The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota
Abstract
:1. Introduction
2. Results
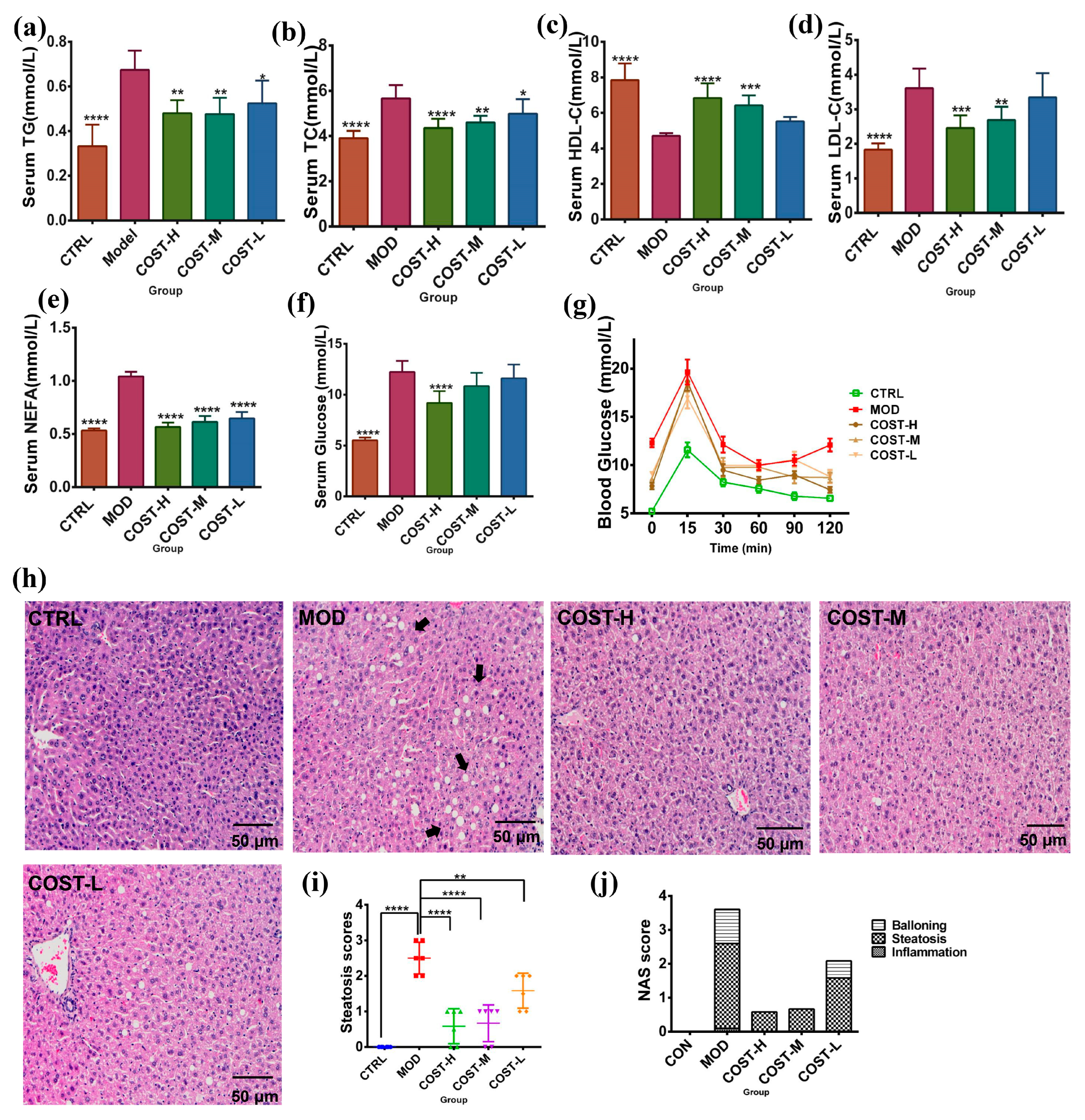
2.1. Effect of COST Intervention on Serum Lipid Levels and Liver Lipid Accumulation in Mice
2.2. Effect of COST Intervention on Intestinal Microflora Structure in Mice
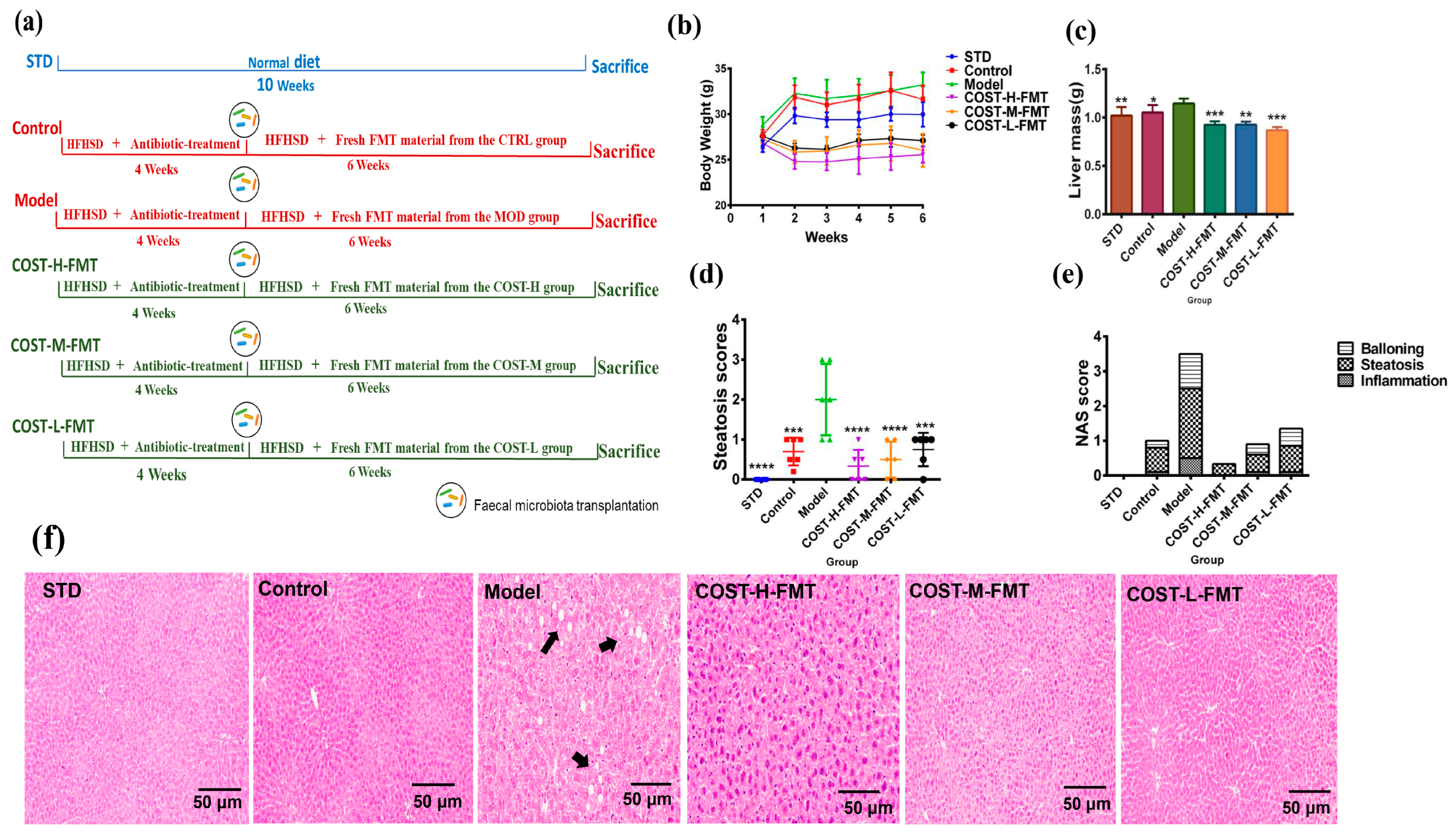
2.3. Effects of FMT on Body Weight and Liver in Mice
2.4. Effects of FMT on Adipose Tissue in Mice
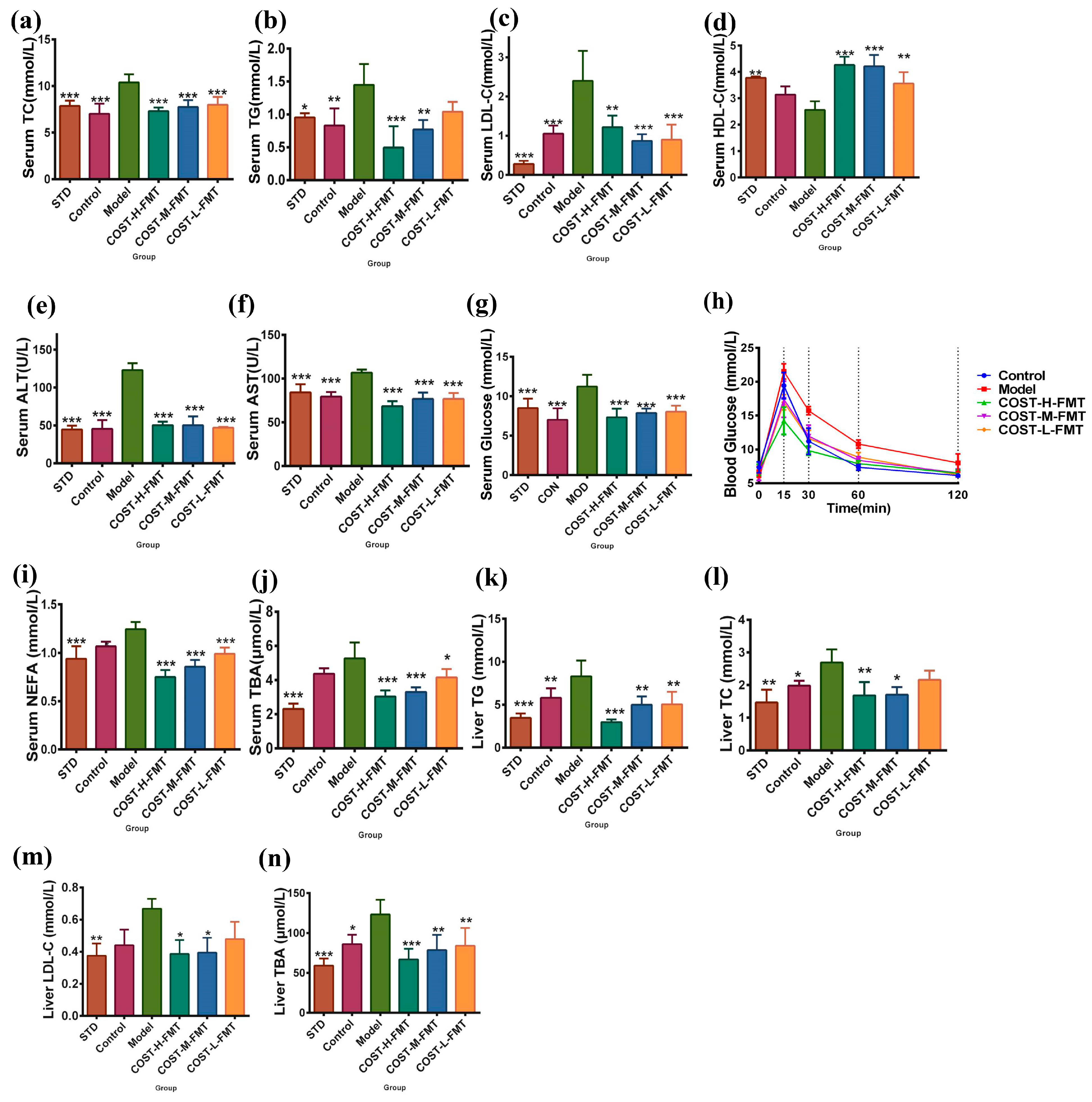
2.5. Effects of FMT on Serum and Liver Biochemical Indexes in Mice
2.6. Structural Characteristics of Intestinal Flora in Mice Treated with FMT
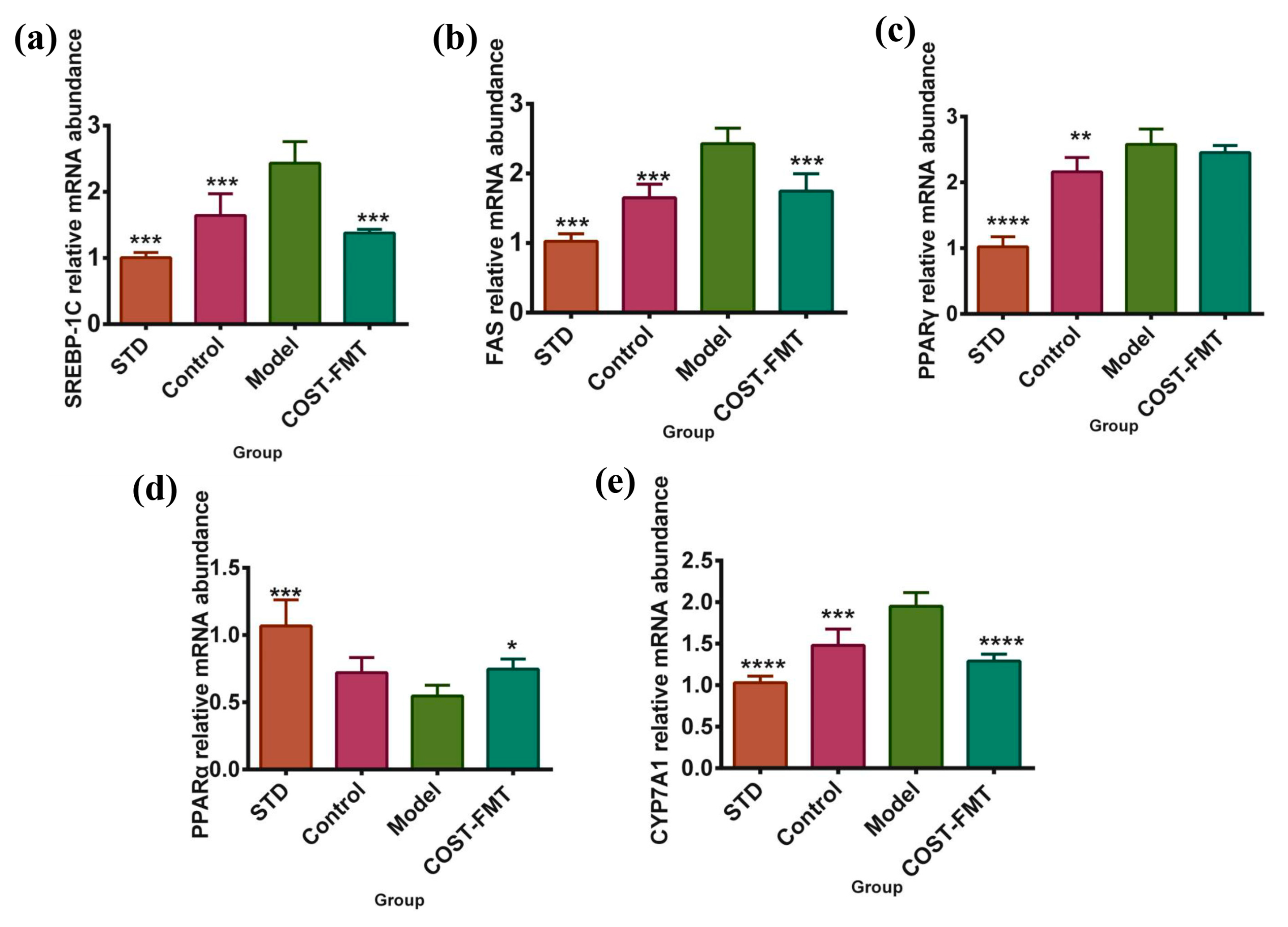
2.7. Effects of COST on Liver Lipid Metabolism-Related Gene Expression in FMT Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experimental Design
4.2. Extraction Method of Fecal Flora Liquid
4.3. Serum and Liver Index Analyses
4.4. Histological Analyses
4.5. Faecal Microbiota 16S rRNA Analysis
4.6. Quantitative RT–PCR
4.7. Glucose Tolerance Test (OGTT)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, B.J.; Perrot, N.; Couture, P. Does Lifestyle Contribute to Disease Severity in Patients with Inherited Lipid Disorders? Curr. Opin. Lipidol. 2017, 28, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, T.A.; Wawro, N.; Breuninger, J.; Reitmeier, S.; Clavel, T.; Six-Merker, J.; Pestoni, G.; Rohrmann, S.; Rathmann, W.; Peters, A.; et al. Associations between Habitual Diet, Metabolic Disease, and the Gut Microbiota Using Latent Dirichlet Allocation. Microbiome 2021, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.E.; Jasarevic, E.; Howard, C.D.; Bale, T.L. It’s the Fiber, Not the Fat: Significant Effects of Dietary Challenge on the Gut Microbiome. Microbiome 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wen, B.; Zhu, K.; Luo, Y.; Li, J.; Li, Y.; Lin, H.; Huang, J.; Liu, Z. Antibiotics-Induced Perturbations in Gut Microbial Diversity Influence Metabolic Phenotypes in a Murine Model of High-Fat Diet-Induced Obesity. Appl. Microbiol. Biotechnol. 2019, 103, 5269–5283. [Google Scholar] [CrossRef]
- He, Y.; You, C. The Potential Role of Gut Microbiota in the Prevention and Treatment of Lipid Metabolism Disorders. Int. J. Endocrinol. 2020, 2020, 8601796. [Google Scholar] [CrossRef]
- Coker, O.O.; Liu, C.; Wu, W.K.K.; Wong, S.H.; Jia, W.; Sung, J.J.Y.; Yu, J. Altered Gut Metabolites and Microbiota Interactions Are Implicated in Colorectal Carcinogenesis and Can Be Non-Invasive Diagnostic Biomarkers. Microbiome 2022, 10, 35. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Chitosan Oligosaccharides Packaged into Rat Adipose Mesenchymal Stem Cells-Derived Extracellular Vesicles Facilitating Cartilage Injury Repair and Alleviating Osteoarthritis. J. Nanobiotechnol. 2021, 19, 343. [Google Scholar] [CrossRef]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and Its Oligosaccharides, a Promising Option for Sustainable Crop Production-a Review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Chang, Q.; Guo, G.; Lan, R. Effects of Chitosan Oligosaccharides on Intestinal Oxidative Stress and Inflammation Response in Heat Stressed Rats. Exp. Anim. 2021, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.T.; Varela, J.E. Bariatric Surgery for Obesity and Metabolic Disorders: State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; Network, N.C.R. Nonalcoholic Fatty Liver Disease (Nafld) Activity Score and the Histopathologic Diagnosis in Nafld: Distinct Clinicopathologic Meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Hjelkrem, M.; Stauch, C.; Shaw, J.; Harrison, S.A. Validation of the Non-Alcoholic Fatty Liver Disease Activity Score. Aliment. Pharmacol. Ther. 2011, 34, 214–218. [Google Scholar] [CrossRef]
- Wang, Z.; Koonen, D.; Hofker, M.; Fu, J. Gut Microbiome and Lipid Metabolism: From Associations to Mechanisms. Curr. Opin. Lipidol. 2016, 27, 216–224. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The Role of the Microbiome in Nafld and Nash. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of Intestinal Lipid Metabolism: Current Concepts and Relevance to Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the Microbiome in Energy Regulation and Metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Lin, K.; Li, K.; Deng, X.; Li, C. Reshaped Fecal Gut Microbiota Composition by the Intake of High Molecular Weight Persimmon Tannin in Normal and High-Cholesterol Diet-Fed Rats. Food Funct. 2018, 9, 541–551. [Google Scholar] [CrossRef]
- Zhang, F.; Su, H.; Song, M.; Zheng, J.; Liu, F.; Yuan, C.; Fu, Q.; Chen, S.; Zhu, X.; Wang, L.; et al. Calcium Supplementation Alleviates High-Fat Diet-Induced Estrous Cycle Irregularity and Subfertility Associated with Concomitantly Enhanced Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. J. Agric. Food Chem. 2019, 67, 7073–7081. [Google Scholar] [CrossRef] [PubMed]
- Montanari, T.; Poscic, N.; Colitti, M. Factors Involved in White-to-Brown Adipose Tissue Conversion and in Thermogenesis: A Review. Obes. Rev. 2017, 18, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial Adaptations and Dysfunctions in Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Gehrmann, W.; Lenzen, S. Peroxisome-Generated Hydrogen Peroxide as Important Mediator of Lipotoxicity in Insulin-Producing Cells. Diabetes 2011, 60, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Meadows, V.; Kennedy, L.; Ekser, B.; Kyritsi, K.; Kundu, D.; Zhou, T.; Chen, L.; Pham, L.; Wu, N.; Demieville, J.; et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis through Farnesoid X Receptor Signaling. Hepatology 2021, 74, 2684–2698. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, Z.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Effect of Different Bile Acids on the Intestine through Enterohepatic Circulation Based on Fxr. Gut Microbes 2021, 13, 1949095. [Google Scholar] [CrossRef]
- Xia, F.; Xiang, S.; Chen, Z.; Song, L.; Li, Y.; Liao, Z.; Ge, B.; Zhou, B. The Probiotic Effects of Ab23a on High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice May Be Associated with Suppressing the Serum Levels of Lipopolysaccharides and Branched-Chain Amino Acids. Arch. Biochem. Biophys. 2021, 714, 109080. [Google Scholar] [CrossRef]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide Is a Prebiotic That Reduces Desulfovibrio Bacteria, Increases Cecal Short-Chain Fatty Acids, and Is Anti-Inflammatory in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.K.; Zhai, Q.; Chen, W. Roles of Intestinal Bacteroides in Human Health and Diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 3518–3536. [Google Scholar] [CrossRef] [PubMed]
- Truax, A.D.; Chen, L.; Tam, J.W.; Cheng, N.; Guo, H.; Koblansky, A.A.; Chou, W.C.; Wilson, J.E.; Brickey, W.J.; Petrucelli, A.; et al. The Inhibitory Innate Immune Sensor Nlrp12 Maintains a Threshold against Obesity by Regulating Gut Microbiota Homeostasis. Cell Host Microbe 2018, 24, 364–378.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribo, S.; Sánchez-Infantes, D.; Martinez-Guino, L.; García-Mantrana, I.; Ramon-Krauel, M.; Tondo, M.; Arning, E.; Nofrarías, M.; Osorio-Conles, Ó.; Fernández-Pérez, A.; et al. Akkermansiaincreasing Breast Milk Betaine Modulates Abundance in Mammalian Neonates and Improves Long-Term Metabolic Health. Sci. Transl. Med. 2021, 13, eabb0322. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A Next Generation Probiotic, Akkermansia Muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium Ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, e2000745. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.; Deng, X.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota. Mar. Drugs 2022, 20, 444. https://doi.org/10.3390/md20070444
You H, Deng X, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota. Marine Drugs. 2022; 20(7):444. https://doi.org/10.3390/md20070444
Chicago/Turabian StyleYou, Huimin, Xiaoyi Deng, Yan Bai, Jincan He, Hua Cao, Qishi Che, Jiao Guo, and Zhengquan Su. 2022. "The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota" Marine Drugs 20, no. 7: 444. https://doi.org/10.3390/md20070444
APA StyleYou, H., Deng, X., Bai, Y., He, J., Cao, H., Che, Q., Guo, J., & Su, Z. (2022). The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota. Marine Drugs, 20(7), 444. https://doi.org/10.3390/md20070444





