Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression
Abstract
:1. Introduction
2. Results
2.1. Effect of SCSP on Immune Organ Indices
2.2. Effect of SCSP on Cytokine Production
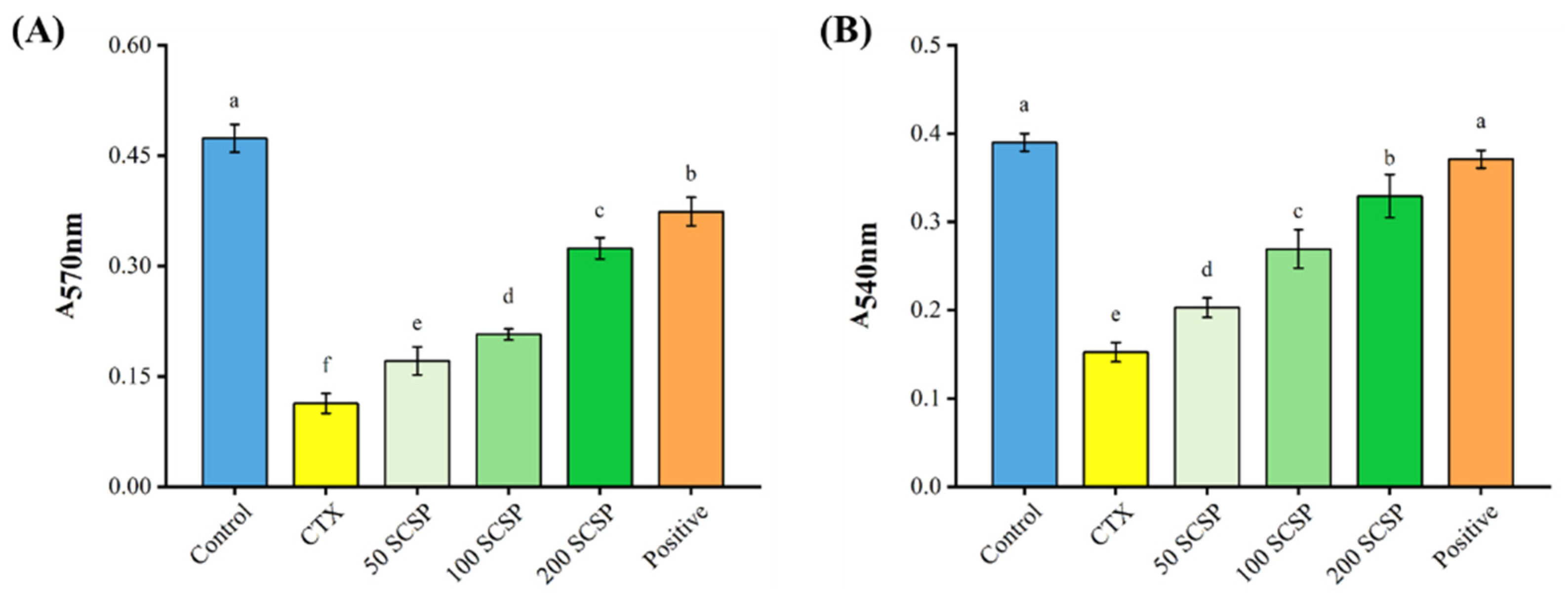
2.3. Effects of SCSP on Cellular Immunity
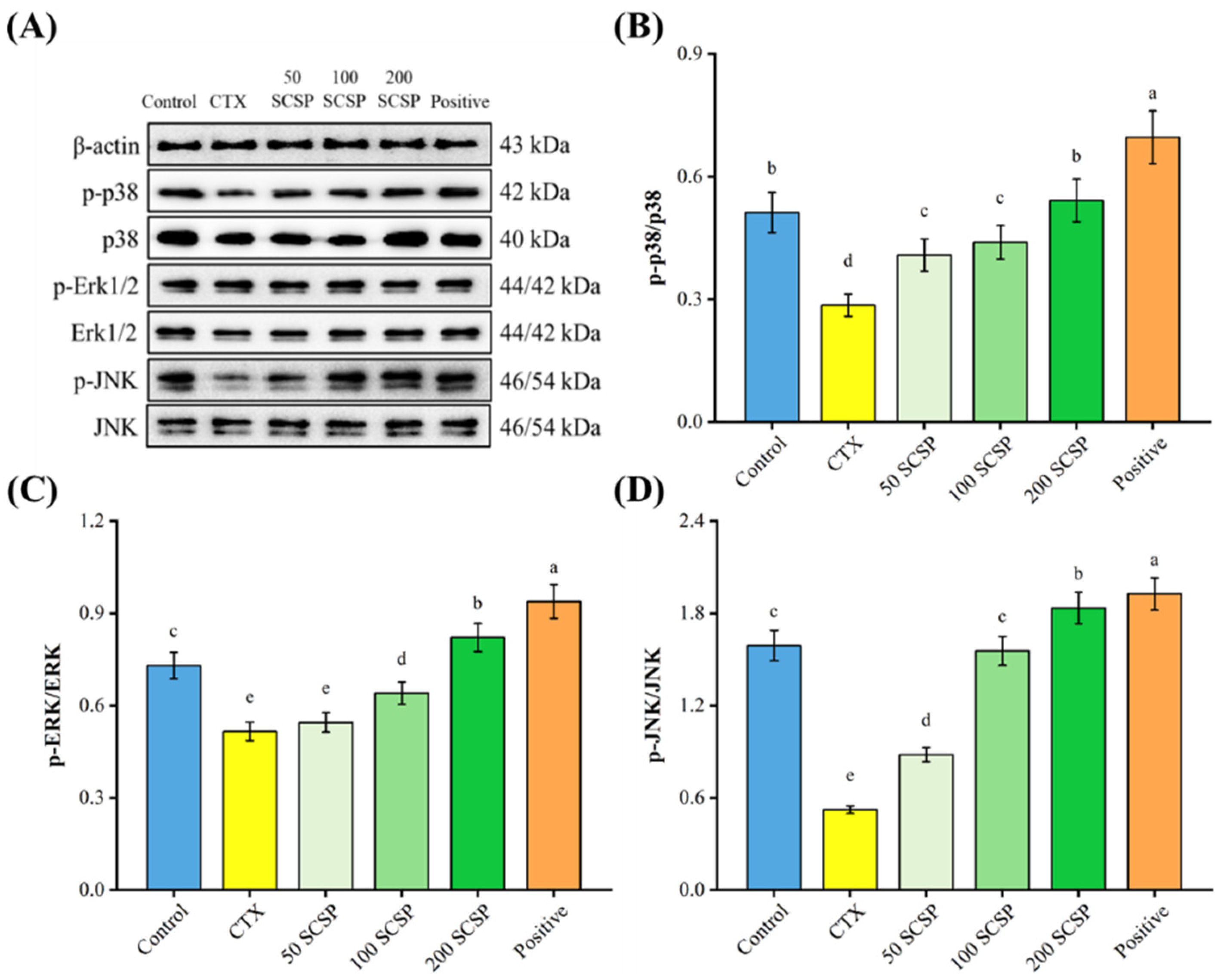
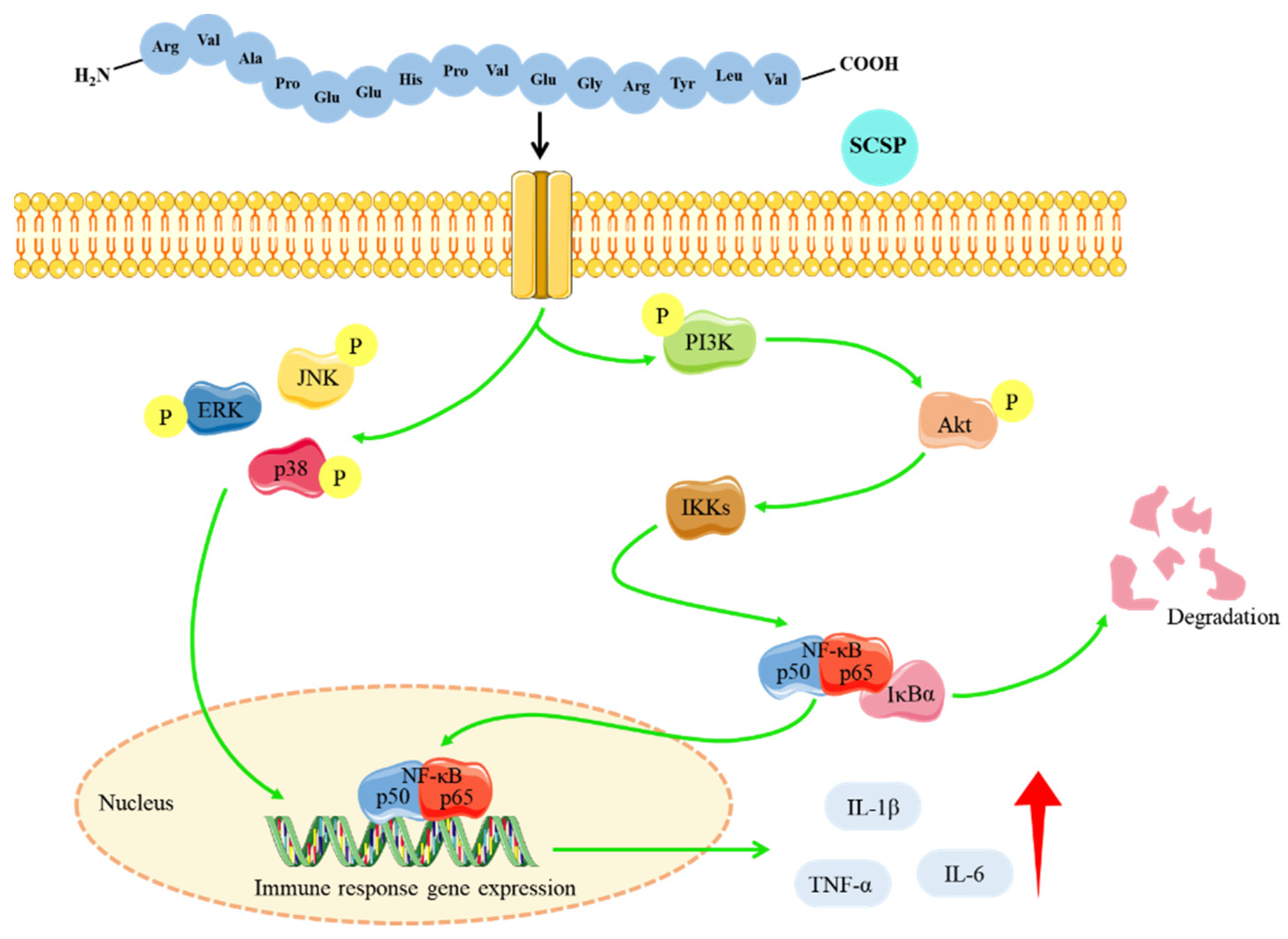
2.4. Western Blot Analysis
2.5. Expression of NF-κB p65 in the Spleen
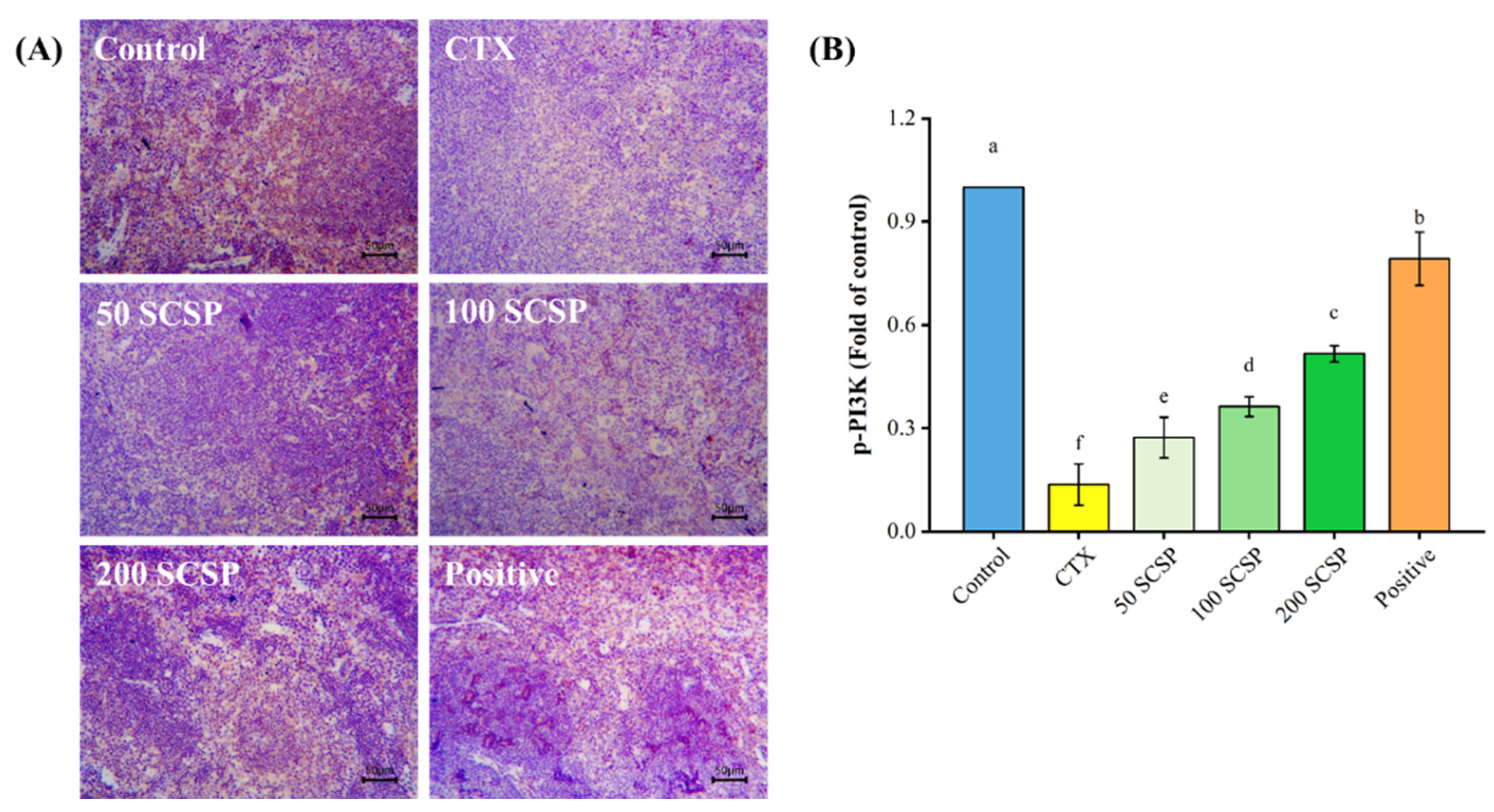
2.6. Expression of p-PI3K in Spleen
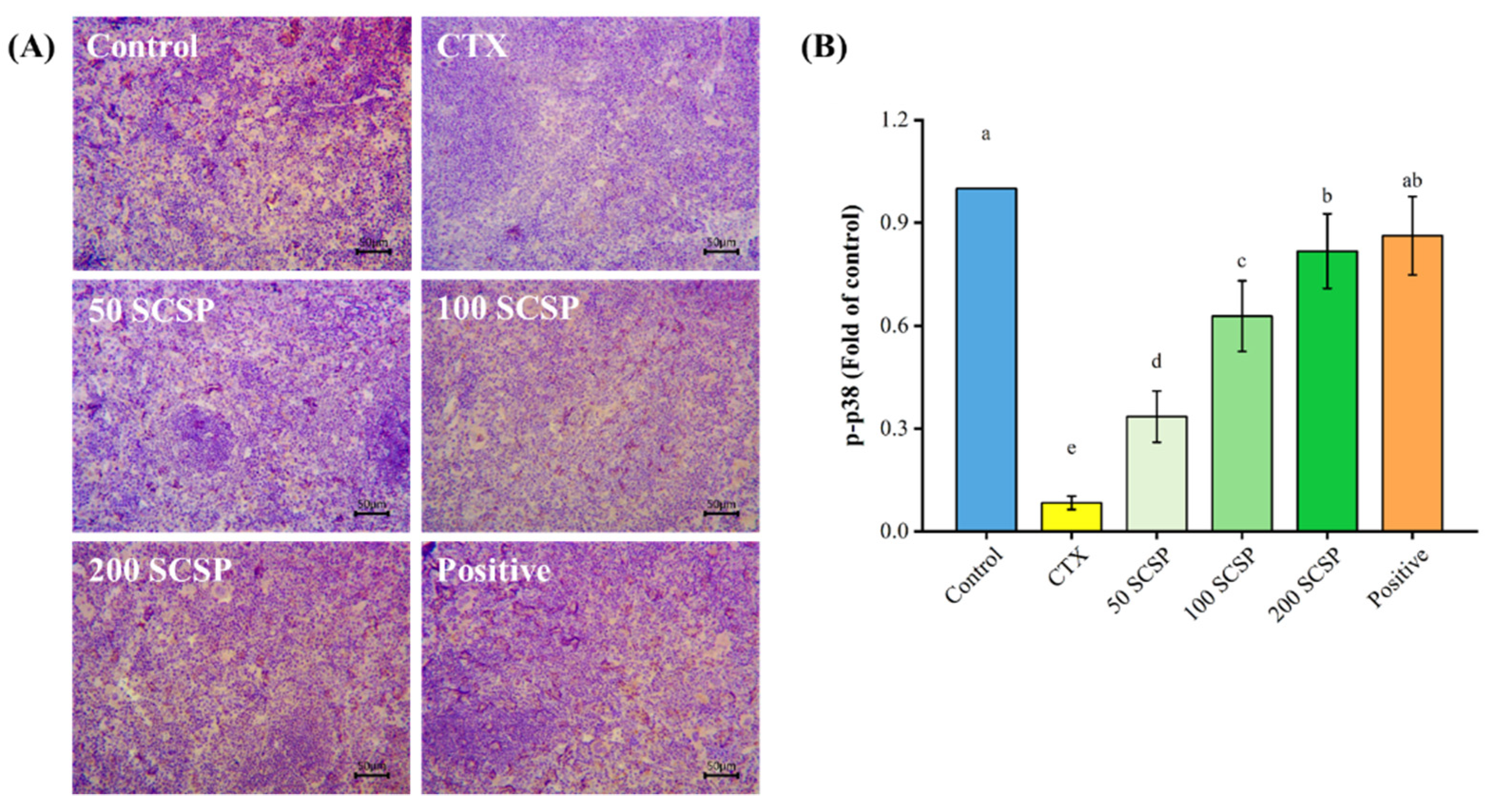
2.7. Expression of p-p38 in the Spleen
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals and Treatment
4.3. Immune Organ Indices
4.4. Cytokines Assays in Serum
4.5. Splenic Lymphocyte Proliferation Assay
4.6. Peritoneal Macrophage Proliferation Assay
4.7. Macrophage Phagocytic Capacity
4.8. Western Blotting
4.9. Immunohistochemical Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
International Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ploegh, H.L. Logic of the immune system. Cancer Immunol. Res. 2013, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.; Almeida, M.E.F. Biochemical and immunological changes in obesity. Arch. Biochem. Biophys. 2021, 708, 108951. [Google Scholar] [CrossRef] [PubMed]
- Ambrée, O.; Ruland, C.; Scheu, S.; Arolt, V.; Alferink, J. Alterations of the innate immune system in susceptibility and resilience after social defeat stress. Front. Behav. Neurosci. 2018, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M.; Kigar, S.L. Contributions of the adaptive immune system to mood regulation: Mechanisms and pathways of neuroimmune interactions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 49–57. [Google Scholar] [CrossRef]
- Filgueira, T.O.; Castoldi, A.; Santos, L.E.R.; de Amorim, G.J.; de Sousa Fernandes, M.S.; Anastácio, W.d.L.d.N.; Campos, E.Z.; Santos, T.M.; Souto, F.O. The relevance of a physical active lifestyle and physical fitness on immune defense: Mitigating disease burden, with focus on COVID-19 consequences. Front. Immunol. 2021, 12, 587146. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Lin, M.; Zhu, J.; Chen, W.R. Recent advances in immunotherapy, immunoadjuvant, and nanomaterial-based combination immunotherapy. Coord. Chem. Rev. 2021, 442, 214009. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, J.; Xiong, Y.; Song, X.; Li, C.; He, Z. Development of broad-spectrum antiviral agents—Inspiration from immunomodulatory natural products. Viruses 2021, 13, 1257. [Google Scholar] [CrossRef]
- Nuzzo, G.; Senese, G.; Gallo, C.; Albiani, F.; Romano, L.; d’Ippolito, G.; Manzo, E.; Fontana, A. Antitumor potential of immunomodulatory natural products. Mar. Drugs 2022, 20, 386. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.-L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [Green Version]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Xing, H.; Tong, M.; Jiang, N.; Zhang, X.; Hu, H.; Pan, H.; Li, D. Antitumour bioactive peptides isolated from marine organisms. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1077–1082. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Chen, Y.; Ding, G. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2020, 68, 103888. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Ren, D.; Wang, M.; Shen, M.; Liu, C.; Liu, W.; Min, W.; Liu, J. In vivo assessment of immunomodulatory activity of hydrolysed peptides from Corylus heterophylla Fisch. J. Sci. Food Agric. 2016, 96, 3508–3514. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef]
- Cai, B.; Chen, H.; Wan, P.; Luo, L.; Ye, Z.; Huang, J.; Chen, D.; Pan, J. Isolation and identification of immunomodulatory peptides from the protein hydrolysate of tuna trimmings (Thunnas albacares). LWT 2022, 164, 113614. [Google Scholar] [CrossRef]
- Xu, B.; Ye, L.; Tang, Y.; Zheng, J.; Tian, X.; Yang, Y.; Yang, Z. Preparation and purification of an immunoregulatory peptide from Stolephorus chinensis of the East Sea of China. Process Biochem. 2020, 98, 151–159. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by Cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, M. Advances in immunosuppressive agents based on signal pathway. Front. Pharmacol. 2022, 13, 917162. [Google Scholar] [CrossRef]
- Arakelyan, A.; Nersisyan, L.; Poghosyan, D.; Khondkaryan, L.; Hakobyan, A.; Löffler-Wirth, H.; Melanitou, E.; Binder, H. Autoimmunity and autoinflammation: A systems view on signaling pathway dysregulation profiles. PLoS ONE 2017, 12, e0187572. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad-S, S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1886844. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, S.; Shen, M.; Chen, Y.; Yu, Q.; Li, Z.; Xie, J. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-κB and PI3K-Akt signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zeng, Y.; Tian, H.; Zhang, Z.; Zhang, H.; Huang, F.; Yu, F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. J. Funct. Foods 2021, 83, 104562. [Google Scholar] [CrossRef]
- Yao, L.; Yang, P.; Luo, W.; Li, S.; Wu, Y.; Cai, N.; Bi, D.; Li, H.; Han, Q.; Xu, X. Macrophage-stimulating activity of European eel (Anguilla anguilla) peptides in RAW264.7 cells mediated via NF-κB and MAPK signaling pathways. Food Funct. 2020, 11, 10968–10978. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Pan, L.; Wu, H.; Zhang, L.; Zhang, Y.; Zhang, Y.; Yang, J.; Lin, Z.; Zhang, M. Isolation, identification, and immunomodulatory mechanism of peptides from Lepidium meyenii (maca) protein hydrolysate. J. Agric. Food Chem. 2022, 70, 4328–4341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Zhao, Q.; Tian, D.; Tang, Y. Protective effects of pentadecapeptide derived from Cyclaina sinensis against cyclophosphamide-induced hepatotoxicity. Biochem. Biophys. Res. Commun. 2019, 520, 392–398. [Google Scholar] [CrossRef]
- Talmadge, J.E. Natural product derived immune-regulatory agents. Int. Immunopharmacol. 2016, 37, 5–15. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.-C.; He, S.-B.; Zhang, X.-F.; Ling, Y.-H.; Li, X.-Y.; Zhang, H.; Hou, D.-D. The immunoenhancement effects of sea buckthorn pulp oil in cyclophosphamide-induced immunosuppressed mice. Food Funct. 2021, 12, 7954–7963. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.; Li, H.; Cao, M.; Li, W.; Liu, X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 6322–6334. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory activity of carboxymethyl pachymaran on immunosuppressed mice induced by cyclophosphamide. Molecules 2021, 26, 5733. [Google Scholar] [CrossRef]
- Wang, S.; Huang, S.; Ye, Q.; Zeng, X.; Yu, H.; Qi, D.; Qiao, S. Prevention of cyclophosphamide-induced immunosuppression in mice with the antimicrobial peptide sublancin. J. Immunol. Res. 2018, 2018, 4353580. [Google Scholar] [CrossRef]
- Khan, A.I.; Rehman, A.U.; Farooqui, N.A.; Siddiqui, N.Z.; Ayub, Q.; Ramzan, M.N.; Zexu, W.; Zhang, X.; Yu, Y.; Xin, Y.; et al. Shrimp peptide hydrolysate modulates the immune response in cyclophosphamide immunosuppressed mice model. J. Food Biochem. 2022, e14251. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, X.; Yu, Z.; Wang, F.; Zhang, Z.; He, K.; Tian, H.; Yu, F. Immune enhancement and antioxidant effects of low molecular-weight peptides derived from Nibea japonica muscles on immune-deficient mice induced by cyclophosphamide. Process Biochem. 2021, 102, 42–50. [Google Scholar] [CrossRef]
- Li, M.-Z.; Huang, X.-J.; Hu, J.-L.; Cui, S.W.; Xie, M.-Y.; Nie, S.-P. The protective effects against cyclophosphamide (CTX)-induced immunosuppression of three glucomannans. Food Hydrocoll. 2020, 100, 105445. [Google Scholar] [CrossRef]
- Niu, Y.; Dong, J.; Jiang, H.; Wang, J.; Liu, Z.; Ma, C.; Kang, W. Effects of polysaccharide from Malus halliana Koehne flowers in cyclophosphamide-induced immunosuppression and oxidative stress on mice. Oxidative Med. Cell. Longev. 2020, 2020, 1603735. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, M.; Cai, X.; Jia, L.; Wang, S. Investigation on activation in RAW264.7 macrophage cells and protection in cyclophosphamide-treated mice of Pseudostellaria heterophylla protein hydrolysate. Food Chem. Toxicol. 2019, 134, 110816. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, J.; Jia, G.; Li, Z.; Xiong, H. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ. Cytokine 2022, 154, 155888. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Wei, X.; Shi, M.; Fan, P.; Xie, W.; Zhang, Y.; Xu, N. Aloin suppresses lipopolysaccharide-induced inflammatory response and apoptosis by inhibiting the activation of NF-κB. Molecules 2018, 23, 517. [Google Scholar] [CrossRef]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the borders of autoimmunity and inflammation. Front. Immunol. 2021, 12, 3169. [Google Scholar] [CrossRef]
- Caforio, M.; de Billy, E.; De Angelis, B.; Iacovelli, S.; Quintarelli, C.; Paganelli, V.; Folgiero, V. PI3K/Akt pathway: The indestructible role of a vintage target as a support to the most recent immunotherapeutic approaches. Cancers 2021, 13, 4040. [Google Scholar] [CrossRef]
- Yang, D.; Yang, L.; Cai, J.; Li, H.; Xing, Z.; Hou, Y. Phosphoinositide 3-kinase/Akt and its related signaling pathways in the regulation of tumor-associated macrophages polarization. Mol. Cell. Biochem. 2022. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Chen, Y.; Wang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The immunomodulatory effects of active ingredients from Nigella sativa in RAW264.7 cells through NF-κB/MAPK signaling pathways. Front. Nutr. 2022, 9, 899797. [Google Scholar] [CrossRef]
- Um, Y.; Eo, H.J.; Kim, H.J.; Kim, K.; Jeon, K.S.; Jeong, J.B. Wild simulated ginseng activates mouse macrophage, RAW264.7 cells through TRL2/4-dependent activation of MAPK, NF-κB and PI3K/AKT pathways. J. Ethnopharmacol. 2020, 263, 113218. [Google Scholar] [CrossRef]
- Xu, D.; Lin, F.; Zhu, X.Y.; Liu, W.Y.; Chen, X.W.; Feng, J.Q.; Fan, A.Q.; Cai, M.Y.; Xu, Y.J. Immunomodulatory effect of oyster peptide on immunosuppressed mice. J. Peking Univ. (Health Sci.) 2016, 48, 392–397. [Google Scholar]
- Tang, Y.P.; Pu, Q.Y.; Zhao, Q.L.; Zhou, Y.F.; Jiang, X.X.; Han, T. Effects of fucoidan isolated from Laminaria japonica on immune response and gut microbiota in cyclophosphamide-treated mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- Han, L.R.; Lei, H.N.; Tian, Z.W.; Wang, X.; Cheng, D.; Wang, C.L. The immunomodulatory activity and mechanism of docosahexenoic acid (DHA) on immunosuppressive mice models. Food Funct. 2018, 9, 3254. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Jiang, X.-X.; Zhao, Q.-L.; Ye, H.-W.; Lin, Y.; Huang, J.; Tang, Y.-P. Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression. Mar. Drugs 2022, 20, 560. https://doi.org/10.3390/md20090560
Zhao R, Jiang X-X, Zhao Q-L, Ye H-W, Lin Y, Huang J, Tang Y-P. Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression. Marine Drugs. 2022; 20(9):560. https://doi.org/10.3390/md20090560
Chicago/Turabian StyleZhao, Rui, Xiao-Xia Jiang, Qiao-Ling Zhao, Han-Wei Ye, Yi Lin, Ju Huang, and Yun-Ping Tang. 2022. "Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression" Marine Drugs 20, no. 9: 560. https://doi.org/10.3390/md20090560
APA StyleZhao, R., Jiang, X. -X., Zhao, Q. -L., Ye, H. -W., Lin, Y., Huang, J., & Tang, Y. -P. (2022). Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression. Marine Drugs, 20(9), 560. https://doi.org/10.3390/md20090560





