaweed Resources
2.1. Brown Seaweeds
2.2. Red Seaweeds
2.3. Green Seaweeds
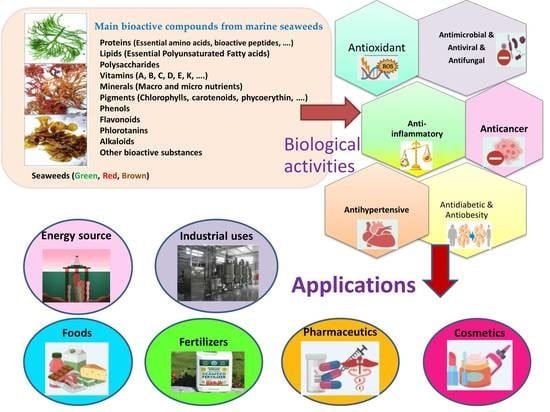
3. Bioactive Compounds
3.1. Polysaccharides
3.1.1. Role of Polysaccharides in Medicine
3.1.2. Role of Polysaccharides in Food Industry
3.1.3. Role of Polysaccharides in Cosmeceuticals
3.2. Protein and Amino Acids
3.2.1. Role of Proteins and Amino Acid in Medicine
3.2.2. Role of Proteins and Amino Acid in Cosmeceuticals
3.3. Fatty Acids
3.3.1. Role of Fatty Acids in Medicine
3.3.2. Role of Fatty Acids in Foods
3.3.3. Role of Fatty Acids in Cosmeceuticals
3.4. Pigments
3.5. Phenolic Compounds
3.6. Minerals
3.7. Vitamins
4. Biological Activities
4.1. Antioxidant Activity
4.2. Antimicrobial Activity
4.3. Anticancer Activity
4.4. Antidiabetics Activity
5. Seaweeds in Bio-Manufacturing Applications
5.1. Fertilizer and Soil Conditioners
5.2. Medical and Pharmaceutical Use
5.2.1. Biomedical Applications of Seaweeds
5.2.2. Pharmaceutical Applications of Seaweeds
5.3. Cosmetic Industry
6. Materials and Methods
Literature Search
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Science 2007, 316, 382–383. Available online: https://www.science.org/doi/10.1126/science.1138042 (accessed on 20 April 2007). [CrossRef] [PubMed]
- Irkin, L.C.; Yayintas, Ö. Pharmacological Properties and Therapeutic Benefits of Seaweeds (A Review). Int. J. Trend Sci. Res. Dev. 2018, 2, 1126–1131. [Google Scholar] [CrossRef]
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Chapman and Hall in Associate with Methuen: London, UK, 1980; p. 334. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204. [Google Scholar]
- Alshehri, M.A.; Al Thabiani, A.; Alzahrani, O.; Ibrahim, A.A.S.; Osman, G.; Bahattab, O. DNA-barcoding and Species Identification for some Saudi Arabia Seaweeds using rbcL Gene. J. Pure Appl. Microbiol. 2019, 13, 2035–2044. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Misurcová Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra species: A mini-review of its pharmacological and nutritional properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Mantri, V.A.; Kavale, M.G.; Kazi, M.A. Seaweed biodiversity of India: Reviewing current knowledge to identify gaps, challenges, and opportunities. Diversity 2020, 12, 13. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Saratale, G.D. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Eppink, M.H.; Olivieri, G.; Reith, H.; van den Berg, C.; Barbosa, M.J.; Wijffels, R.H. From current algae products to future biorefinery practices: A review. Biorefineries 2017, 166, 99–123. Available online: https://link.springer.com/chapter/10.1007/10_2016_64 (accessed on 7 March 2017).
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Pulz, O.; Broneske, J.; Waldeck, P. IGV GmbH experience report, industrial production of microalgae under controlled conditions: Innovative prospects. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Wageningen University: Wageningen, The Netherlands, 2013; pp. 445–460. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Ghosh, R.; Banerjee, K.; Mitra, A. Seaweeds in the Lower Gangetic Delta. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Misurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.-K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 567. [Google Scholar]
- Al-Amoudi, O.A.; Mutawie, H.H.; Patel, A.V.; Blunden, G. Chemical composition and antioxidant activities of Jeddah corniche algae. Saudi J. Biol. Sci. 2009, 16, 23–29. [Google Scholar] [CrossRef]
- Edwards, M.; Hanniffy, D.; Heesch, S.; Hernández-Kantún, J.; Moniz, M.; Queguineur, B.; Ratcliff, J.; Soler-Vila, A.; Wan, A. Macroalgae Fact-Sheets; Soler-Vila, A., Moniz, M., Eds.; Irish Seaweed Research Group, Ryan Institute, NUI Galway: Galway, Ireland, 2012; p. 40. [Google Scholar]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytungaa, D.T.U.; Lee, W.W.; Jeon, Y.-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Hii, S.L.; Lim, J.; Ong, W.T.; Wong, C.L. Agar from Malaysian red seaweed as potential material for synthesis of bioplastic film. J. Eng. Sci. Technol. 2016, 7, 1–15. [Google Scholar]
- Melo, M.R.S.; Feitosa, J.P.A.; Freitas, A.L.P.; De Paula, R.C.M. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491. [Google Scholar] [CrossRef]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. A Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Salman, A.S.; Alharbi, A.A.; Alhasani, R.H.; Elshamy, M.M. Assessment of the Antigenotoxic Effects of Alginate and ZnO/Alginate–Nanocomposites Extracted from Brown Alga Fucus vesiculosus in Mice. Polymers 2021, 13, 3839. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from Filamentous microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.-I.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agr. 2019, 99, 6722–6730. [Google Scholar] [CrossRef]
- Nagamine, T.; Hayakawa, K.; Kusakabe, T.; Takada, H.; Nakazato, K.; Hisanaga, E.; Iha, M. Inhibitory effect of fucoidan on Huh7 hepatoma cells through downregulation of CXCL12. Nutr. Cancer 2009, 61, 340–347. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Kan, J.; Hu, Z.; Liu, Y.; Du, H.; Pang, G.-C.; Cheong, K.-L. Quantification of Neoagaro-Oligosaccharide Production through Enzymatic Hydrolysis and Its Anti-Oxidant Activities. Molecules 2018, 23, 1354. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)- Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2012, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’hara, C.; Magee, P.J.; Mcsorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Venugopal, V. Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. ECronicon Nutr. 2019, 2, 126–141. [Google Scholar]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.Q.; Hu, Z.; Li, S.D.; Quan, W.Y.; Wen, L.L.; Yang, Z.M.; Li, P.W. Thermal degradation of agar: Mechanism and toxicity of products. Food Chem. 2018, 264, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Kurup, G.M. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Biomed. Prev. Nutr. 2011, 1, 294–301. [Google Scholar] [CrossRef]
- Anastyuk, S.; Shervchenko, N.; Ermakova, S.; Vishchuk, O.; Nazarenko, E.; Dmitrenok, P.; Zvyagintseva, T. Anticancer activity in vitro of a fucoidan from the brown algae Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Xu, W.; Liang, J.-W.; Wang, C.-S.; Kang, Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Boil. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Park, J.S.; Hong, S.K. Toxicological evaluation of neoagarooligosaccharides prepared by enzymatic hydrolysis of agar. Regul. Toxicol. Pharmacol. 2017, 90, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.L.; Norton, A.B.; Mills, T.B.; Norton, I.T. Stabilisation of foams by agar gel particles. Food Hydrocoll. 2017, 33, 222–228. [Google Scholar] [CrossRef]
- Hernandez-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Cambridge, UK, 2013; pp. 475–516. [Google Scholar] [CrossRef]
- Pegg, A.M. The application of natural hydrocolloids to foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing: Cambridge, UK, 2012; pp. 175–196. [Google Scholar]
- Scieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Available online: https://www.fao.org/3/y4765e/y4765e00.htm (accessed on 15 July 2003).
- Soukoulis, C.; Chandrinos, I.; Tzia, C. Study of the functionality of selected hydrocolloids and their blends with κ-carrageenan on storage quality of vanilla ice cream. LWT 2008, 41, 1816–1827. [Google Scholar] [CrossRef]
- Pereira, L. Carrageenans—Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2016; p. 293. ISBN 1634855035. Available online: https://novapublishers.com/shop/carrageenans-sources-and-extraction-methods-molecular-structure-bioactive-properties-and-health-effects/ (accessed on 1 September 2016).
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from marine sources. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. ISBN 978-3-642-53970-1. Available online: https://www.springerprofessional.de/en/cosmetics-from-marine-sources/4217512 (accessed on 1 February 2020).
- Mafinowska, P. Algae extracts as active cosmetic ingredients. Zesz. Nauk. 2011, 212, 123–129. [Google Scholar]
- Fabrowska, J.; Łęska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and extracts of algae as material for cosmetics. In Marine Algae Extracts; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH, Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 681–706. ISBN 9783527337088. [Google Scholar]
- Hotchkiss, S.; Campbell, R.; Hepburn, C. Carrageenan: Sources and extraction methods. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Pereira, L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–16. ISBN 978-1-63485-503-7. [Google Scholar]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-N.; Patil, M.P.; Cho, Y.-J.; Kim, G.-D.; Park, Y.B.; Woo, H.-C.; Chun, B.-S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2, 1–50. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Admassu, H.; Abdalbasit, M.; Gasmalla, A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Wijesekara, I.; Lang, M.; Marty, C.; Gemin, M.P.; Boulho, R.; Douzenel, P.; Wickramasinghe, I.; Bedoux, G.; Bourgougnon, N. Different extraction procedures and analysis of protein from Ulva Sp. In Brittany, France. J. Appl. Phycol. 2017, 29, 2503–2511. [Google Scholar] [CrossRef]
- Abdel-fattah, A.F.; Sary, H.H. Glycoproteins from Ulva lactuca. Phytochemistry 1987, 26, 1447–1448. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.R.; Nam, T.J.; Kong, I.S. Antioxidant and DNA protection activities of a glycoprotein isolated from a seaweed, Saccharina japonica. Int. J. Food Sci. Technol. 2012, 47, 1020–1027. [Google Scholar] [CrossRef]
- Chaves, R.P.; Silva, S.R.D.; Nascimento Neto, L.G.; Carneiro, R.F.; Silva, A.L.C.D.; Sampaio, A.H.; Sousa, B.L.D.; Cabral, M.G.; Videira, P.A.; Teixeira, E.H.; et al. structural characterization of two isolectins from the marine red alga Solieria Filiformis (Kützing) P.W. Gabrielson and their anticancer effect on MCF-7 breast cancer cells. Int. J. Biol. Macromol. 2018, 107, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.M.; Monteiro, V.S.; Martins, A.B.S.; Teles, F.B.; Da Conceição Rivanor, R.L.; Mota, É.F.; Macedo, D.S.; de Vasconcelos, S.M.M.; Júnior, J.E.R.H.; Benevides, N.M.B. Involvement of the dopaminergic system in the antidepressant-like effect of the lectin isolated from the red marine alga Solieria Filiformis in mice. Int. J. Biol. Macromol. 2018, 111, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Nam, T.J. Hydrophilic glycoproteins of an edible green alga Capsosiphon fulvescens prevent aging- induced spatial memory impairment by suppressing Gsk-3β-Mediated Er stress in Dorsal hippocampus. Mar. Drugs. 2019, 17, 168. [Google Scholar] [CrossRef] [PubMed]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Soo Moon, I.; Kim, J.M.; Kong, I.S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Ratana-arporn, P.; Chirapart, A. Nutritional Evaluation of Tropical Green Seaweeds Caulerpa Lentillifera and Ulva Reticulata. Kasetsart J. Nat. Sci. 2006, 40, 75–83. [Google Scholar]
- Lumbessy, S.Y.; Andayani, S.; Nursyam, H.; Firdaus, M. Biochemical Study of Amino Acid Profile of Kappaphycus alvarezii and Gracilaria salicornia Seaweeds from Gerupuk Waters, West Nusa Tenggara (NTB). Eur. Asian J. Biosci. 2019, 13, 303–307. [Google Scholar]
- Zubia, M.; Payri, C.E.; Deslandes, E.; Guezennec, J. Chemical Composition of Attached and Drift Specimens of Sargassum Mangarevense and Turbinaria Ornata (Phaeophyta: Fucales) from Tahiti, French Polynesia. Bot. Mar. 2003, 46, 562–571. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Vargas, N.; Pasten, A.; Rodríguez, K.; Ah-Hen, K.S. Phytochemical Components and Amino Acid Profile of Brown Seaweed Durvillaea Antarctica as Affected by Air Drying Temperature. J. Food Sci. Technol. 2018, 55, 4792–4801. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Helmi, A.; Mohamed, H.I. Biochemical and ulturasturctural changes of some tomato cultivars to infestation with Aphis gossypii Glover (Hemiptera: Aphididae) at Qalyubiya, Egypt. Gesunde Pflanzen. 2016, 68, 41–50. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 197–213. [Google Scholar]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.P.; Villaume, C.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Wu, G. (Ed.) Amino Acids: Biochemistry and Nutrition, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients. 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, I.-H.; Nam, T.-J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef]
- Mensi, F.; Nasraoui, S.; Bouguerra, S.; BenGhedifa, A.; Chalghaf, M. Effect of Lagoon and sea water depth on Gracilaria gracilis growth and biochemical composition in the Northeast of Tunisia. Sci. Rep. 2020, 10, 10014. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrin, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture System. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-Like amino acid profiles of wild-Harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of Mycosporine-Like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-derived bioactive compounds with anti-lung cancer potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Pangestutil, R.; Kim, S. Seaweed Proteins, Peptides, and Amino Acids; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 125–140. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. Adv. Bot. Res. 2014, 71, 345–378. [Google Scholar] [CrossRef]
- Pereira, L. Chapter 6—Seaweed Flora of the European North Atlantic and Mediterranean. In Springer Handbook of Marine Biotechnology; Se-Kwon, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 65–178. ISBN 978-3-642-53971-8. [Google Scholar] [CrossRef]
- Notowidjojo, L. Seaweed as novel food for prevention and therapy for life style related disease. World Nutr J. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Verlecar, X.N. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Probst, Y. A review of the nutrient composition of selected rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. Algal proteins, peptides and amino acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 135–180. ISBN 978-0-85709-512-1. [Google Scholar]
- Quitral, V.; Morales, C.; Sepúlveda, M.; Schwartz, M. Propiedades nutritivas y saludables de algas marinas y su potencialidad como ingrediente funcional. Rev. Chil. Neuropsiquiatr. 2015, 53, 35–43. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential Antioxidant and Anticancer Activities of Secondary Metabolites of Nostoc linckia Cultivated under Zn and Cu Stress Conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; De Nys, R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; ¸Sahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; López-Lopez, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S.; Larrea, M.T.; Jiménez-Colmenero, F. Nutritional and antioxidant properties of different brown and red spanish edible seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jager, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of alpha-glucosidase inhibitors by combined use of high-resolution alpha-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Arao, T.; Yamada, M. Positional distribution of fatty acids in galactolipids of algae. J. Phytochem. 1989, 28, 805–810. [Google Scholar] [CrossRef]
- Niaz, A.; Kashif, A. Chemical and Different Nutritional Characteristics of Brown Seaweed Lipids Advances in Science. Technol. Eng. Syst. J. 2016, 1, 23–25. [Google Scholar] [CrossRef]
- Kanazawa, A. Sterols in marine invertebrates. Fish. Sci. 2001, 67, 997–1007. [Google Scholar] [CrossRef]
- Francavilla, M.; Trotta, P.; Luque, R. Phytosterols from Dunaliella tertiolecta and Dunaliella salina: A potentially novel industrial application. Bioresour. Technol. 2010, 101, 4144–4150. [Google Scholar] [CrossRef]
- da Vaz, B.S.; Moreira, J.B.; De Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical Composition of Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 79–124. ISBN 9780124199583. [Google Scholar]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed Minor Constituents; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 193–242. [Google Scholar]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Jia, X.; Yang, J.; Wang, Z.; Liu, R.; Xie, R. Polysaccharides from Laminaria japonica show hypoglycemic and hypolipidemic activities in mice with experimentally induced diabetes. Exp. Biol. Med. 2014, 239, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty Acids of Microalgae: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2021; Volume 3, ISBN 0123456789. [Google Scholar]
- Da Silva, T.L.; Moniz, P.; Silva, C.; Reis, A. The dark side of microalgae biotechnology: A heterotrophic biorefinery platform directed to ω-3 rich lipid production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Wynn, J.; Behrens, P.; Sundararajan, A.; Hansen, J.; Apt, K. Production of single cell oils from dinoflagellates. In Single Cell Oils; Microbial and Algal Oils; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2010; pp. 115–129. [Google Scholar]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Murphy, M.J.; Dow, A.A. Clinical studies of the safety and efficacy of macroalgae extracts in cosmeceuticals. J. Clin. Aesthet. Dermatol. 2021, 14, 37–41. [Google Scholar]
- Yang, M.; Zhou, M.; Song, L. A review of fatty acids influencing skin condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Yamada, K.; Nitta, T.; Atsuji, K.; Shiroyama, M.; Inoue, K.; Higuchi, C.; Nitta, N.; Oshiro, S.; Mochida, K.; Iwata, O.; et al. Characterization of sulfur-compound metabolism underlying wax-ester fermentation in Euglena gracilis. Sci. Rep. 2019, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.; Maktabi, B.; Reddy, C.M.; O’Neil, G.W.; Chandler, M.; Baki, G. Evaluation of alkenones, a renewably sourced, plant-derived wax as a structuring agent for lipsticks. Int. J. Cosmet. Sci. 2020, 42, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Dang, H.T.; Kang, G.J.; Yang, E.J.; Park, S.S.; Yoon, W.J.; Jung, J.H.; Kang, H.K.; Yoo, E.S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-êB and STAT1 activity in lipopolysaccharide-stimulated raw 264.7 cells. Arch. Pharm. Res. 2009, 32, 453–462. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, K.; Jung, S.; Lee, S. Effects of the extracts from the marine algae Pelvetia siliquosa on hyperlipidemia in rats. Korean J. Pharmacogn. 2004, 35, 143–146. [Google Scholar]
- Hwang, E.; Park, S.-Y.; Sun, Z.-W.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-Irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Singh, P.R.; Kumar, D.; Sinha, R.P. Phycobiliproteins in microalgae: Occurrence, distribution, and biosynthesis. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020; pp. 43–68. ISBN 978-3-030-50971-2. [Google Scholar]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May omega-3 fatty acid dietary supplementation help reduce severe complications in COVID-19 patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.; Huang, Y.-H.; Lim, H.-W.; Lee, Y.; Jang, K.; Cho, Y.; Park, J.S.; Kim, D.-D.; Lim, C.-J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; DeLutiis, M.A. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokinessecretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Akhter, S.; Hasan, A.T.; Alam, T.; Nageeb Hasan, S.M.; Saifullah, A.R.; Shohel, C. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain Dis. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Lee, Y.-J.; Chou, M.-C.; Chang, R.; Chiu, C.-H.; Liang, Y.-J.; Wu, L.-S. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse leydig cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [CrossRef]
- Sharoni, Y.; Agemy, L.; Giat, U.; Kirilov, E.; Danilenko, M.; Levy, J. Lycopene and astaxanthin inhibit human prostate cancer cell proliferation induced by androgens. In Proceedings of the 13th International Symposium on Carotenoids, Honolulu, HI, USA, 6–11 January 2002. [Google Scholar]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumoractivity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.-M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2 induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Hamada, M.; Takahashi, S.; Xing, G.; Liu, J.; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep. 2008, 20, 1099–1103. [Google Scholar] [CrossRef]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.-Y.O.; Shang, F. Lutein and zeaxanthin supplementation reduces H2O2 induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. Available online: http://www.molvis.org/molvis/v17/a343 (accessed on 7 December 2011).
- Matsumoto, M.; Hosokawa, M.; Matsukawa, N.; Hagio, M.; Shinoki, A.; Nishimukai, M.; Hara, H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr. 2010, 49, 243–249. [Google Scholar] [CrossRef]
- Allard, J.P.; Royall, D.; Kurian, R.; Muggli, R.; Jeejeebhoy, K.N. Effects of beta-carotene supplementation on lipid peroxidation in humans. Am. J. Clin. Nutr. 1994, 59, 884–890. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. β-Carotene and lycopene affect endothelial response to TNF-a reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Pioneering role of marine macroalgae in cosmeceuticals. Phycology 2022, 2, 172–203. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C.; Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Xu, T.; Sutour, S.; Casabianca, H.; Tomi, F.; Paoli, M.; Garrido, M.; Pasqualini, V.; Aiello, A.; Castola, V.; Bighelli, A. Rapid Screening of Chemical Compositions of Gracilaria dura and Hypnea mucisformis (Rhodophyta) from Corsican Lagoon. Int. J. Phytocosmetics Nat. Ingred. 2015, 2, 8. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental impact on seaweed phenolic production and activity: An important step for compound exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štĕrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Urban, S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.P.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Unusual antimalarial meroditerpenes from tropical red macroalgae. Bioorganic Med. Chem. Lett. 2010, 20, 5662–5665. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Leenen, F.H.H.; Chen, L.; Golovina, V.A.; Hamlyn, J.M.; Pallone, T.L.; Van Huysse, J.W.; Zhang, J.; Wier, W.G. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1031–H1049. [Google Scholar] [CrossRef]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef]
- Desideri, D.; Cantaluppi, C.; Ceccotto, F.; Meli, M.A.; Roselli, C.; Feduzi, L. Essential and toxic elements in seaweeds for human consumption. J. Toxicol. Environ. Health Part A 2016, 79, 112–122. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soci. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Parjikolaei, B.R.; Bruhn, A.; Eybye, K.L.; Larsen, M.M.; Rasmussen, M.B.; Christensen, K.V.; Fretté, X.C. Valuable biomolecules from nine north atlantic red macroalgae: Amino acids, fatty acids, carotenoids, minerals and metals. Nat. Resour. 2016, 7, 157–183. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schäfer, U.; Leiterer, M.; Jahreis, G. Nutritional and toxicological importance of macro, trace, and ultra-trace elements in algae food products. J. Agric. Food Chem. 2007, 55, 10470–10475. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Cañeque, V.; Díaz, M.T.; López, O.; Jiménez-Colmenero, F. Effect of cooking on the chemical composition of low-salt, low-fat Wakame/olive oil added beef patties with special reference to fatty acid content. Meat Sci. 2011, 89, 27–34. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sánchez-Muniz, F.; Cofrades, S.; Jiménez-Colmenero, F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Yakan, A.; Solas, M.T.; Jiménez-Colmenero, F. Frozen storage characteristics of low-salt and low-fat beef patties as affected by Wakame addition and replacing pork backfat with olive oil-in-water emulsion. Food Res. Int. 2010, 43, 1244–1254. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Jacob, L.; Baker, C.; Farris, P. Vitamin-based cosmeceuticals. Cosmet. Dermatol. 2012, 25, 405. [Google Scholar]
- Chakraborty, K.; Praveen, N.K.; Vijayan, K.K.; Rao, G.S. Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2013, 3, 8–16. [Google Scholar] [CrossRef]
- Škrovánková, S. Seaweed vitamins as nutraceuticals. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 64, pp. 357–369. ISBN 9780123876690. [Google Scholar]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, 14095. [Google Scholar] [CrossRef]
- Bissett, D.L.; Oblong, J.E.; Goodman, L.J. Topical Vitamins. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 336–345. [Google Scholar] [CrossRef]
- Kilinç, B.; Semra, C.; Gamze, T.; Hatice, T.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; Muzzalupo, I., Ed.; InTech: Rijeka, Croatia, 2013; pp. 735–748. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Fernández, E.; Sone, I.; Astráin-Redín, L.; Prabhu, L.; Sivertsvik, M.; Álvarez, I.; Cebrián, G. Innovative ultrasound-assisted approaches towards reduction of heavy metals and iodine in macroalgal biomass. Foods 2021, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Falquet, J.; Hurni, J.P. The Nutritional Aspects of Spirulina. Antenna Foundation. 1997. Available online: https://www.antenna.ch/wp-content/uploads/2017/03/AspectNut_UK (accessed on 25 July 2017).
- Kumar, C.S.; Ganesan, P.; Suresh, P.V.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds—A review. J. Food Sci. Technol. 2008, 45, 1. [Google Scholar]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Mathew, S.; Ravishankar, C.N. Seaweeds as a Source of Micro and Macro Nutrients; ICAR-Central Institute of Fisheries Technology: Cochin, India, 2018. Available online: http://krishi.icar.gov.in/jspui/handle/123456789/20485 (accessed on 26 November 2018).
- Vardi, M.; Levy, N.S.; Levy, A.P. Vitamin E in the prevention of cardiovascular disease: The importance of proper patient selection. J. Lipid Res. 2013, 54, 2307–2314. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Butt, M.S.; Amjad, N.; Yasmin, I.; Suleria, H.A.R. Marine-Algal Bioactive Compounds: A Comprehensive Appraisal. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 71–80. [Google Scholar]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 9, 12233. [Google Scholar] [CrossRef]
- Sellimi, S.; Kadri, N.; Barragan-Montero, V.; Laouer, H.; Hajji, M.; Nasri, M. Fucans from a Tunisian brown seaweed Cystoseira barbata: Structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014, 66, 281–288. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and control of its deleterious effects with melatonin and salicylic acid. Plants 2021, 28, 459. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidney functions against lipidemic disease. Elec. J. Environ. Agricult. Food Chem. 2010, 9, 1117–1127. [Google Scholar]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Salama, H.M.H.; Marraiki, N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi J. Biol. Sci. 2010, 17, 57–63. [Google Scholar] [CrossRef]
- Tuney, I.; Cadirci, B.H.; Unal, D.; Sukatar, A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turkish J. Biol. 2006, 30, 171–175. [Google Scholar]
- Kandhasamy, M.; Arunachalam, K.D. Evaluation of in vitro antibacterial property of seaweeds of southeast coast of India. Afr. J. Biotechnol. 2008, 7, 1958–1961. [Google Scholar] [CrossRef]
- Charway, G.N.A.; Yenumula, P.; Kim, Y.-M. Marine algae and their potential application as antimicrobial agents. J. Food Hyg. Saf. 2018, 33, 151–156. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simõs, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid.-Based Complementary Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In Vitro Antimicrobial Effects and Mechanism of Action of Selected Plant Essential Oil Combinations against Four Food-Related Microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Sameeh, M.Y.; Mohamed, A.A.; Elazzazy, A.M. Polyphenolic contents and antimicrobial activity of different extracts of Padina boryana Thivy and Enteromorpha sp. marine algae. J. Appl. Pharm. Sci. 2016, 6, 87–92. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abd El-Mobdy, M.A.; Kamel, M.T.; Mohamed, H.I.; Bayoumi, A.E. Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. Pharmacol. Online 2019, 2, 101–121. [Google Scholar]
- Hussain, E.; Wang, L.J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592. [Google Scholar] [CrossRef]
- Gutierrez-Rodriguez, A.G.; Juarez-Portilla, C.; Olivares-Banuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and in vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; De Almeida, A.A.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.F.; Ji, Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014, 7, 1728–1732. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydras. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Yan, M.D.; Yao, C.J.; Chow, J.M.; Chang, C.L.; Hwang, P.A.; Chuang, S.E.; Whang-Peng, J.; Lai, G.M. Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef]
- Lee, H.E.; Choi, E.S.; Shin, J.; Lee, S.O.; Park, K.S.; Cho, N.P.; Cho, S.D. Fucoidan induces caspase-dependent apoptosis in MC3 human mucoepidermoid carcinoma cells. Exp. Ther. Med. 2014, 7, 228–232. [Google Scholar] [CrossRef]
- Elrggal, M.E.; Alamer, S.I.; Alkahtani, S.A.; Alshrahili, M.A.; Alharbi, A.; Alghamdi, B.A.; Zaitoun, M.F. Dispensing Practices for Weight Management Products in Eastern Saudi Arabia: A Survey of Community Pharmacists. Int. J. Environ. Res. Public Health 2021, 18, 13146. [Google Scholar] [CrossRef]
- Krentz, A.J.; Bailey, C.J. Oral Antidiabetic Agents-Current Role in Type 2 Diabetes Mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Garcimartín, A.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Aqueous extracts and suspensions of restructured pork formulated with Undaria pinnatifida, Himanthaliaelongata and Porphyraumbilicalis distinctly affect the in vitro α-glucosidase activity and glucose diffusion. LWT Food Sci. Technol. 2015, 64, 720–726. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive polyphenols from southern chile seaweed as inhibitors of enzymes for starch digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef]
- Al-Araby, S.Q.; Rahman, M.A.; Chowdhury, M.A.; Das, R.R.; Chowdhury, T.A.; Hasan, C.M.M.; Afroze, M.; Hashem, M.A.; Hajjar, D.; Alelwani, W.; et al. Padina tenuis (marine alga) attenuates oxidative stress and streptozotocin-induced type 2 diabetic indices in Wistar albino rats. S. Afr. J. Bot. 2020, 128, 87–100. [Google Scholar] [CrossRef]
- Abdel-Karim, O.H.; Abo-Shady, A.M.; Ismail, G.A.; Gheda, S.F. Potential effect of Turbinaria decurrens acetone extract on the biochemical and histological parameters of alloxan-induced diabetic rats. Int. J. Environ. Health Res. 2021, 202, 1–22. [Google Scholar] [CrossRef]
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef]
- Muñoz-Ochoa, M.; Murillo-Álvarez, J.I.; Zermeño-Cervantes, L.A.; Martínez-Díaz, S.; Rodríguez-Riosmena, R. Screening of extracts of algae from Baja California Sur, Mexico as reversers of the antibiotic resistance of some pathogenic bacteria. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 739–747. [Google Scholar]
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76. [Google Scholar]
- Panayotova, V.; Merzdhanova, A.; Dobreva, D.A.; Zlatanov, M.; Makedonski, L. Lipids of black sea algae: Unveiling their potential for pharmaceutical and cosmetic applications. J. IMAB Ann. Proc. Sci. Pap. 2017, 23, 1747–1751. [Google Scholar] [CrossRef]
- Kosani´c, M.; Rankovi´c, B.; Stanojkovi´c, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: A study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 2018, 54, 880–890. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharm. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; José, M.; Nathalie, B. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean Coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- SpecialChem—The Universal Selection Source: Cosmetics Ingredients. Available online: https://cosmetics.specialchem.com/ (accessed on 5 May 2020).
- Thomas, N.V.; Kim, S.K. Beneficial e_ects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2018, 31, 1333–1341. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Pezeshk, F.; Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Naseri, M. The effect of supplementing diets with extracts derived from three different species of macroalgae on growth, thermal stress resistance, antioxidant enzyme activities and skin colour of electric yellow cichlid (Labidochromis caeruleus). Aquac. Nutr. 2019, 25, 436–443. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, M.; Dhasarathan, P.; Theriappan, P. Phytochemical characterization and antimicrobial effciency of seaweed samples, Ulva fasciata and Chaetomorpha antennina. Int. J. Pharm. Biol. Sci. 2011, 2, 288–293. [Google Scholar]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- José de Andrade, C.; Maria de Andrade, L. An overview on the application of genus Chlorella in biotechnological processes. J. Adv. Res. Biotechnol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Makpol, S.; Yeoh, T.W.; Ruslam, F.A.C.; Arifin, K.T.; Yusof, Y.A.M. Comparative effect of Piper betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts. BMC Complement. Altern. Med. 2013, 13, 210. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C.H.; Kim, J.R.; Kwon, J.Y.; Seo, S.G.; Han, J.G.; Kim, B.; Kim, J.; Lee, K.W. Chlorella vulgaris attenuates dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int. J. Mol. Sci. 2015, 16, 21021–21034. [Google Scholar] [CrossRef]
- Murthy, K.; Vanitha, A.; Rajesha, J.; Swamy, M.; Sowmya, P.; Ravishankar, G. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Yang, D.J.; Lin, J.T.; Chen, Y.C.; Liu, S.C.; Lu, F.J.; Chang, T.J.; Wang, M.; Lin, H.W.; Chang, Y.Y. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264. 7 cells via NF-B and JNK inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.E.; Pak, K.J.; Kang, J.I.; Kim, T.S.; Lee, S.Y.; Yeo, I.H.; Park, J.H.Y.; Kim, J.H.; Kang, N.J.; et al. A Combination of soybean and Haematococcus extract alleviates ultraviolet B-induced photoaging. Int. J. Mol. Sci. 2017, 18, 682. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Shen, C.T.; Chen, P.Y.; Wu, J.J.; Lee, T.M.; Hsu, S.L.; Chang, C.M.J.; Young, C.C.; Shieh, C.J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculate using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962. [Google Scholar] [CrossRef]
- Wu, H.L.; Fu, X.Y.; Cao, W.Q.; Xiang, W.Z.; Hou, Y.J.; Ma, J.K.; Wang, Y.; Fan, C.D. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J. Agric. Food Chem. 2019, 67, 2212. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.; Incharoensakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 16, 110–118. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Daboor, S.M.; Swelim, M.A.; Mohamed, S. Production and characterization of antimicrobial active substance from Spirulina platensis. Iran. J. Microbiol. 2014, 6, 112–119. [Google Scholar]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Jae-Llane, D.; Carlos Braisv, C. Versatility of the Humble Seaweed in Biomanufacturing. Procedia Manuf. 2019, 32, 87–94. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubble and microbubble. J. Soil Sci. Plant Nutr. 2021, 21, 389–403. [Google Scholar] [CrossRef]
- Eissa, M.A.; Nasralla, N.N.; Gomah, N.H.; Osman, D.M.; El-Derwy, Y.M. Evaluation of natural fertilizer extracted from expired dairy products as a soil amendment. J. Soil Sci. Plant Nutr. 2018, 18, 694–704. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Exploring strategies for seaweed hydrolysis: Effect on methane potential and heavy metal mobilisation. Process Biochem. 2012, 47, 2523–2526. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Abdel Khalik, K.; Osman, G. Genetic analysis of Plectranthus L. (Lamiaceae) in Saudi Arabia based on RAPD and ISSR markers. Pak. J. Bot. 2017, 49, 1073–1084. [Google Scholar]
- Anisimov, M.; Chaikina, E.; Klykov, A.; Rasskazov, V. Effect of seaweeds extracts on the growth of seedling roots of buckwheat (Fagopyrum esculentum Moench) is depended on the season of algae collection. Agric. Sci. Dev. 2013, 2, 67–75. [Google Scholar]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef]
- Myers, S.P.; O’Connor, J.; Fitton, J.H.; Brooks, L.; Rolfe, M.; Connellan, P.; Wohlmuth, H.; Cheras, P.A.; Morris, C. A combined Phase I and II open-label study on the Immunomodulatory effects of seaweed extract nutrient complex. Biol. Targets Ther. 2011, 5, 45–60. [Google Scholar] [CrossRef]
- Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A.; Deters, A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107. [Google Scholar] [CrossRef]
- EMA. Community Herbal Monograph on Fucus vesiculosus L., Thallus; EMA: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourão, P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007, 342, 2326–2330. [Google Scholar] [CrossRef]
- Drozd, N.N.; Tolstenkov, A.S.; Makarov, V.A.; Kuznetsova, T.A.; Besednova, N.N.; Shevchenko, N.M.; Zvyagintseva, T.N. Pharmacodynamic parameters of anticoagulants based on sulfated polysaccharides from marine algae. Bull. Exp. Biol. Med. 2006, 142, 591–593. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alsaqufi, A.S.; Omar, E.A.; El-Beltagi, H.S.; Srour, T.M.; Yousef, M.I. Ginseng, Tribulus extracts and pollen grains supplementation improves sexual state, testes redox status, and testicular histology in Nile Tilapia Males. Antioxidants 2022, 11, 875. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Celikler, S.; Tas, S.; Vatan, O.; Ziyanok-Ayvalik, S.; Yildiz, G.; Bilaloglu, R. Anti-hyperglycemic and antigenotoxic potential of Ulva rigida ethanolic extract in the experimental diabetes mellitus. Food Chem. Toxicol. 2009, 47, 1837–1840. [Google Scholar] [CrossRef]
- Kang, J.Y.; Khan, M.N.A.; Park, N.H.; Cho, J.Y.; Lee, M.C.; Fujii, H.; Hong, Y.K. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharmacol. 2008, 116, 187–190. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J. Preparation, structural characterization and in vitro antitumor activity of kappa-carrageenan oligosaccharide fraction from Kappaphycus striatum. J. Appl. Phycol. 2005, 17, 7–13. [Google Scholar] [CrossRef]
- Tannoury, M.Y.; Saab, A.M.; Elia, J.M.; Harb, N.N.; Makhlouf, H.Y.; Diab-Assaf, M. In vitro cytotoxic activity of Laurencia papillosa, marine red algae from the Lebanese coast. J. Appl. Pharm. Sci. 2017, 7, 175–179. [Google Scholar] [CrossRef]
- Patra, S.; Muthuraman, M.S. Gracilaria edulis extract induces apoptosis and inhibits tumor in Ehrlich Ascites tumor cells in vivo. BMC Complement. Altern. Med. 2013, 13, 331. [Google Scholar] [CrossRef]
- Alarif, W.M.; Al-Lihaibi, S.S.; Ayyad, S.E.N.; Abdel-Rhman, M.H.; Badria, F.A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtusa as potential antitumor-antimicrobial agents. Eur. J. Med. Chem. 2012, 55, 462–466. [Google Scholar] [CrossRef]
- Celikler, S.; Yildiz, G.; Vatan, O.; Bilaloglu, R. In vitro antigenotoxicity of Ulva rigida C. Agardh (Chlorophyceae) extract against induction of chromosome aberration, sister chromatid exchange and micronuclei by mutagenic agent MMC. Biomed. Environ. Sci. 2008, 21, 492–498. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid_production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Myung, C.S.; Shin, H.C.; Hai, Y.B.; Soo, J.Y.; Bong, H.L.; Jong, S.K. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Mei, C.H.; Zhou, S.C.; Zhu, L.; Ming, J.X.; Zeng, F.D.; Xu, R. Antitumor effects of laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef]
- Atya, M.E.; El-Hawiet, A.; Alyeldeen, M.A.; Ghareeb, D.A.; Abdel-Daim, M.M.; El-Sadek, M.M. In vitro biological activities and in vivo hepatoprotective role of brown algae-isolated fucoidans. Environ. Sci. Pollut. Res. 2021, 28, 19664–19676. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; White, W.; Lu, J. Extracts from New Zealand Undaria pinnatifida containing fucoxanthin as potential functional biomaterials against cancer in vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Cao, M.J.; Liu, G.M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the barents sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef]
- Zhu, W.; Chiu, L.C.M.; Ooi, V.E.C.; Chan, P.K.S.; Ang, P.O. Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against Herpes simplex virus type 2. Int. J. Antimicrob. Agents 2004, 24, 279–283. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Histopathological studies on liver, kidney and heart of normal and dietary induced hyperlipidaemic rats fed with tropical red seaweed Gracilaria changii. J. Funct. Foods 2015, 17, 202–213. [Google Scholar] [CrossRef]
- Kim, M.M.; Kim, S.K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulfated Rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant Activity of Sulfated Ulvan Isolated from the Green Macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- De Zoysa, M.; Nikapitiya, C.; Jeon, Y.J.; Jee, Y.; Lee, J. Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J. Appl. Phycol. 2008, 20, 67–74. [Google Scholar] [CrossRef]
- Gwon, W.G.; Lee, M.S.; Kim, J.S.; Kim, J.I.; Lim, C.W.; Kim, N.G.; Kim, H.R. Hexane fraction from Sargassum fulvellum inhibits lipopolysaccharide- induced inducible nitric oxide synthase expression in RAW 264.7 cells via NF-_B pathways. Am. J. Chin. Med. 2013, 41, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- de Almeida, C.L.F.; Falcão, D.S.H.; Lima, D.M.G.R.; Montenegro, D.A.C.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza, M.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.C.D. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia fish fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef]
- Gheda, S.F.; El-Adawi, H.I.; El-Deeb, N.M. Antiviral Profile of Brown and Red Seaweed Polysaccharides against Hepatitis C Virus. Iran. J. Pharm. Res. IJPR 2016, 15, 483–491. [Google Scholar]
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Braz. J. Pharmacogn. 2012, 22, 714–723. [Google Scholar] [CrossRef]
- Lakshmi, V.; Goel, A.K.; Srivastava, M.N.; Raghubir, R. Bioactivity of marine organisms: Part X-Screening of some marine fauna from the Indian coasts. Indian J. Exp. Biol. 2006, 44, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.D.S.; Soares, A.R.; Martins, F.O.; De Albuquerque, M.C.M.; Costa, S.S.; Yoneshigue-Valentin, Y.; Gestinari, L.M.D.S.; Santos, N.; Romanos, M.T.V. Antiviral activity of the green marine alga Ulva fasciata on the replication of human metapneumovirus. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective effect of Anethole against Renal Ischemia/Reperfusion: The role of the TLR2,4/MYD88/NF_B pathway. Antioxidants 2022, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Gellenbeck, K.W. Utilization of algal materials for nutraceutical and cosmeceutical applications—What do manufacturers need to know? J. Appl. Phycol. 2012, 24, 309–313. [Google Scholar] [CrossRef]
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory effect of papaya extract against chlorpyrifos-induced oxidative stress, immune suppression, endocrine disruption, and dna damage in female Clarias gariepinus. Int. J. Environ. Res. Public Health 2022, 19, 4640. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried brown seaweed’s phytoremediation potential for methylene blue dye removal from aquatic environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abou El-Enain, M.M. Role of secondary metabolites from seaweeds in the context of plant development and crop production. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; Pereira, L., Bahcevandziev, K., Joshi, N.H., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 64–79. [Google Scholar]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresen. Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- El-desoky, A.H.; Abdel-Rahman, A.H.; Rehab, F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Riani Mansauda, K.L.; Anwar, E.; Nurhayati, T. Antioxidant and anti-collagenase activity of Sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacogn. Mag. 2018, 10, 932–936. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- Unnikrishnan, P.S.; Jayasri, M.A. Marine algae as a prospective source for antidiabetic compounds—A Brief Review. Curr. Diabetes Rev. 2018, 14, 237–245. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. In Vitro Cell. Dev. Biol. Plant. 2022. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.-G.; Yang, H.-W.; Jeon, Y.-J.; Lee, S. Dieckol, an algae-derived phenolic compound, suppresses UVB-induced skin damage in human dermal fibroblasts and its underlying mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Mahendra, C.K.; Goh, B.-H.; Gew, L.T.; Yow, Y.-Y. UVB radiation protective effect of brown Alga Padina australis: A potential cosmeceutical application of Malaysian Seaweed. Cosmetics 2021, 8, 58. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Evaluation of fractions extracted from Polycladia myrica: Biological activities, UVR protective effect, and stability of cream formulation based on it. J. Appl. Phycol. 2022. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef]



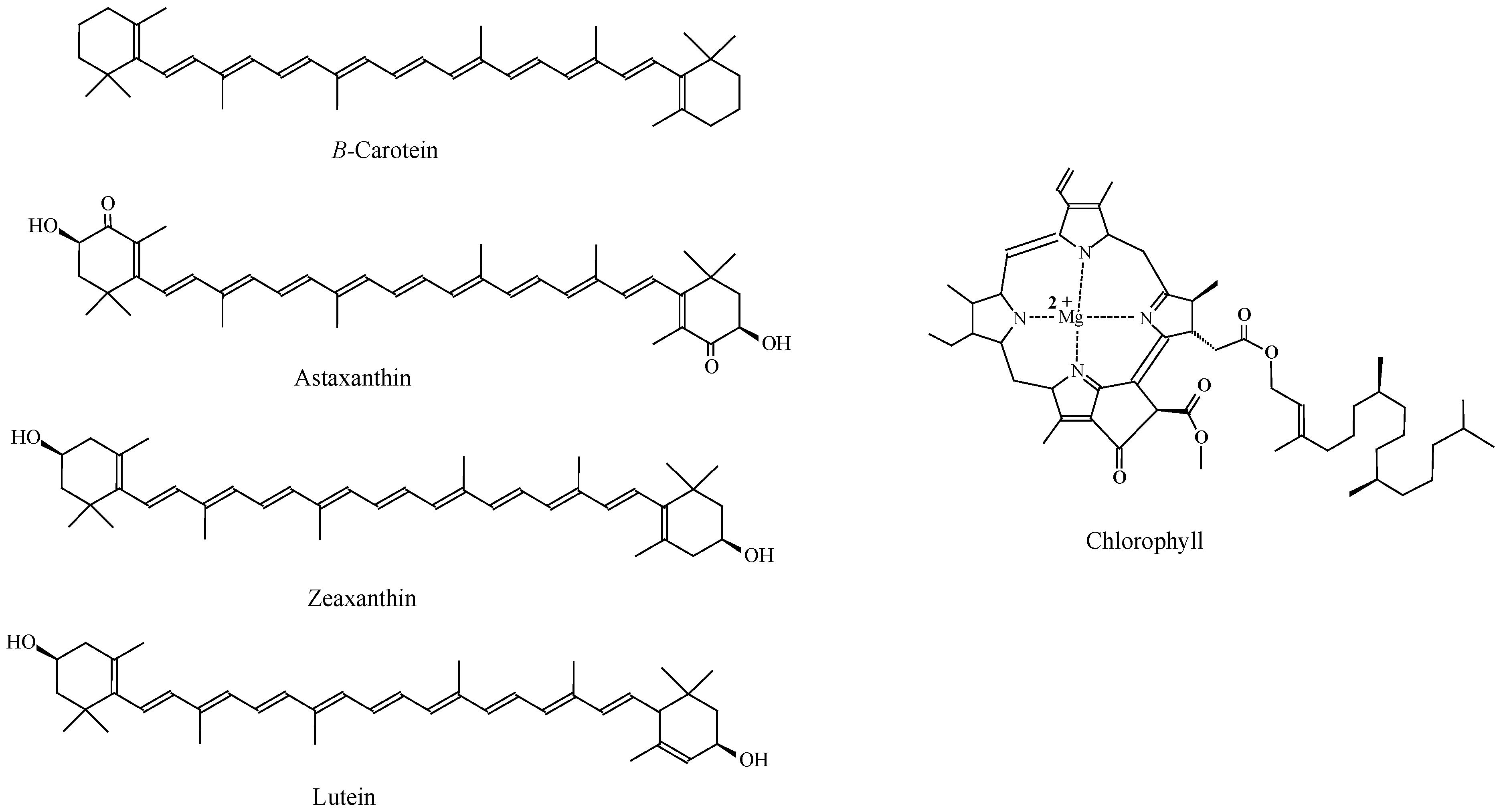





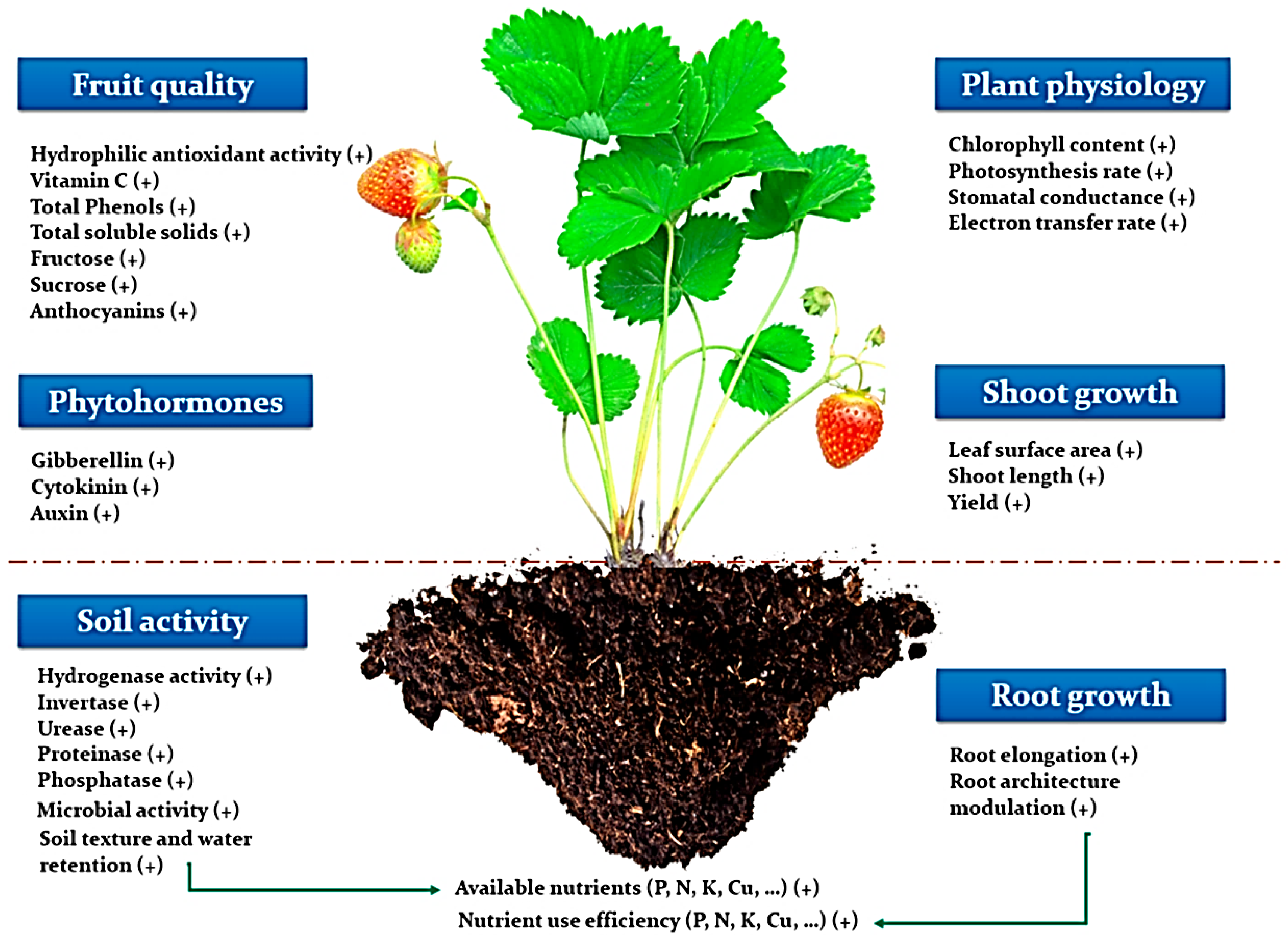

| Component | Species | Molecular Weight | Chemical Composition | Doses | Properties/Activities | References |
|---|---|---|---|---|---|---|
| Carrageenan | Tribonema minus | 197 kDa | Heteropolysaccharide composed mainly of galactose | 250 μg mL−1 | Anticancer activity | [33] |
| Porphyran | Chondrus armatus | f 9.7–34.6 kDa | Mainly composed of 3,6-anhydro-L-galactose | 327.3 μg mL−1 | Anticancer activity | [34] |
| Fucoidans | Cladosiphon okamuranus | 75.0 kDa | 5.01 mg mL−1 of l-fucose, 2.02 mg mL−1 of uronic acids and 1.65 ppm of sulfate | 1 mg mL−1 | Anticancer activities | [35] |
| Fucus vesiculosus | - | Fucose and Xylose | - | Antioxidant activity | [36] | |
| Agar | Gelidium amansii | 1.21 × 104 Da and 1.85 × 105 Da | (1–4)-linked 3,6-anhydro-α-L-galactose alternating with (1–3)-linked β-D-galactopyranose | 25.6 mg L−1 | Antioxidant activity | [37] |
| Laminaran | Laminaria digitata | - | β-(1,3)-glucan | 10 µg mL−1 | Antioxidant protection | [38] |
| Ulvan | Ulva pertusa | 83.1 from 143.2 kDa | Rhamnose, and xylose | 500 mg kg−1 | Antioxidant and antihyperlipidemic activity | [39] |
| 143.47 kDa | Rhamnose and xylose | 1.5 mg mL−1 | Antioxidant Activity | [40] |
| Component | Species | Models | Doses | MW | Activity | Results | References |
|---|---|---|---|---|---|---|---|
| Carrageenan | Padina tetrastromatic | Paw edema in rats | 20 mg kg−1 | 25 kDa | Anti-inflammation | COX-2 and iNOS inhibitions | [49] |
| Fucoidan | Fucus vesiculosus | Human malignant melanoma cells | 100–400 µg mL−1 | 60 kDa | Anticancer activity | Inhibit cell proliferation | [50] |
| B16 murine melanoma cells | 550 µg mL−1 | - | Anti-melanogenic | Inhibit tyrosinase and melanin | [51] | ||
| Ulvans | Ulva sp. | Human dermal fibroblast | 100 and 500 µg mL−1 | 4–57 kDa | Anti-aging | Increase hyaluronan production | [52] |
| Laminaran | Laminaria japonica | In vitro | 15 mg mL−1 | 250 kDa | antioxidant activity | ROS scavenging potential | [53] |
| Fucoidan | Chnoospora minima | RAW macrophages | 27.82 µg mL−1 | 60 kDa | Anti-inflammation | Inhibition of LPS-induced NO production, iNOS, COX-2, and PGE2 levels | [54] |
| Fucoidan | Sargassum hemiphyllum | RAW 264.7 macrophage cells | dose-dependent manner | - | Anti-inflammation | Inhibit LPS-induced inflammatory response | [55] |
| Fucoidan | Sargassum hemiphyllum | B16 melanoma cells | dose-dependent manner | - | Anticancer | Activation of caspase-3 | [56] |
| Seaweed | Species | Name of the Protein | Protein Yield % | References |
|---|---|---|---|---|
| Ulva sp. | Green algae | Glycoproteins (GP) | “UvGP-1” (0.54) “UvGP-2 DA”(0.52) “UvGP-2-DS”(1.98) | [81] |
| Ulva lactuca | Green algae | GP fraction G | ND | [82] |
| Saccharina japonica | Brown algae | Glycoprotein | 0.27 | [83] |
| Solieria filiformis | Red algae | Lectins “SfL-1” “SfL-2” | ND | [84] |
| Solieria filiformis | Red algae | Lectin “SfL” | ND | [85] |
| Capsosiphon fulvescens | Green algae | “Cf-hGP” | ND | [86] |
| Undaria pinnatifida | Brown algae | “UPGP” | ND | [87] |
| No. | Amino Acids (AA) | Caulerpa lentillifera (Green Algae) [88] | Ulva reticulate (Green Algae) [88] | Kappaphycus alvarezii (Red Algae) [89] | Gracilaria salicornia (Red Algae) [89] | Turbinaria ornata (Brown Algae) [90] | Durvillaea antarctica (Brown Algae) [91] |
|---|---|---|---|---|---|---|---|
| Essential AA | |||||||
| 1 | Threonine | 6.38 | 5.41 | 2.49 | 2.25 | 0.15 | 5.84 |
| 2 | Valine | 7.03 | 6.30 | 2.49 | 2.20 | 0.23 | 9.97 |
| 3 | Lysine | 6.63 | 6.02 | 1.51 | - | 0.20 | 4.22 |
| 4 | Isoleucine | 5.01 | 4.23 | 2.14 | 1.98 | 0.18 | 8.05 |
| 5 | Leucine | 8.00 | 7.90 | 2.34 | 2.16 | 0.26 | 15.88 |
| 6 | Phenylalanine | 4.93 | 5.26 | 2.11 | 1.79 | 0.19 | 9.97 |
| 7 | Methionine | - | - | 1.69 | 1.61 | 0.05 | 3.89 |
| Non essential AA | |||||||
| 8 | Aspartic | 11.56 | 12.50 | 3.33 | - | 0.53 | 4.17 |
| 9 | Serine | 6.14 | 6.39 | 2.68 | 2.90 | 0.10 | 5.38 |
| 10 | Glutamic | 14.39 | 12.98 | 11.67 | 2.79 | 0.58 | 17.87 |
| 11 | Glycine | 6.87 | 6.49 | 2.97 | 2.18 | 0.22 | 18.36 |
| 12 | Arginine | 7.03 | 8.65 | 2.40 | 2.40 | 0.19 | 4.83 |
| 13 | Histidine | 0.65 | 1.08 | 1.60 | 2.29 | 0.07 | 2.26 |
| 14 | Alanine | 6.87 | 8.09 | 2.93 | 2.51 | 0.23 | 9.57 |
| 15 | Tyrosine | 3.88 | 3.62 | 1.81 | 1.74 | 0.05 | 4.45 |
| 16 | Proline | 4.61 | 5.08 | - | - | 0.17 | 7.95 |
| 17 | Cystin | - | - | - | - | 0.00 | 0.78 |
| Component | Properties/Activities | Seaweed | Doses | Molecular Weight | References |
|---|---|---|---|---|---|
| Peptide PPY1 | Anti-aging | Pyropia yezoensis | 250–1000 ng mL−1 | 532 Da | [100] |
| Peptides PYP1-5 and porphyra 334 | Boost synthesis of elastin | Porphyra yezoensis f. coreana Ueda | 0–200 μM | 1622 kDa | [101] |
| Lactate and progerin | Reduce synthesis, anti-elastase, anti-collagenase | Alaria esculenta | - | 112 KDa | [102] |
| Phycobiliproteins | Antioxidant | Gracilaria gracilis | 0.5–30 mg mL−1 | 240 KDa | [103] |
| Deoxygadusol, palythene and usujirene | Antioxidant | Rhodymenia pseudopalmata | - | - | [104] |
| Palythine, palythinol, porphyra-334, asterina-330, shinorine, or usujirene | Antioxidant, antiproliferative | Palmaria palmate, Mastocarpus stellatus, Chondrus crispus | 2.0–4.0 mg mL−1 | 244.24 KDa | [105] |
| Porphyra-334, shinorine, palythine and asterina-330 | Antioxidant; UV-protective effect | Gracilaria vermiculophylla | - | 346.33 KDa | [106] |
| Seaweed | Species | Lipids g/100 g | EPA (%) | DHA (%) | References |
|---|---|---|---|---|---|
| Caulerpa lentillifera | Green algae | 1.11 ± 0.05 | 0.86 | - | [127] |
| Codium fragile | Green algae | 1.5 ± 0.0 | 2.10 ± 0.00 | - | [128] |
| Ulva lactuca | Green algae | 1.27 ± 0.11 | 0.87 ± 0.16 | 0.8 ± 0.01 | [129] |
| Agarophyton chilense | Red algae | 1.3 ± 0.0 | 1.3 ± 0.01 | - | [128] |
| Porphyra/Pyropia spp. (China) | Red algae | 1.0 ± 0.2 | 10.4 ± 7.46 | - | [128] |
| Ascophyllum nodosum | Brown algae | 3.62 ± 0.17 | 7.24 ± 0.08 | - | [130] |
| Bifurcaria bifurcata | Brown algae | 6.54 ± 0.27 | 4.09 ± 0.08 | 11.10 ± 1.13 | [130] |
| Durvillaea antarctica | Brown algae | 0.8 ± 0.1 | 4.95 ± 0.11 | 1.66 ± 0.02 | [129] |
| Fucus vesiculosus | Brown algae | 3.75 ± 0.20 | 9.94 ± 0.14 | - | [130] |
| Himanthalia elongata | Brown algae | <1.5 | 7.45 | - | [131] |
| Laminaria spp. | Brown algae | 1.0 ± 0.3 | 16.2 ± 8.9 | - | [132] |
| Macrocystis pyrifera | Brown algae | 0.7 ± 0.1 | 0.47 ± 0.01 | - | [128] |
| Sargassum fusiforme | Brown algae | 1.4 ± 0.1 | 42.4 ± 11.9 | - | [132] |
| Undaria pinnatifida | Brown algae | 4.5 ± 0.7 | 413.2 ± 0.66 | - | [132] |
| Component | Molecular Mass | Properties/Activities | Seaweed | References |
|---|---|---|---|---|
| E-9-oxooctadec-10-enoic acid E-10-oxooctadec-8-enoic acid | 282.46 g mol−1 | Anti-inflammatory | Gracilaria verrucosa | [156] |
| Essential oil (tetradeconoic acid, hexadecanoic acid, (9Z)-hexadec-9-enoic acid) (9Z,12Z)-9,12-octadecadienoic acid | 280.447 g mol−1 | Antioxidant: radical scavenging Antibacterial activity upon Staphylococcus aureus and Bacillus cereus | Laminaria japonica | [157] |
| Fucosterol | 412.69 g mol−1 | Antioxidant: increased antioxidative enzymes (glutathione peroxidase, superoxide dismutase, catalase) | Pelvetia siliquosa | [158] |
| Fucosterol | 412.69 g mol−1 | Anti-inflammatory, Ati-photodamage: decreased UVB-induced MMPs | Hizikia fusiformis | [159] |
| Palmitic acid | 256.430 g mol−1 | Enzyme inhibition, Antioxidant | Ulva rigida, Gracilaria sp., Saccharina latissima, Fucus vesiculosus | [160] |
| Omega 3 fatty acids | 909.4 g mol−1 | Antioxidant | Brown algae | [161] |
| Arachidonic acid (ARA) | - | Improves growth and development of neonates | P. purpureum, P. cruentum | [162] |
| Eicosapentaenoic acid (EPA) | 500 mg/day | Cognition, heart health, protection against arthrosclerosis, anti-inflammatory | Nannochloropsis, P. tricornutum, P. cruentum | [163,164] |
| Docosahexaenoic acid (DHA) | 500 mg/day | Brain and eye health, cardiovascular benefits, nervous system development | C. cohnii, Schizochytrium sp., Ulkenia sp. | [162,163,164] |
| Fucosterol | 1 and 10 μg mL−1 | Anti-aging Inhibit MMP expression | Hizikia fusiformis | [165] |
| Polyunsaturated fatty acid | 10.3 mg mL−1 | Anti-inflammation | Undaria pinnatifida | [166] |
| Carotenoid | Seaweed Source | Effect | Model | Bioactive Concentration | Target | Reference |
|---|---|---|---|---|---|---|
| Astaxanthin | Hematococcus pluvialis | Antioxidant | Human monocytes (U-937) | 10 μM | SHP-1 | [168] |
| Mice brain | 2 mg/kg/day | MDA, NO, APOP, GSH. | [169] | |||
| Leydig cells | 10 μg/mL | StAR | [170] | |||
| Antiproliferative | human prostatic adenocarcinoma (LNCaP) | 10 μM | prostate specific antigen (PSA) | [171] | ||
| immune system stimulation | transplantable methylcholanthrene-induced fibrosarcoma (Meth-A tumor) | 40 mg/kg/day | interferon-g (IFN-γ) | [172] | ||
| anti-obesity | Humans | 0, 6, 12 and 18 mg/day | adiponectin | [173] | ||
| Cardiovascular protective | spontaneously hypertensive rats (SHR) | 50 mg/kg | blood pressure (BP) | [174] | ||
| Fucoxanthin | Sargassum horneri | antioxidant and protective | Vero cells | 5, 50, 100 and 200 µM (50 µM H2O2) | DNA | [175] |
| UV protection | Human fibroblasts | 5, 50 and 100 µM (50 mJ/cm2 UV-B) | DNA | [176] | ||
| Antioxidant | Retinol deficiency rats | 0.83 µM | CAT, GST and Na+K+ATPase activity | [177] | ||
| Antiproliferative | leukemia cells (HD-60) | 11.3 and 45.2 μM | DNA fragmentation | [178] | ||
| colorectal adenocarcinoma cells (Caco-2) | 15.2 μM | DNA fragmentation | [178] | |||
| colorectal adenocarcinoma cells (DLD-1) | 15.2 μM | DNA fragmentation | [178] | |||
| colorectal adenocarcinoma cells (CHT-29) | 15.2 μM | DNA fragmentation | [178] | |||
| human colorectal carcinoma (HCT116) | 5 and 10 μM | Bcl-xL, PARP and caspase 3 and 7 | [179] | |||
| Antiproliferative | human urinary bladder cancer cells (EJ-1) | 20 μM | [180] | |||
| anti-obesity | Rats | 2 mg | absorption of triglycerides, pancreatic lipase | [181] | ||
| Fucoxanthinol | Corbicula fluminea | Antiproliferative | human prostate cancer (PC-3) | 2.0 μM | Bcl-xL, PARP and caspase 3 and 7 | [179] |
| anti-obesity | Rats | 2 mg | absorption of triglycerides, pancreatic lipase | [181] | ||
| Halocynthiaxanthin | Mastocarpus stellatus | Antiproliferative | human neuroblastoma cells (GOTO) | 5 μg/mL | [182] | |
| β-carotene | Kappaphycus alvarezii | Antioxidant | Smokers | 20 mg | Breath pentane | [183] |
| Cure of erythema | Humans | 30 to 90 mg/day | [184] | |||
| Antiproliferative | murine osteosarcoma (LM8) | 30 µM | [185] | |||
| Antiinfiammatory | human umbilical vein endothelial cells (HUVECs) | 0.02 µmol/L | VCAM-1, ICAM-1 and E-Selectin | [186] | ||
| Lutein | Zostera noltii | ADM prevention | Human Dermal Lymphatic Endothelial Cells (HLEC) | 5 µM | DNA, lipid and protein level | [187] |
| Cardiovascular protective | Human monocytes | 0.1, 1, 10 and 100 nM | LDL associated with artery wall | [182] | ||
| Zeaxanthin | Pyropia yezoensis | ADM prevention | Human Dermal Lymphatic Endothelial Cells (HLEC) | 5 µM | DNA, lipid and protein level | [187] |
| Algae Species | Bioactive Compound/Extract | Beneficial Activity | Mechanism of Action | Experimental Model | Reference |
|---|---|---|---|---|---|
| Brown algae | |||||
| Ascophyllum nodosum | Ascophyllan | Anticancer | Inhibit MMP expression | B16 melanoma cells | [268] |
| Bifurcaria bifurcata | Eleganonal | Antioxidant | DPPH inhibition | In vitro | [269] |
| Chnoospora implexa | Ethanol extract | Antimicrobial | Bacterial growth inhibition | Staphylococcus aureus, Staphylococcus pyogenes | [270] |
| Chnoospora minima | Fucoidan | Anti-inflammation | Inhibition of LPS-induced NO production, iNOS, COX-2, and PGE2 levels | RAW macrophages | [53] |
| Cladosiphon okamuranus | Fucoxanthin | Antioxidant | DPPH inhibition | In vitro | [271] |
| Colpomenia sinuosa | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Cystoseira barbata | Fat-soluble vitamin and carotenoids | Antioxidant | High fat-soluble vitamin and carotenoid content | In vitro | [272] |
| Dictyopteris delicatula | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Dictyota dichotoma | Algae extract | Antimicrobial | Inhibit the synthesis of the peptidoglycan layer of bacterial cell walls | Penicillium purpurescens, Candida albicans, Aspergillus flavus | [273] |
| Eisenia arborea | Phlorotannin | Anti-inflammation | Inhibit release of histamine | Rat basophile leukemia cells (RBL-2HE) | [274] |
| Fucus evanescens | Fucoidan | Anticancer | Inhibit cell proliferation | Human malignant melanoma cells | [50] |
| Halopteris scoparia | Ethanol extract | Anti-inflammation | COX-2 inhibition | COX inhibitory screening assay kit | [275] |
| Laminaria japonica | Fucoxanthin | Anti-melanogenic | Suppress tyrosinase activity | UVB-irradiated guinea pig | [276] |
| Padina concrescens | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Saccharina latissima | Phenol | Antioxidant | High total phenolic content, DPPH scavenging activity and FRAP | In vitro | [277] |
| Red algae | |||||
| Alsidium corallinum | Methanol extract | Antimicrobial | Bacterial growth inhibition | Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus | [278] |
| Ceramium rubrum | Methanol extract | Antimicrobial | Bacterial growth inhibition | Escherichia coli, Enterococcus faecalis, Staphylococcus aureus | [278] |
| Ganonema farinosum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Gelidium robustum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Jania rubens | Glycosaminoglycan | Anti-aging | Collagen synthesis | Unknown | [279] |
| Laurencia luzonensis | Sesquiterpenes | Antimicrobial | Bacterial growth inhibition | Bacillus megaterium | [280] |
| Palisada flagellifera | Methanol extract | Antioxidant | β-carotene bleaching activity | In vitro | [281] |
| Porphyra haitanensis | Sulfated Polysaccharide | Antioxidant | ROS scavenging potential | Mice | [282] |
| Schizymenia dubyi | Phenol | Anti-melanogenic | Inhibit tyrosinase activity | In vitro | [283] |
| Green algae | |||||
| Bryopsis plumose | Polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [54] |
| Cladophora sp. | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [270] |
| Entromorpha intestinalis | Chloroform and methanol extract | Antioxidant | SOD activity is reduced | Labidochromis caeruleus | [284] |
| Gayralia oxysperma | Fucoxanthin | Antioxidant | High FRAP value (>6 μM/μg of extract) | In vitro | [285] |
| Ulva dactilifera | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, Streptococcus pyogenes | [270] |
| Ulva fasciata | Fucoxanthin | Antioxidant | DPPH inhibition (83.95%) | In vitro | [286] |
| Ulva pertusa | Polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [54] |
| Microalgae/Cyanobacteria | |||||
| Anabaena vaginicola | Lycopene | Antioxidant Anti-aging | N/A | In vitro | [287] |
| Arthrospira platensis | Methanol extracts of exopolysaccharides | Antioxidant | N/A | In vitro | [287] |
| Chlorella fusca | Sporopollenin | Anti-aging | Protect cells from UV radiation | N/A | [288] |
| Chlorella minutissima | MAA | Anti-aging | Protect cells from UV radiation | N/A | [288] |
| Chlorella sorokiniana | MAA | Anti-aging | Protect cells from UV radiation | N/A | [288] |
| Lutein | Anti-aging | Reduce UV induced damage | N/A | [289] | |
| Chlorella vulgaris | Hot water extract | Anti-aging | Reduced activity of SOD | Human diploid fibroblast | [290] |
| Anti-inflammation | Down-regulated mRNA expression levels of IL-4 and IFN-γ | NC/Nga mice | [291] | ||
| Dunaliella salina | β-carotene | Antioxidant | Protect against oxidative stress | Rat | [292] |
| β-cryptoxanthin | Anti-inflammation | Reduced the production of IL-1β, IL-6, TNF-ɑ, the protein expression of iNOS and COX-2 | LPS-stimulated RAW 264.7 cells | [293] | |
| Haematococcus pluvialis | Astaxanthin (carotenoid) | Anti-aging | Inhibit MMP expression | Mice and human dermal fibroblasts | [294] |
| Anticancer | ROS scavenging potential | Mice | [295] | ||
| Nannochloropsis granulata | Carotenoid | Antioxidant | DPPH inhibition | In vitro | [296] |
| Nannochloropsis oculata | Zeaxanthin | Anti-melanogenic | Inhibit tyrosinase | In vitro | [297] |
| Nitzschia sp. | Fucoxanthin | Antioxidant | Reduced oxidative stress | Human Glioma Cells | [298] |
| Nostoc sp. | MAA | Antioxidant | ROS scavenging potential | In vitro | [299] |
| Odontella aurita | EPA | Antioxidant | Reduce oxidative stress | Rat | [300] |
| Planktochlorella nurekis | Fatty acid | Antimicrobial | Bacterial growth inhibition | Campylobacter jejuni, E. coli, Salmonella enterica var. | [301] |
| Porphyridium sp. | Sulfated polysaccharide | Anti-inflammation Antioxidant | Inhibit proinflammatory modulator Inhibited oxidative damage | Unknown 3T3 cells | [282] |
| Rhodella reticulata | Sulfated polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [282] |
| Skeletonema marinoi | Polyunsaturated aldehyde and fatty acid | Anticancer | Inhibit cell proliferation | Human melanoma cells (A2058) | [302] |
| Spirulina platensis | β-carotene and phycocyanin | Antioxidant Anti-inflammation | Inhibit lipid peroxidation Inhibit TNF-ɑ and IL-6 expressions | Mouse Human dermal fibroblast cells (CCD-986sk) | [303] |
| Ethanol extract | Antimicrobial | Bacterial growth inhibition | E. coli, Pseudomonas aeruginosa, Bacillus subtilis, and Aspergillus niger | [304] | |
| Synechocystis spp. | Fatty acids and phenols | Antimicrobial | Bacterial growth inhibition | E. coli, S. aureus | [305] |
| Seaweed | Compound Extracted | Cell Lines/Animals Surveyed | Route of Administration | Dosage (μg/mL) | Effect | Reference |
|---|---|---|---|---|---|---|
| Laminaria cichorioides (Phaeophyceae) | Sulfated fucan | Human plasma | The lyophilized crude polysaccharide was dissolved in human plasma | 10, 30, 50 | In vitro anticoagulant activity | [320] |
| Fucus evanescens (Phaeophyceae) | Fucoidans | Human plasma Rat plasma | Intravenous Injection | 125, 250, 500, 1000 | In vitro and in vivo anticoagulant activity | [321] |
| Gracilaria edulis (Rhodophyceae) | Phenolic, Flavonoid and Alkaloid compounds | Bovine serum albumin (protein) | The extracts were tested on the protein | 20, 40, 60, 80, 100, 120 | Hypoglycemic activity | [322] |
| Sargassum fulvellum (Phaeophyceae) | Phlorotannins, grasshopper ketone, fucoidan and polysaccharides | Mice | Oral administration | Based on weight of mice | Antioxidant, anticancer, antiinflammatory, antibacterial, and anticoagulant activities | [323] |
| Griffithsia sp. (Rhodophyceae) | Griffithsin (protein) | MERS-CoV and SARS-CoV glycoproteins | The extracts were tested on the proteins | 0.125, 0.25, 0.5, 1, 2 | Antiviral activity against MERS-CoV virus and SARS-CoV glycoprotei | [324] |
| Ulva rigida (Chlorophyceae) | Ethanolic extract | Twenty-four male Wistar rats | Oral administration | 500 mL of water with extracts in 2% wt/vol as drinking water for exposed groups per each day (from 3 to 30 days). | In vivo antihyperglycaemic, antioxidative and genotoxic/ antigenotoxic activities | [325] |
| Saccharina japonica (Phaeophyceae) | polysaccharides | SARS-CoV-2 S-protein | The extracts were tested on the proteins | 50–500 | In vitro inhibition to SARS-CoV-2 | [326] |
| Component | Properties/Activities | Seaweed | Doses | Models | References |
|---|---|---|---|---|---|
| Fucoxanthins | Antitumoral activity on lung cancer cells | Laminaria japonica | 12.5–100 μM | Female and male (1:1 ratio) BALB/c nude mice (18–20 g; 6–8 weeks of age) | [341] |
| Antitumoral activity on MCF-7, HepG-2, HCT-116 cells | Colpomenia sinuosa, Sargassum prismaticum | 100 and 200 mg/kg | Paracetamol-administered rats (one dose of 1 g/kg) | [342] | |
| Antitumoral activity on SiHa, Malme-3M cells | Undaria pinnatifida | 1.5625, 6.25, 12.5, 25, 50, 80, 100 µM | Human cell lines | [343] | |
| Antimicrobial activity | Cladosiphon okamuranus | 2–2000 µg/mL. | Helicobacter pylori | [344] | |
| Antimicrobial activity | Laminaria japonica | 2, 3, 4, 5, 6, 7, and 7.5 mg/mL | Staphylococcus aureus, Escherichia coli | [345] | |
| Antimicrobial activity | Fucus vesiculosus | 2, 4, 6, 8 and 10 mg/mL | Staphylococcus aureus, Bacillus licheniformis, Escherichia coli, Staphylococcus epidermidis | [346] | |
| Antiviral activity against ECHO-1, HIV-1, HSV-1, HSV-2 | Fucus evanescens | 200 μg/mL | Female outbred mice (16–20 g) | [347] | |
| Sulfate polysaccharide | Antiviral activity against HSV-1, HVS-2 | Sargassum patens | 0.78–12.5 μg/mL | Vero cells (African green monkey kidney cell line) | [348] |
| Anti-obesity, antidiabetic activities | Gracilaria lemaneiformis | 5–10% Seaweed powder | Dawley laboratory rats (4 to 5 months old, 250–300 g) | [349] | |
| Phloroglucinol | Anti-inflammatory activity | Ecklonia cava | 1, 5, 10, 50 100 µM | HT1080 and RAW264.7 cells | [350] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. https://doi.org/10.3390/md20060342
El-Beltagi HS, Mohamed AA, Mohamed HI, Ramadan KMA, Barqawi AA, Mansour AT. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Marine Drugs. 2022; 20(6):342. https://doi.org/10.3390/md20060342
Chicago/Turabian StyleEl-Beltagi, Hossam S., Amal A. Mohamed, Heba I. Mohamed, Khaled M. A. Ramadan, Aminah A. Barqawi, and Abdallah Tageldein Mansour. 2022. "Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review" Marine Drugs 20, no. 6: 342. https://doi.org/10.3390/md20060342
APA StyleEl-Beltagi, H. S., Mohamed, A. A., Mohamed, H. I., Ramadan, K. M. A., Barqawi, A. A., & Mansour, A. T. (2022). Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Marine Drugs, 20(6), 342. https://doi.org/10.3390/md20060342