1-O-Alkylglycerol Ethers from the Marine Sponge Guitarra abbotti and Their Cytotoxic Activity
Abstract
:1. Introduction
2. Results
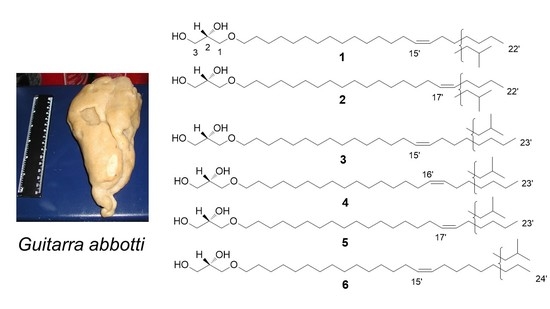
2.1. Composition of the Isolated AGE Fraction
2.2. Anticancer Effects of the Isolated AGE Fraction
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Reagents
4.3. Animal Material
4.4. Extraction and Isolation
4.5. Characterization of the Purified 1-O-Alkylglycerol Ether (AGE) Mixture
4.6. Preparation of Trimethylsilyl Derivatives (TMS-d) and Their GC/MS Analysis
4.7. Preparation of Acetate Derivatives
4.8. Preparation of Dimethyldisulfide Acetate Derivatives (DMDS-d) of AGEs and Their GC/MS Analysis
4.9. Cell Culture
4.10. Cell Viability Test
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Jung, J.H.; Ji, H.; Zhang, S. Glicerolipids from a Sarcotragus species sponge. Molecules 2006, 11, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-Y.; Zhao, Q.; Choi, K.; Hong, J.; Lee, D.S.; Lee, C.-O.; Jung, J.H. A new glycerol ether from a marine sponge Stelleta species. Nat. Prod. Sci. 2003, 9, 232–234. [Google Scholar]
- Seo, Y.; Cho, K.W.; Lee, H.-S.; Rho, J.-R.; Shin, J. New acetylenic enol ethers of glycerol from the sponge Petrosia sp. J. Nat. Prod. 1999, 62, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.D.; Wahidullah, S.; D’Souza, L.D.; Kamat, S.Y. Steroids from marine sponges Suberites vestigium and Chrotella australiensis. Indian J. Chem. 1997, 36B, 719–721. [Google Scholar]
- Quijano, L.; Cruz, F.; Navarrete, I.; Gomez, P.; Rios, T. Alkyl glycerol monoethers in the marine sponge Desmapsamma anchorata. Lipids 1994, 29, 731–734. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Aknin, M.; Fall, A.; Samb, A.; Miralles, J. An unusual ether glycolipid from the senegalese sponge Trikentrion loeve Carter. Tetrahedron 1993, 49, 2711–2716. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Blunt, J.W.; Munro, M.H.G. A new sterol sulfate from the marine sponge Stylopus australis. J. Nat. Prod. 1989, 52, 657–659. [Google Scholar] [CrossRef]
- Smith, G.M.; Djerassi, C. Phospholipid studies of marine organisms: 14. Ether lipids of the sponge Tethya aurantia. Lipids 1987, 22, 236–240. [Google Scholar] [CrossRef]
- Guella, G.; Mancini, I.; Pietra, F. (+)-Raspailyne-A, a novel, acid-sensitive acetylenic enol ether glyceride from the marine sponge Raspailia pumila. J. Chem. Soc. Chem. Commun. 1986, 1, 77–78. [Google Scholar] [CrossRef]
- Myers, B.L.; Crews, P. Chiral ether glycerides from a marine sponge. J. Org. Chem. 1983, 48, 3583–3585. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Vanderah, D.J.; Hollenbeak, K.H.; Enwall, C.E.L.; Gopichand, Y.; SenGupta, P.K.; Hossain, M.B.; van der Helm, D. Metabolites from the marine sponge Tedania ignis. A new atisanediol and several known diketopiperazines. J. Org. Chem. 1983, 48, 3941–3945. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Graden, C.J.; Greer, B.J. 17Z-Tetracosenyl 1-glycerol ether from the sponges Cinachyra alloclada and Ulosa ruetzleri. Lipids 1983, 18, 107–110. [Google Scholar] [CrossRef]
- Do, M.N.; Erickson, K.L. Branched chain mono-glycerol ethers from a Taiwanese marine sponge of the genus Aaptos. Tetrahedron Lett. 1983, 24, 5699–5702. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J.; Slattery, M.; Neine, J.N.; Bryan, P.J.; Yoshida, W.; Davies-Coleman, M.T.; Faulkner, D.J. Chemical defence of common Antarctic shallow-water nudibranch Tritoniella belli Eliot (Mollusca: Tritonidae) and its prey, Clavularia frankliniana Rouel (Cnidaria: Octocorallia). J. Chem. Ecol. 1994, 20, 3361–3372. [Google Scholar] [CrossRef]
- Imbs, A.B.; Demidkova, D.A.; Dautova, T.N. Lipids and fatty acids of cold-water soft corals and hydrocorals: A comparison with tropical species and implications for coral nutrition. Mar. Biol. 2016, 163, 202. [Google Scholar] [CrossRef]
- Diaz, Y.M.; Laverde, G.V.; Gamba, L.R.; Wandurraga, H.M.; Arevalo-Ferro, C.; Rodriguez, F.R.; Bertran, C.D.; Hernandez, L.C. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev. Bras. Farm. 2015, 25, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Han, A.-R.; Song, J.-I.; Jang, D.S.; Min, H.-Y.; Lee, S.K.; Seo, E.-K. Cytotoxic constituents of the octocoral Dendronephthya gidantea. Arch. Pharm. Res. 2005, 28, 290–293. [Google Scholar] [CrossRef]
- Pettit, G.R.; Fujii, Y. Antineoplastic agents. 81. The glycerol ethers of Palythoa liscia. J. Nat. Prod. 1982, 45, 640–643. [Google Scholar] [CrossRef]
- Kind, C.A.; Bergmann, W. Contributions to the study of marine products. XI. The occurence of octadecyl alcohol, batyl alcohol, and cetyl palmitate in gorgonias. J. Org. Chem. 1942, 7, 424–427. [Google Scholar] [CrossRef]
- Gustafson, K.; Andersen, R.J. Chemical studies of British Columbia nudibranchs. Tetrahedron 1985, 41, 1101–1108. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Ermakova, S.P.; Ermolenko, E.V.; Imbs, A.B.; Kasyanov, S.P.; Sultanov, R.M. 1-O-alkylglycerols from the hepatopancreas of the crab Paralithodes camtschaticus, liver of the squid Berryteuthis magister, and liver of the skate Bathyraja parmifera, and their anticancer activity on human melanoma cells. J. Food Biochem. 2019, 43, e12828. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Sultanov, R.M.; Ermolenko, E.V. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem. 2017, 119, 812–821. [Google Scholar] [CrossRef]
- Hayashi, K.; Okawa, Y.; Kawasaki, K. Liver lipids of gonatid squid Berryteuthis magister: A rich source of alkyi glyceryl ethers. Bull. Jap. Soc. Sci. Fish. 1985, 51, 1523–1526. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nichols, P.D.; Virtue, P. Lipids and buoyancy in Southern ocean pteropods. Lipids 1997, 32, 1093–1100. [Google Scholar] [CrossRef]
- Thompson, G.A.; Lee, P. Studies of the α-glyceryl ether lipids occurring in molluscan tissues. Biochim. Biophys. Acta 1965, 98, 151–159. [Google Scholar] [CrossRef]
- Hayashi, K. Content and composition of glyceryl ethers in the pyloric ceca and ovaries of the starfish Distolasterias nippon, Asterina pectinifera, and Lysastrosoma anthosticta. Fish. Sci. 1998, 64, 852–853. [Google Scholar] [CrossRef] [Green Version]
- Rybin, V.; Pavel, K.; Mitrofanov, D. 1-O-Alkylglycerol ether lipids in two holothurian species: Cucumaria japonica and C. okhotensis. Nat. Prod. Commun. 2007, 2, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Santos, V.L.C.; Billett, D.S.M.; Wolff, G.A. 1-O-Alkylglyceryl ether lipids of the gut walls and contents of an Abyssal holothurian (Oneirophanta mutabilis). J. Braz. Chem. Soc. 2002, 13, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Takeara, R.; Jimenez, P.C.; Wilke, D.V.; de Moraes, M.O.; Pessoa, C.; Lopes, N.P.; Lopes, J.L.C.; da Cruz Lotufo, T.M.; Costa-Lotufo, L.V. Antileukemic effects of Didemnum psammatodes (Tunicata: Ascidiacea) constituents. Comp. Biochem. Physiol. Part A 2008, 151, 363–369. [Google Scholar] [CrossRef]
- Othmani, A.; Bunet, R.; Bonnefont, J.-L.; Briand, J.-F.; Culioli, G. Settlement inhibition of marine biofilm bacteria and barnacle larvae by compounds isolated from the Mediterranean brown alga Taonia atomaria. J. Appl. Phycol. 2016, 28, 1975–1986. [Google Scholar] [CrossRef]
- Magnusson, C.D.; Haraldsson, G.G. Ether lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Urate, K.; Takaishi, N. Ether lipids based on the glyceryl ether skeleton: Present state, future potential. JAOCS 1996, 73, 819–830. [Google Scholar] [CrossRef]
- Baer, E.; Fisher, H.O.L. Studies on acetone-glyceraldehyde, and optically active glycerides IX. Configuration of the natural batyl, chimil, and selachyl alcohols. J. Biol. Chem. 1941, 140, 397–410. [Google Scholar] [CrossRef]
- IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids (Recommendations 1976). J. Lipid Res. 1978, 19, 114–128. [Google Scholar] [CrossRef]
- Taguchi, H.; Armarego, W.L.F. Glyceryl-ether monooxygenase [EC 1.14.16.5]. A microsomal enzyme of ether lipid metabolism. Med. Res. Rev. 1998, 18, 43–89. [Google Scholar] [CrossRef]
- Watschinger, K.; Keller, M.A.; Golderer, G.; Hermann, M.; Maglione, M.; Sarg, B.; Lindner, H.H.; Hermetter, A.; Werner-Felmayer, G.; Konrat, R.; et al. Identification of the gene encoding alkylglycerol monooxygenase defines a third class of tetrahydrobiopterin-dependent enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 13672–13677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniau, A.-L.; Mosset, P.; Le Bot, D.; Legrand, A.B. Which alkylglycerols from shark liver oil have anti-tumour activities? Biochimie 2011, 93, 1–3. [Google Scholar] [CrossRef]
- Deniau, A.-L.; Le Bot, D.; Mosset, P.; Legrand, A.B. Activités antitumorale et antimétastasique des alkylglycérols naturels: Relation structure-activité. OCL Ol. Corps Gras Lipides 2010, 17, 236–237. (In French) [Google Scholar] [CrossRef]
- Pedrono, F.; Saıag, B.; Moulinoux, J.-P.; Legrand, A.B. 1-O-Alkylglycerols reduce the stimulating effects of bFGF on endothelial cell proliferation in vitro. Cancer Lett. 2007, 251, 317–322. [Google Scholar] [CrossRef]
- Pedrono, F.; Martin, B.; Leduc, C.; Le Lan, J.; Saiag, B.; Legrand, P.; Moulinoux, J.-P.; Legrand, A.B. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr. Cancer 2004, 48, 64–69. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Przybyszewska, M.; Janik, P. Cytostatic and cytotoxic effects of alkylglycerols (Ecomer). Med. Sci. Monit. 2003, 9, 131–135. [Google Scholar]
- Hernández-Colina, M.; Cermeño, A.M.; García, A.D. Selective cytotoxic effect of 1-O-undecylglycerol in human melanoma cells. J. Pharm. Pharmacog. Res. 2016, 4, 84–94. [Google Scholar]
- Brohult, A.; Brohult, J.; Brohult, S. Regression of tumor growth after administration of alkoxyglycerols. Acta Obstet. Gynecol. Scand. 1978, 57, 79–83. [Google Scholar] [CrossRef]
- Brohult, A.; Brohult, J.; Brohult, S. Biochemical effects of alkylglycerols and their use in cancer therapy. Acta Chem. Scand. 1970, 24, 730–732. [Google Scholar] [CrossRef] [Green Version]
- Di Iannitti, T.; Capone, S.; Palmieri, B. A telephone interview to assess alkylglycerols effectiveness in preventing influenza-like symptoms in Modena, Emilia Romagna, Italy, in the season 2009–2010. Clin. Ter. 2011, 162, e115–e118. [Google Scholar]
- Daza, K.E.C.; Gómez, E.S.; Murillo, B.D.M.; Wandurraga, H.M. Natural and enantiopure alkylglycerols as antibiofilms against clinical bacterial isolates and quorum sensing inhibitors of Chromobacterium violaceum ATCC 12472. Antibiotics 2021, 10, 430. [Google Scholar]
- Montoya, D.J.F.; Jordan, L.A.C.; Murillo, B.M.; Gomez, E.S.; Wandurraga, H.M. Enantiomeric synthesis of natural alkylglycerols and their antibacterial and antibiofilm activities. Nat. Prod. Res. 2021, 35, 2544–2550. [Google Scholar] [CrossRef]
- Hayens, M.P.; Buckley, H.R.; Higgins, M.L.; Pieringer, R.A. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agent. Chemother. 1994, 38, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, T.S.; Monteiro, L.G.; Braga, E.F.; Batista, W.R.; Albert, A.L.M.; Chantre, L.G.F.; de Machado, S.P.; Lopes, R.S.C.; Lopes, C.C. Synthesis of natural ether lipids and 1-O-hexadecylglycero-arylboronates via an epoxide-ring opening approach: Potential antifouling additives to marine paint coatings. Int. J. Adv. Res. Sci. Eng. Technol. 2018, 5, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Parri, A.; Fito, M.; Torres, C.F.; Munoz-Aguayo, D.; Schroder, H.; Cano, J.F.; Vazquez, L.; Reglero, G.; Covas, M.-I. Alkylglycerols reduce serum complement and plasma vascular endothelial growth factor in obese individuals. Inflammopharmacology 2016, 24, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brohult, A.; Brohult, J.; Brohult, S.; Joelsson, I. Effect of alkoxyglycerols on the frequency of fistulas following radiation therapy for carcinoma of the uterine cervix. Acta Obstet. Gynecol. Scand. 1979, 58, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.; Connell, D.I.; Brohult, A.; Brohult, S. Reduction of radiation induced shortening of life span by a diet augmented with alkoxy glycerol esters and essential fatty acids. Gerontologia 1959, 3, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Bronhult, A.; Holmberg, J. Alkoxyglycerols in the treatment of leukopenia caused by irradiation. Nature 1954, 174, 1102–1103. [Google Scholar] [CrossRef]
- Yu, H.; Dilbaz, S.; Cobmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Hofer, D.C.; Pessentheiner, A.R.; Pelzmann, H.J.; Schlager, S.; Madreiter-Sokolowski, C.T.; Kolb, D.; Eichmann, T.O.; Rechberger, G.; Bilban, M.; Graier, W.F.; et al. Critical role of the peroxisomal protein PEX16 in white adipocyte development and lipid homeostasis. Biochim. Biophys. Acta 2017, 1862, 358–368. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, S.; Tang, N.; Cai, W.; Qian, L. Oral administration of alkylglycerols differentially modulates high-fat diet-induced obesity and insulin resistance in mice. Evid. Based Complement. Alter. Med. 2013, 2013, 834027. [Google Scholar] [CrossRef]
- Homan, E.A.; Kim, Y.-G.; Cardia, J.P.; Saghatelian, A. Monoalkylglycerol ether lipids promote adipogenesis. J. Am. Chem. Soc. 2011, 133, 5178–5181. [Google Scholar] [CrossRef] [Green Version]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Kipryushina, Y.; Ermolenko, E.V. Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int. J. Mol. Med. 2019, 43, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, R.; Gil, D.; del Campo, J.; Bracho, G.; Valdes, Y.; Perez, O. The adjuvant potential of synthetic alkylglycerols. Vaccine 2006, 24, S32–S33. [Google Scholar] [CrossRef]
- Ngwenya, B.Z.; Foster, D.M. Enhancement of antibody production by lysophosphatidylcholine and alkylglycerol. Proc. Soc. Exp. Biol. Med. 1991, 196, 69–75. [Google Scholar] [CrossRef]
- Marigny, K.; Pedrono, F.; Martin-Chouly, C.A.E.; Youmine, H.; Saiag, B.; Legrand, A.B. Modulation of endothelial permeability by 1-O-alkylglycerols. Acta Physiol. Scand. 2002, 176, 263–268. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, M.; Wu, S.; Zhong, Y.; Van Tol, E.; Cai, W. Alkylglycerols modulate the proliferation and differentiation of non-specific agonist and specific antigen-stimulated splenic lymphocytes. PLoS ONE 2014, 9, e96207. [Google Scholar] [CrossRef] [Green Version]
- Homma, S.; Yamamoto, N. Activation process of macrophages after in vitro treatment of mouse lymphocytes with dodecylglycerol. Clin. Exp. Immunol. 1990, 79, 307–313. [Google Scholar] [CrossRef]
- Yamamoto, N.; St Claire, D.A., Jr.; Homma, S.; Ngwenya, B.Z. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988, 48, 6044–6049. [Google Scholar]
- Brohult, A.; Brohult, J.; Brohult, S. Effect of irradiation and alkoxyglycerol treatment on the formation of antibodies after Salmonella vaccination. Experientia 1972, 8, 954–955. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Alipour, M.; Fricker, G.; Miller, D.S.; Kugler, W.; Eibl, H.; Lakomek, M. Alkylglycerol opening of the blood–brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharm. 2003, 140, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Erdlenbruch, B.; Jendrossek, V.; Eibl, H.; Lakomek, M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp. Brain Res. 2000, 135, 417–422. [Google Scholar]
- Bernal-Chávez, S.A.; Pérez-Carreto, L.Y.; Nava-Arzaluz, M.G.; Ganem-Rondero, A. Alkylglycerol derivatives, a new class of skin penetration modulators. Molecules 2017, 22, 185. [Google Scholar] [CrossRef] [Green Version]
- Cheminade, C.; Gautier, V.; Hichami, A.; Allaume, P.; Le Lannou, D.; Legrand, A.B. 1-O-Alkylglycerols improve boar sperm motility and fertility. Biol. Reprod. 2002, 66, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Shubina, L.K.; Makarieva, T.N.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; Dmitrenok, P.S.; von Amsberg, G.; Stonik, V.A. Gracilosulfates A–G, monosulfated polyoxygenated steroids from the marine sponge Haliclona gracilis. Mar. Drugs 2020, 18, 454. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Kaune, M.; Kriegs, M.; Hauschild, J.; Busenbender, T.; Shubina, L.K.; Makarieva, T.N.; Hoffer, K.; Bokemeyer, C.; Graefen, M.; et al. Marine alkaloid monanchoxymycalin C: A new specific activator of JNK1/2 kinase with anticancer properties. Sci. Rep. 2020, 10, 13178. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Kuzmich, A.S.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and studies of acetylthioglycoside conjugates of 4-chloro-1,2-dithiole-3-thione as potential antitumor agents. Russ. Chem. Bull. 2021, 70, 573–579. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Agafonova, I.G.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and study of thioglycoside conjugates of 4-chloro-1,2-dithiol-3-one as potential cancer-preventive substances in vitro and in vivo. Russ. Chem. Bull. 2022, 71, 489–495. [Google Scholar] [CrossRef]
- Baltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, innersalt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Gunstone, F.D.; Pollard, M.R.; Scrimgeour, C.M.; Vedanayagam, H.S. Fatty acids. Part 50. 13C Nuclear magnetic resonance studies of olefinic fatty acids and esters. Chem. Phys. Lipids 1977, 18, 115–129. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Wimmer, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond—ether lipids in signaling and neurodegeneration. Neurobiol. Disease 2020, 145, 105061. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From peroxisomal disorders to common neurodegenerative diseases—the role of ether phospholipids in the nervous system. FEBS Lett. 2017, 591, 2761–2788. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Paul, S.; Rasmiena, A.A.; Huynh, K.; Smith, A.A.T.; Mellett, N.A.; Jandeleit-Dahm, K.; Lancaster, G.I.; Meikle, P.J. Oral supplementation of an alkylglycerol mix comprising different alkyl chains effectively modulates multiple endogenous plasmalogen species in mice. Metabolites 2021, 11, 299. [Google Scholar] [CrossRef]
- Hichami, A.; Duroudier, V.; Leblais, V.; Vernhet, L.; Le Goffic, F.; Ninio, E.; Legrand, A. Modulation of platelet-activating-factor production by incorporation of naturally occurring 1-O-alkylglycerols in phospholipids of human leukemic monocyte-like THP-1 cells. Eur. J. Biochem. 1997, 250, 242–248. [Google Scholar] [CrossRef]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part I). Drugs 1991, 42, 9–29. [Google Scholar] [CrossRef]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part II). Drugs 1991, 42, 174–204. [Google Scholar] [CrossRef]
- da Silva, T.F.; Eira, J.; Lopes, A.T.; Malheiro, A.R.; Sousa, V.; Luoma, A.; Avila, R.L.; Wanders, R.J.A.; Just, W.W.; Kirschner, D.A.; et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Investig. 2014, 124, 2560–2570. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; Mineno, K.; Katafuchi, T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS ONE 2016, 11, e0150846. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Hossain, M.S.; Sejimo, S.; Akashi, K. Plasmalogens inhibit endocytosis of toll-like receptor 4 to attenuate the inflammatory signal in microglial cells. Mol. Neurobiol. 2019, 56, 3404–3419. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Pedrono, F.; Khan, N.A.; Legrand, A.B. Regulation of calcium signaling by 1-O-alkylglycerols in human Jurkat T lymphocytes. Life Sci. 2004, 74, 2793–2801. [Google Scholar] [CrossRef]
- McNeely, T.B.; Rosen, G.; Londner, M.V.; Turco, S.J. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem. J. 1989, 259, 601–604. [Google Scholar] [CrossRef] [Green Version]



| # of Compound | Retention Time | Fatty Alcohol Residue | Peak Area, % of Total | Characteristic Ion Fragment | ||||
|---|---|---|---|---|---|---|---|---|
| M+–CH3 | M+–HOSi(CH3)3–CH2OSi(CH3)3 | M+–HOSi(CH3)3 | M+–HOSi(CH3)3–C4H9 | M+ | ||||
| 13.06 | N/I* | 0.647 | ||||||
| 13.78 | N/I* | 0.890 | ||||||
| 7 | 16.07 | 14:0 | 0.436 | 417 | - | - | 285 | - |
| 8 (iso) | 16.58 | 15:0 | 0.404 | 431 | - | 356 | 299 | - |
| 9 (n) | 16.87 | 15:0 | 1.113 | 431 | - | 356 | 299 | - |
| 10 (iso) | 17.35 | 16:0 | 2.514 | 445 | - | 370 | 313 | - |
| 17 or 18 or 19 or 20 | 17.51 | 16n:1 | 1.973 | 443 | 265 | - | 311 | 458 |
| 17 or 18 or 19 or 20 | 17.59 | 16n:1 | 1.168 | - | 265 | - | 311 | 458 |
| 11 (n) | 17.64 | 16:0 | 21.116 | 445 | 370 | 313 | - | |
| 12 (iso) | 18.10 | 17:0 | 13.648 | 459 | 384 | 327 | - | |
| 13 (anteiso) | 18.18 | 17:0 | 2.181 | 459 | 384 | 327 | - | |
| 14 (n) | 18.38 | 17:0 | 1.414 | 459 | 384 | 327 | - | |
| 15 (iso) | 18.80 | 18:0 | 0.700 | 473 | 398 | 341 | - | |
| 21 or 22 or 23 or 24 | 18.93 | 18n:1 | 1.243 | 471 | 293 | 396 | 339 | 486 |
| 21 or 22 or 23 or 24 | 18.99 | 18n:1 | 2.118 | 471 | 293 | 396 | 339 | 486 |
| 16 (n) | 19.10 | 18:0 | 5.403 | 473 | - | 398 | 341 | - |
| 25 or 26 or 27 | 20.38 | 20n:1 | 0.400 | - | 321 | - | - | 514 |
| 1 | 21.74 | 22n:1 | 16.036 | 527 | 349 | - | 395 | 542 |
| 2 | 21.81 | 22n:1 | 2.860 | 527 | 349 | - | 395 | 542 |
| 3 or 4 or 5 | 22.56 | 23n:1 | 0.731 | - | 363 | - | - | - |
| 23.13 | N/I* | 1.496 | ||||||
| 6 | 23.35 | 24n:1 | 1.722 | - | 377 | 480 | - | - |
| 28 | 23.43 | 24n:1 | 18.241 | - | 377 | 480 | 423 | - |
| 25.35 | N/I* | 1.548 | ||||||
| # of Compound | Retention Time | Fatty Alcohol Residue | Ion Fragment | ||
|---|---|---|---|---|---|
| Ion A | Ion B | M+ | |||
| 17 | 44.69 | 16:1n-7 | 173 | 259 | 492 |
| 18 | 45.00 | 16:1n-9 | 145 | 287 | 492 |
| 19 | 45.69 | 16:1n-11 | 117 | 315 | 492 |
| 20 | 47.69 | 16:1n-13 | 89 | 343 | 492 |
| 21 | 51.10 | 18:1n-9 | 173 | 287 | 520 |
| 22 | 51.40 | 18:1n-11 | 145 | 315 | 520 |
| 23 | 51.62 | 18:1n-12 | 131 | 329 | 520 |
| 24 | 52.13 | 18:1n-13 | 117 | 343 | 520 |
| 25 | 58.48 | 20:1n-11 | 173 | 315 | 548 |
| 26 | 59.39 | 20:1n-13 | 145 | 343 | 548 |
| 27 | 60.29 | 20:1n-15 | 117 | 371 | 548 |
| 1 | 70.67 | 22:1n-15 | 145 | 371 | 576 |
| 2 | 72.07 | 22:1n-17 | 117 | 399 | 576 |
| 3 | 77.50 | 23:1n-15 | 159 | 371 | 590 |
| 4 | 77.90 | 23:1n-16 | 145 | 385 | 590 |
| 5 | 78.67 | 23:1n-17 | 131 | 399 | 590 |
| 6 | 86.27 | 24:1n-15 | 173 | 371 | 604 |
| 28 | 87.30 | 24:1n-17 | 145 | 399 | 604 |
| Cell Line | Cancer Type | IC50 (AGE), μg/mL | IC50 (Cisplatin), μg/mL |
|---|---|---|---|
| HL-60 | promyelocytic leukemia | 87.4 ± 23.9 | 0.7 ± 0.09 |
| THP-1 | monocytic leukemia | 35.9 ± 4.4 | 3.31 ± 0.74 |
| HeLa | cervix carcinoma | 85.9 ± 17 | 1.55 ± 0.21 |
| DLD-1 | colon cancer | 103.3 ± 21.9 | 9.24 ± 1.43 |
| SNU C4 | colon cancer | 117.4 ± 33.1 | 4.01 ± 1.21 |
| SK-MEL-28 | melanoma | 85.8 ± 4.7 | 0.89 ± 0.04 |
| MDA-MB-231 | breast cancer | 137 ± 23.8 | 60.6 ± 26.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyshlovoy, S.A.; Fedorov, S.N.; Svetashev, V.I.; Makarieva, T.N.; Kalinovsky, A.I.; Moiseenko, O.P.; Krasokhin, V.B.; Shubina, L.K.; Guzii, A.G.; von Amsberg, G.; et al. 1-O-Alkylglycerol Ethers from the Marine Sponge Guitarra abbotti and Their Cytotoxic Activity. Mar. Drugs 2022, 20, 409. https://doi.org/10.3390/md20070409
Dyshlovoy SA, Fedorov SN, Svetashev VI, Makarieva TN, Kalinovsky AI, Moiseenko OP, Krasokhin VB, Shubina LK, Guzii AG, von Amsberg G, et al. 1-O-Alkylglycerol Ethers from the Marine Sponge Guitarra abbotti and Their Cytotoxic Activity. Marine Drugs. 2022; 20(7):409. https://doi.org/10.3390/md20070409
Chicago/Turabian StyleDyshlovoy, Sergey A., Sergey N. Fedorov, Vasily I. Svetashev, Tatiana N. Makarieva, Anatoliy I. Kalinovsky, Olga P. Moiseenko, Vladimir B. Krasokhin, Larisa K. Shubina, Alla G. Guzii, Gunhild von Amsberg, and et al. 2022. "1-O-Alkylglycerol Ethers from the Marine Sponge Guitarra abbotti and Their Cytotoxic Activity" Marine Drugs 20, no. 7: 409. https://doi.org/10.3390/md20070409
APA StyleDyshlovoy, S. A., Fedorov, S. N., Svetashev, V. I., Makarieva, T. N., Kalinovsky, A. I., Moiseenko, O. P., Krasokhin, V. B., Shubina, L. K., Guzii, A. G., von Amsberg, G., & Stonik, V. A. (2022). 1-O-Alkylglycerol Ethers from the Marine Sponge Guitarra abbotti and Their Cytotoxic Activity. Marine Drugs, 20(7), 409. https://doi.org/10.3390/md20070409