Abstract
Mangrove-associated fungi are rich sources of novel and bioactive compounds. A total of 102 fungal strains were isolated from the medicinal mangrove Acanthus ilicifolius collected from the South China Sea. Eighty-four independent culturable isolates were identified using a combination of morphological characteristics and internal transcribed spacer (ITS) sequence analyses, of which thirty-seven strains were selected for phylogenetic analysis. The identified fungi belonged to 22 genera within seven taxonomic orders of one phyla, of which four genera Verticillium, Neocosmospora, Valsa, and Pyrenochaeta were first isolated from mangroves. The cytotoxic activity of organic extracts from 55 identified fungi was evaluated against human lung cancer cell lines (A-549), human cervical carcinoma cell lines (HeLa), human hepatoma cells (HepG2), and human acute lymphoblastic leukemia cell lines (Jurkat). The crude extracts of 31 fungi (56.4%) displayed strong cytotoxicity at the concentration of 50 μg/mL. Furthermore, the fungus Penicillium sp. (HS-N-27) still showed strong cytotoxic activity at the concentration of 25 µg/mL. Integrating cytotoxic activity-guided strategy and fingerprint analysis, a well-known natural Golgi-disruptor and Arf-GEFs inhibitor, brefeldin A, was isolated from the target active strain HS-N-27. It displayed potential activity against A549, HeLa and HepG2 cell lines with the IC50 values of 101.2, 171.9 and 239.1 nM, respectively. Therefore, combining activity-guided strategy with fingerprint analysis as a discovery tool will be implemented as a systematic strategy for quick discovery of active compounds.
1. Introduction
Cancer stands in the frontline among leading killers worldwide and the annual mortality rate is expected to reach 16.4 million by 2040 [1,2]. The marine environment has the potential to produce candidate compounds (structures) as leads to drugs, or actual drugs, as has been actively discussed for the last 50 or so years [3,4,5]. Nowadays, several compounds have led to drugs, especially in the area of cancer, such as trabectedin, and eribulin, which were discovered under the cytotoxic activity-guided approach [3,4,5,6]. Brefeldin A (BFA), a well-known natural Golgi-disruptor and Arf-GEFs inhibitor, was first isolated from Penicillium decumbens in 1958 [7,8] and subsequently identified only from the marine-derived genus Penicillium [9]. Previous studies reported that BFA showed strong anticancer activity in a variety of cancers, including colorectal, prostate, lung, and breast cancers [10,11]. BFA is considered as a promising leading molecule for developing anticancer drugs.
The mangrove forests are a complex ecosystem growing in tropical and subtropical intertidal estuarine zones and nourish a diverse group of microorganisms [12,13]. Microorganisms associated with mangrove environments are a major source of antimicrobial agents and also produce a wide range of important medicinal compounds, including enzymes, antitumor agents, insecticides, vitamins, immunosuppressants, and immune modulators [13,14,15,16,17]. Among the mangrove microbial community, mangrove associated fungi were the second-largest ecological group of the marine fungi [13,14]. Up to December 2020, at least 1387 new structures have been isolated and identified from a diverse range of mangrove-derived fungi (325 strains), which belong to about 69 genera. Furthermore, about 40.7% (530) of the 1300 new compounds displayed a wide range of pharmacological activities, and the antitumor (mainly cytotoxicity) function is noteworthy and visible, accounting for 34% (196 compounds) of the active compounds. Therefore, mangrove associated fungi are a rich source of structurally unique and diverse bioactive secondary metabolites [13].
Acanthus ilicifolius is widely distributed from India to southern China, tropical Australia and the Western Pacific islands, throughout Southeast Asia [18]. Various classes of bioactive compounds including alkaloids, benzoxazinoids, lignans, flavanoids, triterpenoids and steroids have been obtained from A. ilicifolius [18,19,20]. In addition, up to December 2020, a total of 22 strains belonging to 9 genera have been reported, which produced 95 new secondary metabolites. The endophytic fungi derived from A. ilicifolius are one of the most favored to be studied [13], yet little attention has been paid to the fungal communities associated with A. ilicifolius.
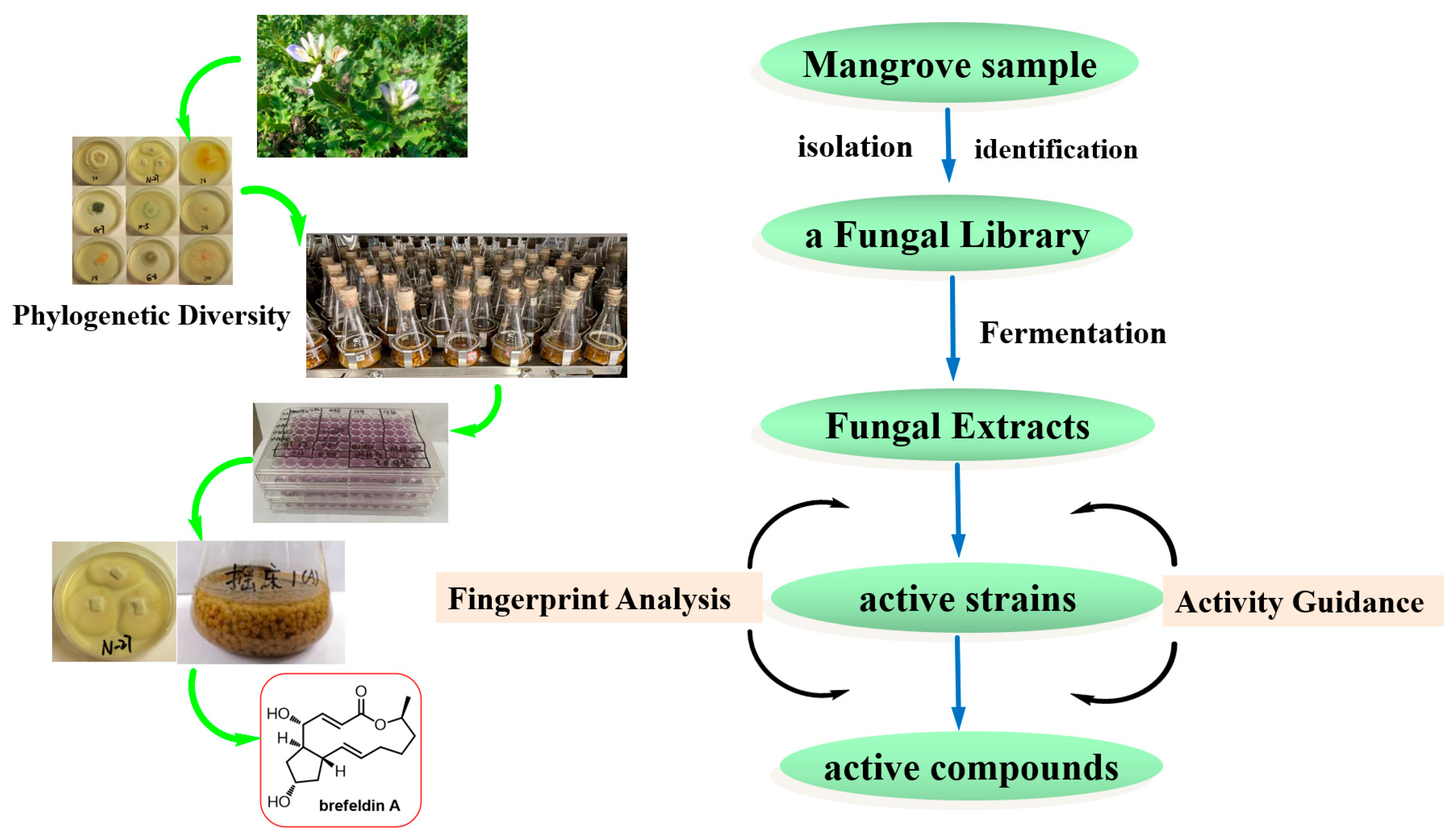
Investigating new bioactive natural products from marine fungi is a major and constant research focus in our laboratory [21,22,23,24]. Natural product researchers also face the challenge of targeting the discovery of bioactive compounds from a microbial resource library. The present work aims to integrate activity-guided strategy and fingerprint analysis to target the potent cytotoxic compounds from a fungal library of the medicinal mangrove A. ilicifolius (Figure 1). The cultivable fungi associated with the medicinal mangrove A. ilicifolius from the South China Sea were firstly systematic evaluated for their diversity. Furthermore, integrating the cytotoxic activity-guided strategy, the target active strains were quickly identified. Combined with fingerprint analysis, a potent cytotoxic activity compound, brefeldin A, was isolated from the target active strains. The combination of activity-guided strategy and fingerprint analysis could improve the efficiency of discovering active compounds in crude extracts from a complex and diverse fungal library.
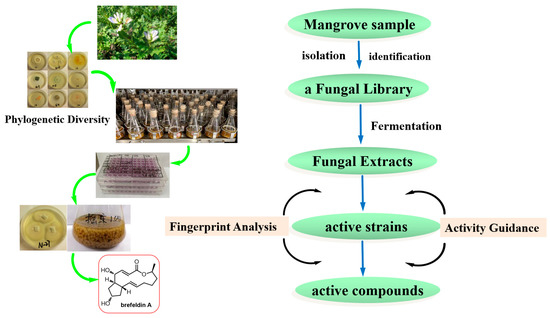
Figure 1.
The detailed flowchart of this study.
2. Results
2.1. Cultivable Fungi’s Phylogeny and Diversity
A total of 102 fungal isolates were obtained from Acanthus ilicifolius using the PDA medium with four salt gradients of 3%, 5%, 7% and 10%. Duplicated strains were removed using a detailed morphological approach. Consequently, eighty-four independent strains were selected for sequencing and identification based on ITS sequences. According to the sequences deposited into NCBI, the 84 strains belonged to the phylum Ascomycota including seven taxonomic orders: Hypocreales, Xylariales, Diaporthales, Eurotiales, Pleosporales, Capnodiales, Botryosphaeriaceae and 22 genera: Trichoderma, Hypocrea, Acremonium, Verticillium, Fusarium, Neocosmospora, Pestalotiopsis, Diaporthe, Phomopsis, Valsa, Colletotrichum, Penicillium, Eupenicillium, Aspergillus, Talaromyces, Pyrenochaeta, Pleosporales, Curvularia, Alternaria, Cladosporium, Phyllosticta, and Lasiodiplodia (Table 1). These identified fungi and their best matches in the NCBI database are summarized in Table S1. Most of the isolates matched their closest relatives with 98 to 100% similarity, except for HS-G-02 (97%) and HS-G-06 (95%), which indicated that they were new species. Both of the fungi HS-G-06 and HS-G-02 further enriched the diversity of mangrove fungi. Further analysis of the isolated fungi showed that Eurotiales was the dominant group with identified fungi, followed by Hypocreales. The fungal community was dominated by Penicillium, comprising 21 isolates, followed by Fusarium, Aspergillus, and Eupenicillium with 15, 14, and 10 isolates, respectively. Some of the genera, such as Trichoderma, Phomopsis and Cladosporium obtained six, five and five, respectively. Most of the remaining genera occurred as singletons or doubletons.

Table 1.
The classification of cultivable fungi associated with Acanthus ilicifolius.
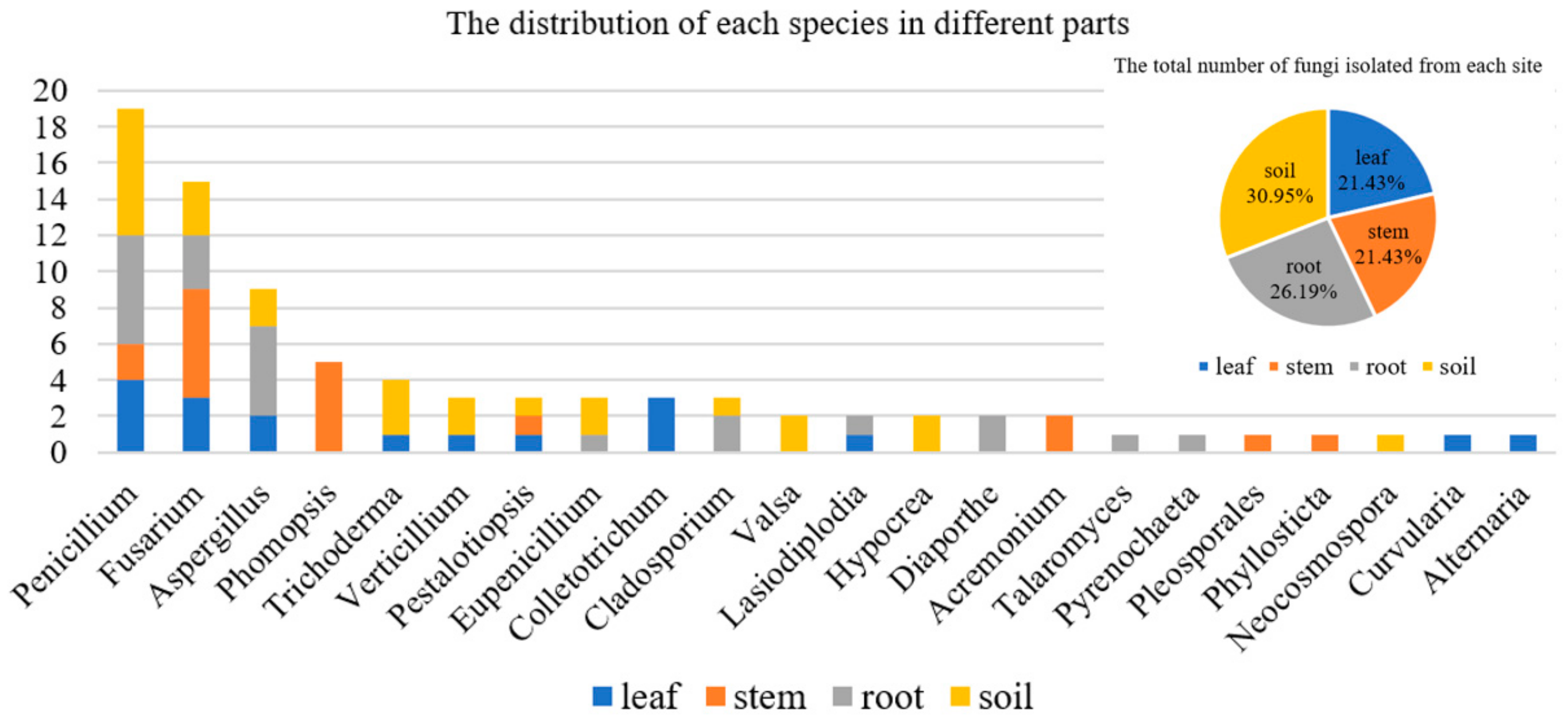
In addition, the species of fungi isolated from different parts of A. ilicifolius were quite different (Figure 2). The results showed that some genera of fungi were isolated only from one part. For example, Phomopsis and Acremonium were isolated only from the stem. Colletotrichum, Curvularia, and Alternaria were isolated only from the leaf. Valsa, Hypocrea, and Neocosmospora were isolated only from the soil. Diaporthe, Talaromyces, and Pyrenochaeta were isolated only from the leaf.
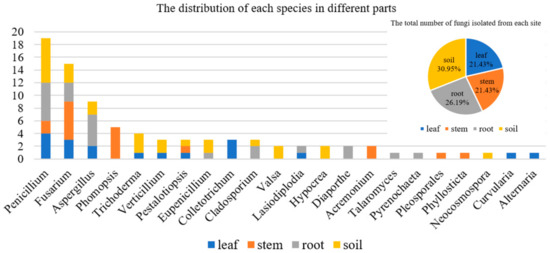
Figure 2.
The distribution of each species in different parts.
Further phylogenetic analysis was carried out on 37 strains. These 37 independent individuals were selected as the representative strains because they belong to different fungal species after we aligned the sequences with the BioEdit software (Figure S1). The phylogenetic tree of fungi in the order Hypocreales based on ITS gene sequence is presented in Figure S2. Furthermore, the fingerprints of secondary metabolites of fungi from different species and genera were analyzed (Figure S4).
2.2. The Cytotoxicity of Cultivable Fungal Extracts
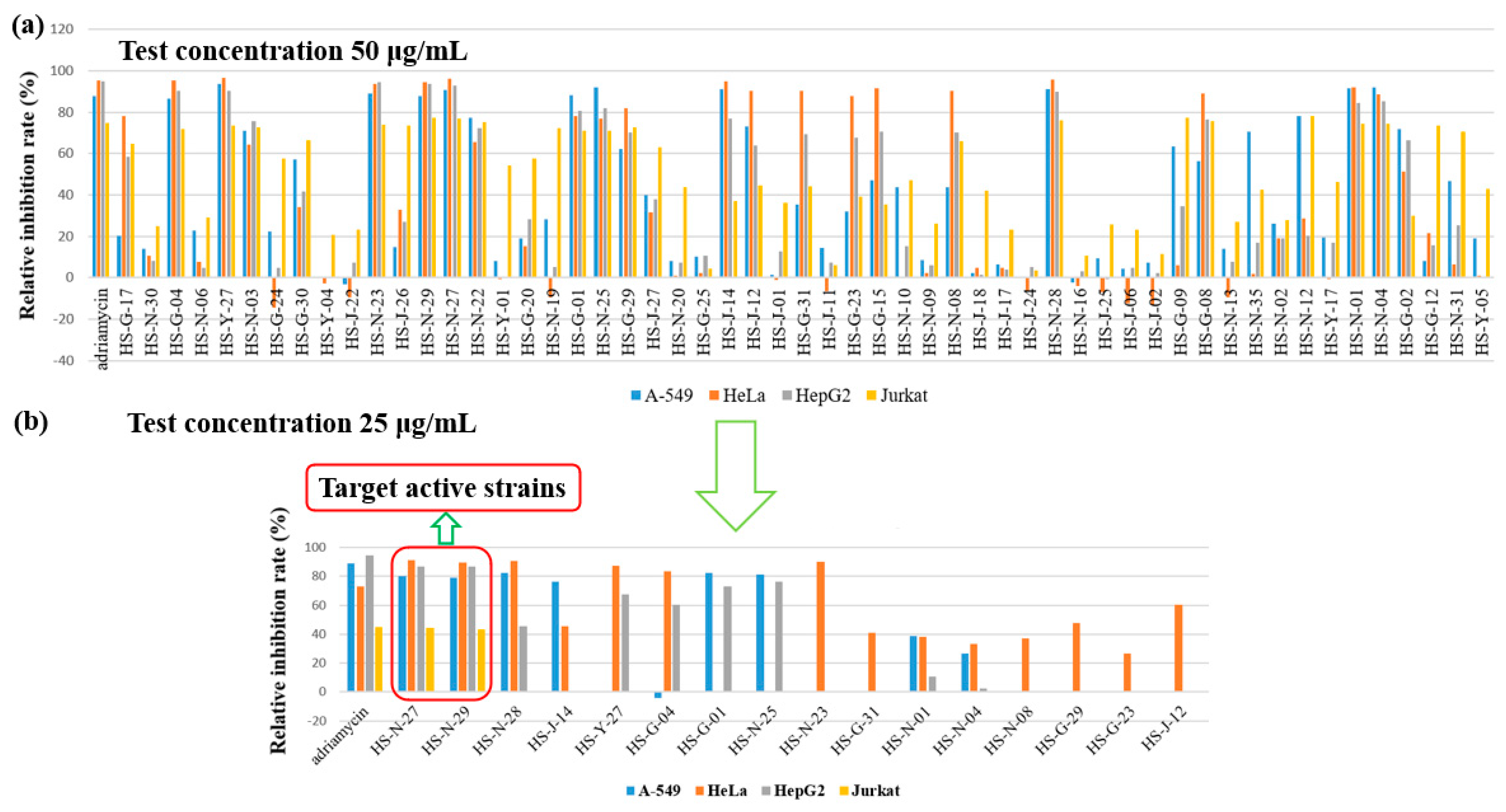
The organic extracts of 55 identified fungi were evaluated for their cytotoxic activities against human lung cancer cell line (A-549), human cervical carcinoma cell (HeLa), human hepatoma cells (HepG2) and Jurkat tumour cell lines at the concentration of 50 μg/mL (Figure 3a). To identify active strains for further research as potential cytotoxic strains, the relative inhibition rate of A-549, HeLa and HepG2 cell lines should greater than 70%, and the relative inhibition rate of Jurkat cell line should greater than 60%. The results showed that these fungi showed different inhibition rates to different cell lines. The number of the fungi showing activity against A-549, HeLa, HepG2, and Jurkat tumour cell lines were 17, 17, 19 and 24, respectively (Figure S3). The crude extracts of 31 fungi displayed cytotoxicity against the test cell lines, of which 21 fungi showed selective inhibitory activity on different tested cell lines,; for example, Fusarium sp. showed selective inhibitory activity on HeLa cell lines. Most fungi showed strong selective inhibitory activity on Jurkat cell lines. Interestingly, the remaining 10 fungi belonging to the two orders Eurotiales and Hypocreales, displayed a broad-spectrum strong cytotoxic activity, such as Penicillium sp. (HS-N-23, HS-N-27, HS-N-29, and HS-G-01), Eupenicillium sp. (HS-N-25), Trichoderma sp. (HS-01 and HS-N-04), Aspergillus sp. (HS-G-04 and HS-Y-27), and Verticillium sp. (HS-N-28).
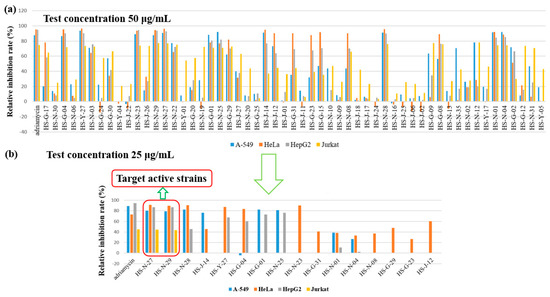
Figure 3.
Cytotoxicity of organic extracts of 55 identified fungi. (a) The cytotoxic activities of 55 identified fungi against A-549, HeLa, HepG2 and Jurkat tumour cell lines at the concentration of 50 μg/mL. (b) The cytotoxic activities of the active strains at the concentration of 25 μg/mL.
The crude extracts were further reduced in concentration for the activity test. The results showed that only the two active strains of Penicillium sp. (HS-N-27 and HS-N-29) still showed strong inhibitory activity against all the texted cell lines at the concentration of 25 μg/mL. Cytotoxic metabolites were isolated from the endophytic fungus Penicillium chermesinum, leading to the discovery of a cysteine-targeted Michael acceptor as a pharmacophore for fragment-based drug discovery, bioconjugation and click reactions [25]. The heteroatom-containing new compounds 2-hydroxyl-3-pyrenocine-thio propanoic acid and 5,5-dichloro-1-(3,5-dimethoxyphenyl)-1,4-dihydroxypentan-2-one, which were isolated from a deep-sea Penicillum citreonigrum XT20-134, showed potent cytotoxicity to the human hepatoma tumor cell Bel7402 [26]. Additionally, the active strains HS-N-28, HS-G-01, and HS-N-25 showed strong selective inhibitory activity against A-549, HeLa and HepG2 cell lines. The active strains HS-Y-27, HS-N-23, HS-G-04, and HS-N-28 showed strong selective inhibitory activity against HeLa cell lines. The active strains HS-G-01, and HS-N-25 showed strong selective inhibitory activity against HepG2 and A549 cell lines. Obviously, these active strains are important microbial resources and have the potential for interesting cytotoxic compounds (Figure 3b).
2.3. Isolation and Identifcation of Compounds 1–7
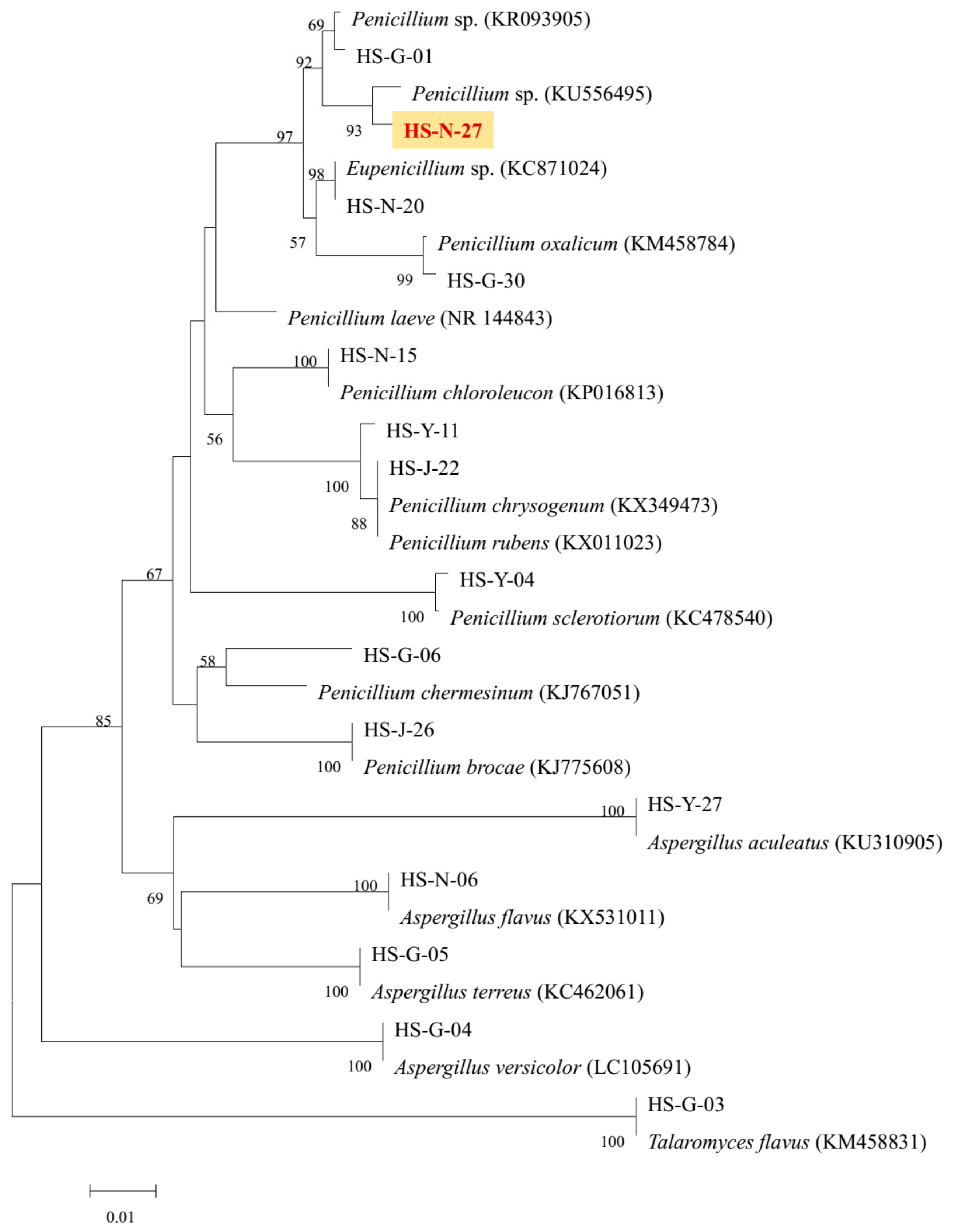
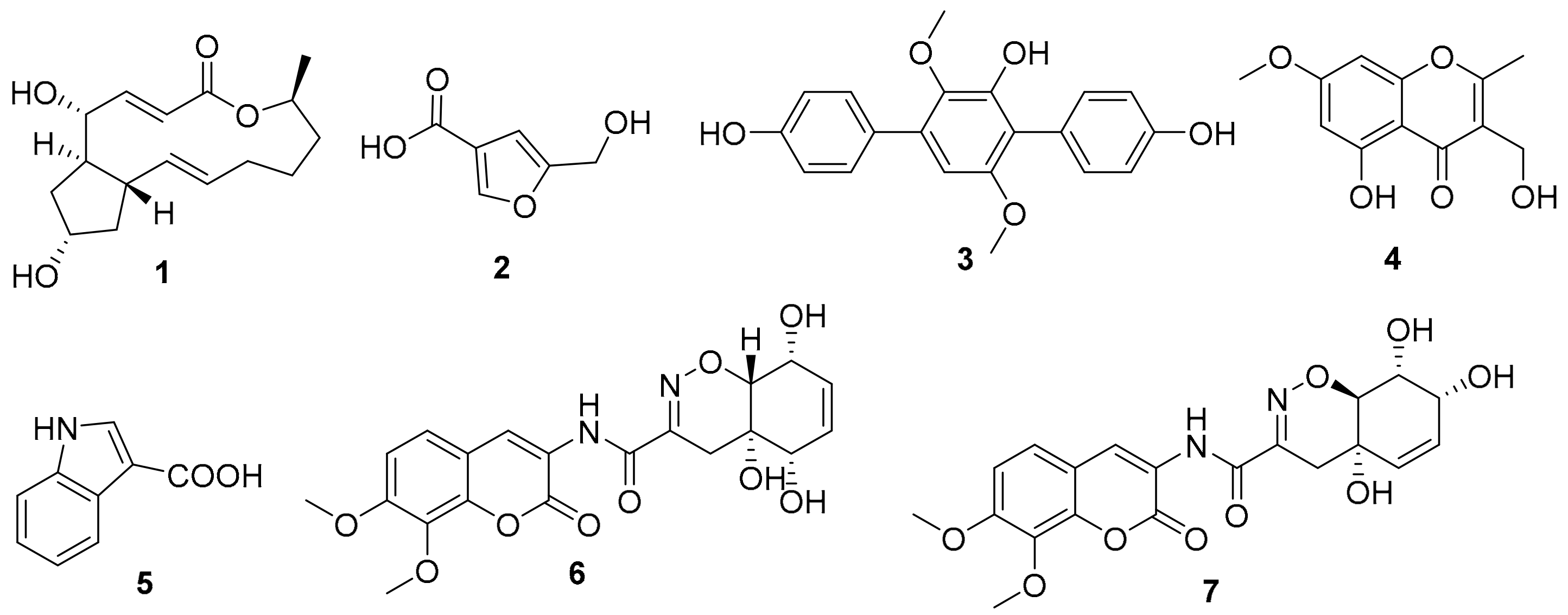
As the two active strains Penicillium sp. (HS-N-27 and HS-N-29) showed strong cytotoxic activity against all the tested cell lines at the concentration of 25 μg/mL, both of the Penicillium sp. fungi were selected as the target strains. Combining cytotoxic activity-guided strategy with fingerprint analysis, compound 1 was obtained from the fermentation broth of the two active strains HS-N-27 and HS-N-29. By comparison of NMR data with the reported literature, the structure was identified as brefeldin A (Figure 5), which was a 13-membered macrolactone with a cyclopentane substituent [7]. BFA is a well-known natural Golgi-disruptor and Arf-GEFs inhibitor [8]. Combining morphological characteristics and fingerprint analysis of metabolites (Figure S5), the two fungi HS-N-27 and HS-N-29 were identified as different individuals of the same Penicillium sp. species. The neighbor-joining of the phylogenetic tree of the target active strain Penicillium sp. (HS-N-27) in Hypocreales order fungi from A. ilicifolius based on ITS sequences is shown in Figure 4.
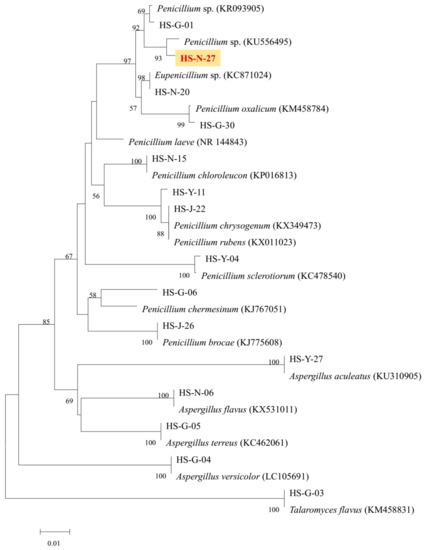
Figure 4.
The neighbor-joining of phylogenetic tree of HS-N-27 fungi in Hypocreales order fungi. The values at each node represent the bootstrap values from 1000 replicates, and the scale bar = 0.01 substitutions per nucleotide.
The genus Aspergillus is one of the dominant producers of new natural products [13]. The fingerprint analysis showed that the metabolites of Aspergillus flavus (HS-N-06) were relatively single and that A. candidus (HS-Y-23) was rich in metabolites with strong special UV absorption peak (Figure S4). The secondary metabolites of the two fungal strains were further studied. Under the guidance of chemical technology, 5-hydroxymethylfuran-3-carboxylic acid (2) was obtained from the fermentation broth of A. flavus (HS-N-06) [27]. Terphenyllin (3) was obtained from the fermentation broth of A. candidus (HS-Y-23), which showed weak cytotoxic activity against HeLa cell lines with the IC50 value of 19.0 µM [28]. In addition, 5-hydroxy-3-hydroxymethyl-2-methyl-7-methoxychromone (4), indolyl-3-carboxylic acid (5), and trichodermamides A (6) and D (7) were obtained from the fermentation broth of Trichoderma harzianum (HS-N-04) [29,30,31]. The structures of isolated and identified compounds were in Figure 5.
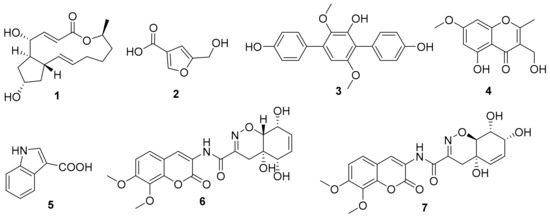
Figure 5.
Structures of isolated and identified compounds.
3. Discussion
Mangrove-associated fungi are rich in diversity and can produce impressive quantities of metabolites with promising biological activities that may be useful to humans as novel physiological agents [13,14,15,16,17]. The phylogenetic diversity of culturable fungi derived mangrove species Rhizophora stylosa and R. mucronata collected from the South China Sea has been reported [32]. The endophytic fungi derived from A. ilicifolius areamong the most favored to be studied. Up to December 2020, only 22 strains associated with A. ilicifolius belonging to 9 genera have been reported [13]. Investigation on phylogenetic diversity of A. ilicifolius associated fungi is relatively rare. In this study, 84 of the 102 isolates were successfully classified at the genus level based on ITS sequences with relatives in the NCBI database (Table S1). The identified fungi belonged to 22 genera, of which four genera Verticillium, Neocosmospora, Valsa, and Pyrenochaeta were first isolated from mangroves. (Table S1). Two strains HS-G-02 (97%) and HS-G-06 (95%) with low similarity indicated that they should be new species, which further enriched the diversity of mangrove fungi. The new strains may produce a variety of commercially interesting and potentially useful products. The above results indicated that a high diversity of fungi can be recovered from A. ilicifolius in the South China Sea.
Further analysis of the isolated fungi showed that Eurotiales was the dominant group with identified fungi accounted for 45.1%, followed by Hypocreales. The fungal community comprising Penicillium accounted for 20.6%, followed by Fusarium, and Aspergillus. It was reported that Penicillium (283, 20%), Aspergillus (246, 18%), and Pestalotiopsis (88, 6%) are the dominant producers of new natural products (1384) isolated from mangrove-associated fungi, comprising more than 45% of the total molecules [13]. The fungi obtained from A. ilicifolius could provide abundant microbial resources for the discovery of new compounds.
Natural product researchers face the challenge of maximizing the discovery of new or potent compounds from a microbial resource library. Combining activity-guided strategy with fingerprint analysis as a discovery tool will be implemented as a systematic strategy for quick discovery of active compounds. The crude extracts of 56.4% fungi displayed strong cytotoxicity. Interestingly, the remaining 10 fungi belonging to the two orders Eurotiales and Hypocreales, displayed a broad-spectrum strong cytotoxic activity. Furthermore, integrating cytotoxic activity-guided strategy and fingerprint analysis, a strong cytotoxic active compound brefeldin A was isolated from the target active strain HS-N-27. Brefeldin A is a well-known natural Golgi-disruptor and Arf-GEFs inhibitor, and shows strong anticancer activity in a variety of cancers [8,9,10,11]. BFA is considered as a promising leading molecule for developing anticancer drugs. As the metabolites of the fungi Penicillium sp. (HS-N-27) are relatively simple and BFA is easily separated and purified, this provides the source of compounds for the study of the medicinal properties of BFA. A series of BFA derivatives with antileukemia activity had been reported in terms of the semi-synthesis, cytotoxic evaluation, and structure-activity relationships [9]. This method, combining activity-guided strategy with fingerprint analysis, could improve the efficiency of discovering active compounds.
4. Materials and Methods
4.1. Sampling Site and Plant Material
The medicinal mangrove A. ilicifolius, which was authenticated by Prof. Fengqin Zhou (Shandong University of Traditional Chinese Medicine) was collected from the South China Sea. The samples were stored at the Key Laboratory of Marine Drugs, the Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China.
4.2. Isolation of Cultivable Fungi
To obtain the fungi associated with medicinal mangrove A. ilicifolius within different parts of the plant, the surface sterilization of each part from A. ilicifolius was carried out following an isolation as Qin et al. described with some modifications [33]. The root, stem and leaf of A. ilicifolius samples were washed with sterile artificial seawater for three times to remove the microorganisms and sediment attached to the surface. Appropriate samples were taken, using scissors or scalpel to cut all parts, including root, stem and leaf, with attention to the integrity of sampling. Then, the sample was soaked in 75% alcohol for 30 s, and the water on the sample was sucked up with sterile filter paper. The sample was cut into 1 cm³ pieces for fungal isolation.
The methods of tissue sectioning and tissue homogenization were used to isolate fungi. Tissue sectioning method: The tissues of 1 cm³ pieces were inoculated into PDA medium (200.0 g of potato extract, 20.0 g of glucose in 1 L of seawater with four salinities of 3%, 5%, 7% and 10% respectively) in a sterile environment. In order to improve the utilization of the plate and to separate more microorganisms, the medium plate was generally divided into three areas, and 2–3 pieces of tissue were placed in each area of the PDA medium with four salt gradients of 3%, 5%, 7% and 10%. Tissue homogenization method: The tissue was ground in 2 mL of sterile artificial seawater with a mortar in a sterile environment, and then the resulting homogenate was diluted with sterile artificial seawater at three dilutions (1:10, 1:100, and 1:1000). 100 μL of each dilution was plated in quadruplicate onto corresponding medium for fungal cultivation. The inoculated plates were cultured at 25 °C for 2 days. The fungi were replated onto new PDA plates several times until the morphology of the fungi could be distinguished. The obtained fungal strains were deposited at the Key Laboratory of Marine Drugs, the Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China.
4.3. Genomic DNA Extraction, PCR Amplifcation, Sequencing and Phylogenetic Analysis
The genomic DNA extraction was conducted using the Fungal DNA kits (E.Z.N.A., Omega, Norcross, GA, USA) according to the manufacturer’s protocol. The internal transcribed spacer (ITS1-5.8S-ITS2) regions of the fungi were amplified with the universal ITS primers, ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) using the polymerase chain reaction (PCR) [34]. The PCR was performed through the following cycle: initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C denaturation for 40 s min, 52 °C annealing for 40 s, 72 °C extension for 1 min; with a final extension at 72 °C for 10 min. Finally, the amplified products were submitted for sequencing (Invitrogen, Shanghai, China) and a BLASTN search was used to search for sequences of the closest match in the GenBank by Basic Local Alignment Search Tool (BLAST) programs database.
The sequences of fungal ITS regions obtained from A. ilicifolius were compared with the related sequences in the National Center for Biotechnology Information (NCBI). Each of these sequences was then aligned to sequences available in the NCBI database to determine the identity of the sequence, which further determined the species and genera of fungi. All fungal ITS sequences were aligned using the BioEdit software, applying the default parameters. The phylogenetic tree was generated using neighbor-joining (NJ) algorithms in the MEGA 7 software (version 7.0, Mega Limited) combined with bootstrap analysis using 1000 replicates incorporating fungal sequences showing the highest homology to sequences amplified.
4.4. General Experimental Procedures
The Agilent DD2 NMR spectrometer (JEOL, Tokyo, Japan) at 500 MHz and 125 MHz frequency was used for 1H and 13C NMR spectra respectively. The vacuum column chromatography silica gel (200–300 mesh, Qing Dao Hai Yang Chemical Group Co, Qingdao, China), silica gel plates for thin layer chromatography (G60, F-254, and Yan Tai Zi Fu Chemical Group Co, Yan Tai, China), and reverse phase octadecylsilyl silica gel column were used for the separation of compounds. UPLCMS spectra were measured on Waters UPLC® system (Waters Ltd., Milford, MA, USA) using a C18 column (ACQUITY UPLC® BEH C18, 2.1 × 50 mm, 1.7 μm; 0.5 mL/min) and ACQUITY QDA ESIMS scan from 150 to 1000 Da was used for the analysis of fungal extracts and ESI-MS spectra of the compounds. Semipreparative HPLC was performed on a Hitachi L-2000 system (Hi-tachi Ltd., Tokyo, Japan) using a C18 column (Kromasil 250 × 10 mm, 5 μm, 2.0 mL/min).
4.5. Fungal Fermentation and Chemical Extraction
The 55 fungal isolates were fermented in a 500 mL conical flask containing 250 mL PDA liquid medium. The fungi were shaken at 28 °C, 120 rpm for 7 days. Each exper-iment was conducted in three parallels. The fermentation broth was extracted three times with an equal volume of EtOAc and the whole EtOAc solutions were evaporated under reduced pressure to give the dried extracts.
4.6. Cytotoxic Assay
The cytotoxic activity was evaluated against human lung cancer cell line (A-549), human cervical carcinoma cells (HeLa), human hepatoma cells (HepG2) and Jurkat tumor cell lines by the MTT method, with adriamycin as a positive control [35]. The organic extracts, and adriamycin (the positive control) were dissolved in DMSO with the concentration of 50 µg/mL, 25 µg/mL, and 1 µM, respectively for bioassay.
4.7. Extraction and Isolation of Compounds
The organic extract of Penicillium sp. (HS-N-27) showed strong cytotoxic activity. The organic extract of the Penicillium sp. (HS-N-27) was subjected to silica gel column chromatography (CC) and eluted by a gradient of petroleum ether (PE)/ethyl acetate (EA) and then EA/MeOH to generate nine fractions (Fr. 1–9). All the fractions were further evaluated for cytotoxic activity. The Fr. 5 showed strong cytotoxic activity. Combined with fingerprint analysis,-the composition of Fr. 5 is relatively simple (Figure S5); it was further purified by semipreparative HPLC (MeOH−H2O, 80%; 2 mL/min) to obtain 1 (BFA) (19.0 mg).
The organic extract of the Aspergillus flavus (HS-N-06) was subjected to silica gel vacuum liquid chromatography (VLC) and eluted by a gradient of PE/EA and then EA/MeOH to afford four subfractions (Fr. 1−Fr. 4). Fr. 3 was separated by ODS CC (MeOH−H2O, 30–50%) to afford 2 (17.0 mg).
The organic extract of the A. aculeatus (HS-Y-23) was subjected to silica gel vacuum liquid chromatography (VLC) and eluted by a gradient of PE/EA and then EA/MeOH to afford seven subfractions (Fr. 1−Fr. 7). Fr. 4 was separated by ODS CC (MeOH−H2O, 30–100%) and then purified by semipreparative HPLC (MeOH−H2O, 70%; 2 mL/min) to afford 3 (7.0 mg).
The organic extract of the Trichoderma harzianum (HS-N-04) was subjected to silica gel column chromatography (CC) and eluted by a gradient of PE/EA and then EA/MeOH to generate six fractions (Fr. 1–6). Fr. 2 was further purified by using CC to generate five fractions (Fr. 2-1–2-5). Fr.2-2 was separated by normal phase silica gel column chromatography and purified by semi preparative HPLC (MeOH−H2O, 85%; 2 mL/min) to obtain 4 (10.0 mg). Fr.2-4 was separated by normal phase silica gel column chromatography and semipreparative HPLC to obtain 5 (2.4 mg). Fr.4 was separated into six fractions by Sephadex LH-20 eluting with MeOH gel column. Fr.4-2 was purified by semipreparative HPLC (MeOH−H2O, 70%; 2 mL/min) to yield 6 (5.3 mg). Fr.4-4 was purified by semipreparative HPLC (MeOH−H2O, 76%; 2 mL/min) to obtain 7 (5.0 mg).
5. Conclusions
This is the first systematic report on the phylogenetic diversity of fungi from mangrove A. ilicifolius. Four genera Verticillium, Neocosmospora, Valsa, and Pyrenochaeta, which were first isolated from mangroves, further enriched the diversity of mangrove fungi. Thirty-one strains of fungi displayed strong cytotoxicity to different cell lines, which was the important microbial resource for the discovery of cytotoxic compounds. Furthermore, by integrating cytotoxic activity-guided strategy and fingerprint analysis, a potent cytotoxic activity compound was quickly isolated from target active strains. This method, combining activity-guided strategy with fingerprint analysis, could improve the efficiency of discovering active compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20070432/s1, Table S1: Phylogenetic affiliations of cultivable fungi associated with A. ilicifolius. Figure S1: Phylogenetic tree of partial ITS gene sequences of mangrove-derived fungal strains. Reference sequences were downloaded from NCBI with the accession numbers indicated in parentheses. Figure S2: Phylogenetic tree of fungi in the order Hypocreales based on ITS gene sequence homology. Figure S3: The cytotoxicity relative inhibition rate data of the organic extracts in cancer cell lines. Figure S4. The fingerprint analysis of the organic extract of fungi from different species and genera. Figure S5. The fingerprint analysis of the organic extract of Penicillium sp. (HS-N-27).
Author Contributions
C.-F.W. contributed to isolation and identification of fungi, and manuscript preparation; J.M. and X.-X.H. contributed to related work of cytotoxic activity; Q.-Q.J., X.-Z.C. and L.C. contributed to fungal fermentation and chemical extraction. R.C. contributed to phylogenetic analysis. C.-L.S. contributed to NMR analysis and structure elucidation; C.-L.S., J.-Y.Z., X.-X.H. and M.-Y.W. were the project leader organizing and guiding the experiments and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (No. 202264001), the Program of National Natural Science Foundation of China (Nos. 41906090, 42006092, U1706210), Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education, Hainan Normal University (RDZH2021003 and RDZH2022002), the Research Fund of State Key Laboratory for Marine Corrosion and Protection of Luoyang Ship Material Research Institute (LSMRI) [No. KF190402], and the Taishan Scholars Program, China (No. tsqn20161010).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Acknowledgments
We thank Xiu-Li Zhang and Cong Wang at School of Medicine and Pharmacy, Ocean University of China for NMR test.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Jadimurthy, R.; Wang, L.; Sethi, G.; Garg, M.; Rangappa, K.S. Bacteria as a treasure house of secondary metabolites with anticancer potential. Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from Marine Sources:An Assessment of the Current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnell, O.J. Antitumor Tetrahydroisoquinoline Alkaloids from the Colonial Ascidian Ecteinascidia turbinate. J. Org. Chem. 1990, 55, 4509–4512. [Google Scholar] [CrossRef]
- Singleton, V.L.; Bohonos, N.; Ullstrup, A.J. Decumbin, a new compound from a species of Penicillium. Nature 1958, 181, 1072–1073. [Google Scholar] [CrossRef]
- Renault, L.; Guibert, B.; Cherfils, J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 2003, 426, 525–530. [Google Scholar] [CrossRef]
- Lu, X.X.; Jiang, Y.Y.; Wu, Y.W.; Chen, G.Y.; Shao, C.L.; Gu, Y.C.; Liu, M.; Wei, M.Y. Semi-Synthesis, Cytotoxic Evaluation, and Structure—Activity Relationships of Brefeldin a Derivatives with Antileukemia Activity. Mar. Drugs 2022, 20, 26. [Google Scholar] [CrossRef]
- Prieto-Dominguez, N.; Parnell, C.; Teng, Y. Drugging the small GTPase pathways in cancer treatment: Promises and challenges. Cells 2019, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Anadu, N.O.; Davisson, V.J.; Cushman, M. Synthesis and anticancer activity of Brefeldin A ester derivatives. J. Med. Chem. 2006, 49, 3897–3905. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Lead compounds from mangrove-associated microorganisms. Mar. Drugs 2018, 16, 319. [Google Scholar] [CrossRef] [Green Version]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Xiao, Q.; Li, Q.; Huang, J.; Long, L.; Huang, L. Phenylethanoid and aliphatic alcohol glycosides from Acanthus ilicifolius. Phytochemistry 2003, 63, 491–495. [Google Scholar] [CrossRef]
- Rutvi, P.; Nilay, P.; Krushil, P.; Meet, P.; Kunj, P.; Preeti, V.; Mamta, S. Acanthus ilicifolius: A true mangrove with biomedical potential. J. Pharm. Pharm. Sci. 2020, 9, 472–489. [Google Scholar] [CrossRef]
- Cai, Y.S.; Sun, J.Z.; Tang, Q.Q.; Fan, F.; Guo, Y.W. Acanthiline A, a pyrido[1,2-a]indole alkaloid from Chinese mangrove Acanthus ilicifolius. J. Asian. Nat. Prod. Res. 2018, 20, 1088–1092. [Google Scholar] [CrossRef]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Xu, W.F.; Wu, N.N.; Wu, Y.W.; Qi, Y.X.; Wei, M.Y.; Pineda, L.M.; Ng, M.G.; Spadafora, C.; Zheng, J.Y.; Lu, L.; et al. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022, 4, 88–97. [Google Scholar] [CrossRef]
- Hai, Y.; Cai, Z.M.; Li, P.D.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Trends of antimalarial marine natural products: Progresses, challenges and opportunities. Nat. Prod. Rep. 2022, 39, 969–990. [Google Scholar] [CrossRef]
- Guo, F.W.; Mou, X.F.; Qu, Y.; Wei, M.Y.; Chen, G.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Scalable total synthesis of (+)-aniduquinolone A and its acid-catalyzed rearrangement to aflaquinolones. Commun. Chem. 2022, 5, 35. [Google Scholar] [CrossRef]
- Darsih, C.; Prachyawarakorn, V.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic metabolites from the endophytic fungus Penicillium chermesinum: Discovery of a cysteine-targeted Michael acceptor as a pharmacophore for fragment-based drug discovery, bioconjugation and click reactions. RSC Adv. 2015, 5, 70595–70603. [Google Scholar] [CrossRef]
- Tang, X.X.; Liu, S.Z.; Yan, X.; Tang, B.W.; Fang, M.J.; Wang, X.M.; Wu, Z.; Qiu, Y.K. Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134. Mar. Drugs 2019, 17, 509. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.M.; Ma, C.C.; Li, T.; Wang, J. A new furan derivative from an endophytic Aspergillus flavus of Cephalotaxus fortunei. Nat. Prod. Res. 2016, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Yoshihira, K.; Natori, S.; Umeda, M. The structures of toxic metabolites of Aspergillus candidus. I. The compounds A and E, cytotoxic p-terphenyls. Chem. Pharm. Bull. 1976, 24, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Hamada, N. 2,3-dialkylchromones from mycobiont cultures of the lichen Graphis Scripta. Heterocycles 2000, 53, 1589–1593. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Z.; Feng, H.; Gan, Q.; Che, Q.; Zhu, T.; Gu, Q.; Han, B.; Li, D. Trichodermamides D–F, heterocyclic dipeptides with a highly functionalized 1, 2-oxazadecaline core isolated from the endophytic fungus Penicillium janthinellum HDN13-309. RSC Adv. 2017, 7, 48019–48024. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Diao, X.; Wang, T.; Chen, G.; Lin, Q.; Yang, X.; Xu, J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PLoS ONE 2018, 13, e0197359. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.Y.; Yang, K.L.; Li, J.; Wang, C.Y.; Shao, C.L. Phylogenetic diversity and antibacterial activity of culturable fungi derived from the Zoanthid Palythoa haddoni in the South China Sea. Mar. Biotechnol. 2015, 17, 99–109. [Google Scholar] [CrossRef]
- Qadri, M.; Rajput, R.; Abdin, M.Z.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. Diversity, molecular phylogeny, and bioactive potential of fungal endophytes associated with the Himalayan blue pine (Pinus wallichiana). Microb. Ecol. 2014, 67, 877–887. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paul, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).