Evaluation of Haloferax mediterranei Strain R4 Capabilities for Cadmium Removal from Brines
Abstract
:1. Introduction
2. Results
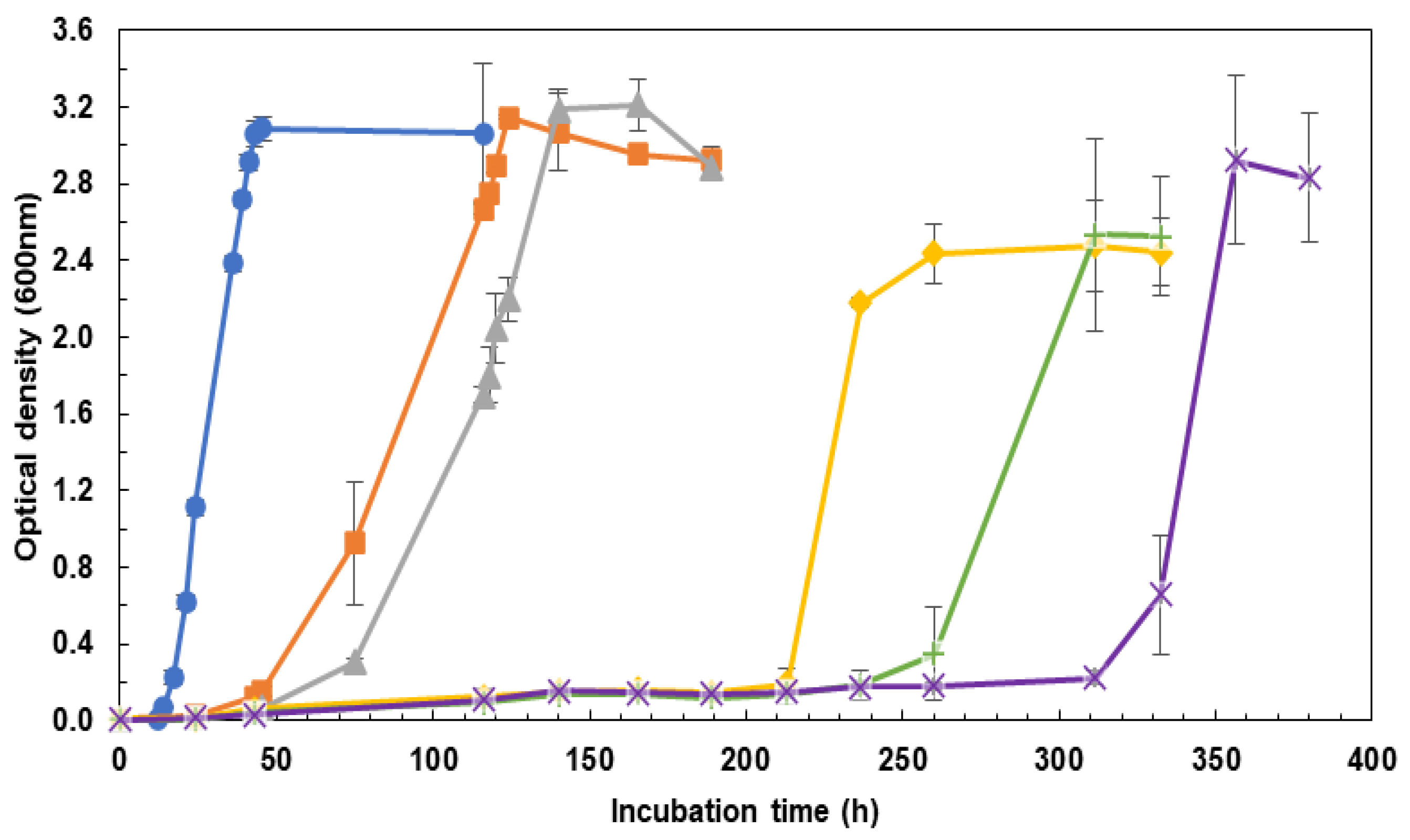
2.1. Influence of the Incubation Medium on Cell Growth Rate: CM vs. DM
2.2. Cd Accumulation by Haloferax mediterranei in DM and Assessment of Bioremediation Capacity
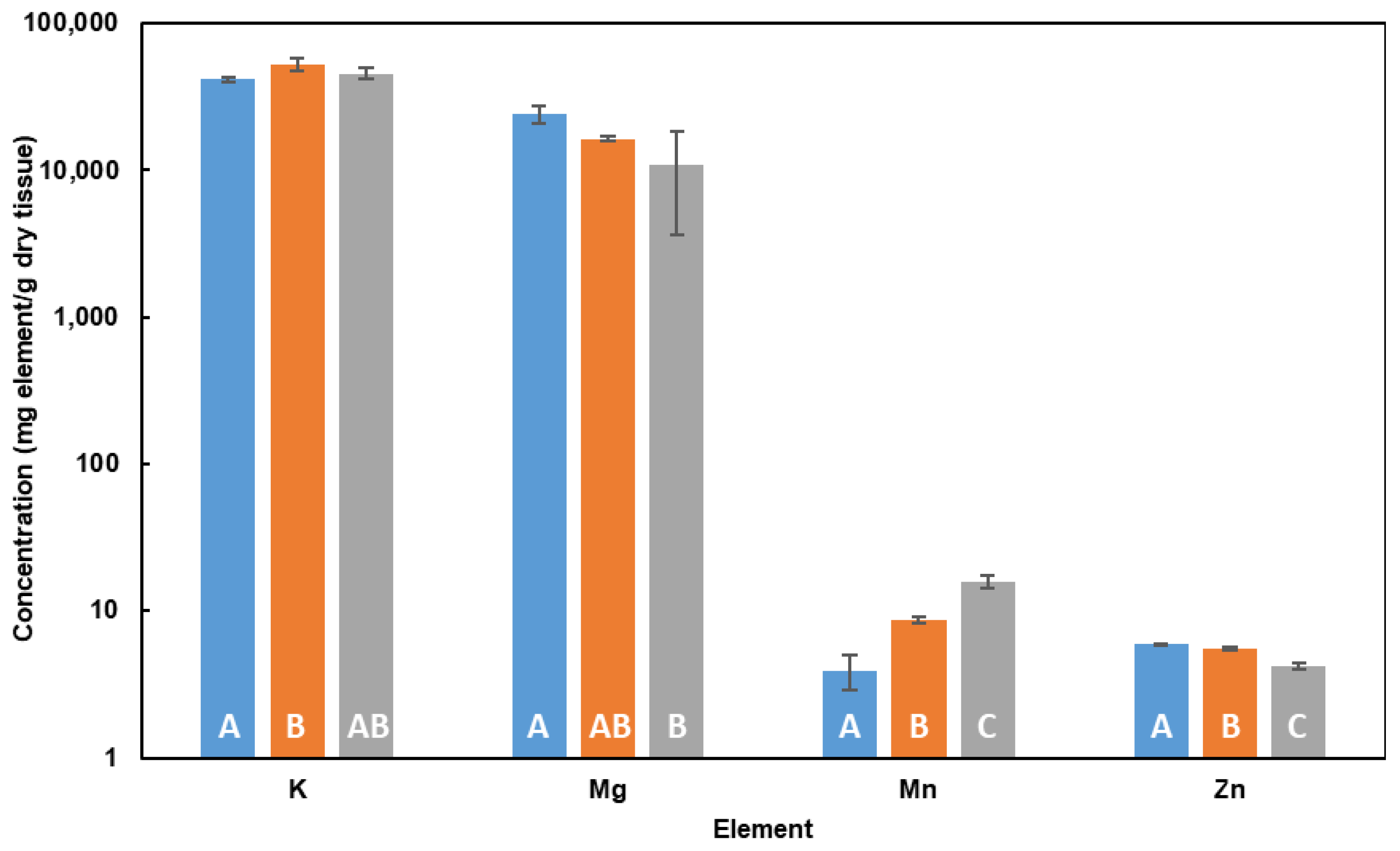
2.3. Influence of Cd in Cell Elemental Profile in DM
3. Discussion
3.1. Influence of the Incubation Medium on Cell Growth Rate: CM vs. DM
3.2. Cd Accumulation by Haloferax mediterranei in DM
3.3. Influence of Cd in Cell Elemental Profile in DM
4. Materials and Methods
4.1. Reagents
4.2. Cd Resistance and Growth Kinetics
4.3. Sample Treatment for Elemental Analysis
4.4. Elemental Analysis
4.5. Data Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, P.; Sharma, R. Microorganism as a tool of bioremediation technology for cleaning environment: A review. Proc. Int. Acad. 2014, 4, 1–6. [Google Scholar]
- Kaushik, S.; Alatawi, A.; Djiwanti, S.R.; Pande, A.; Skotti, E.; Soni, V. Potential of Extremophiles for Bioremediation. In Microbial Rejuvenation of Polluted Environment, 1st ed.; Panpatte, D.G., Jhala, Y.K., Eds.; Springer Nature: Singapore, 2021; Volume 25, pp. 293–328. [Google Scholar] [CrossRef]
- Hussein, H.; Farag, S.; Kandil, K.; Moawad, H. Tolerance and uptake of heavy metals by Pseudomonas. Process. Biochem. 2005, 40, 955–961. [Google Scholar] [CrossRef]
- Volesky, B. Advances in biosorption of metals: Selection of biomass types. FEMS Microbiol. Rev. 1994, 14, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy Metal Pollution: Source, Impact, and Remedies. In Biomanagement of Metal-Contaminated Soils, 1st ed.; Khan, M.S., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer Nature: Singapore, 2011; Volume 20, pp. 1–28. [Google Scholar] [CrossRef]
- Tyagi, B.; Kumar, N. Bioremediation: Principles and applications in environmental management. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–28. [Google Scholar] [CrossRef]
- Perpetuo, E.A.; Souza, C.B.; Nascimento, C.A.O. Engineering Bacteria for Bioremediation. Curr. Opin. Biotechnol. 2011, 11, 262–270. [Google Scholar] [CrossRef]
- Bernard, A. Renal dysfunction induced by cadmium: Biomarkers of critical effects. BioMetals 2004, 17, 519–523. [Google Scholar] [CrossRef]
- Cheng, K.; Tian, H.Z.; Zhao, D.; Lu, L.; Wang, Y.; Chen, J.; Liu, X.G.; Jia, W.X.; Huang, Z. Atmospheric emission inventory of cadmium from anthropogenic sources. Int. J. Environ. Sci. Technol. 2013, 11, 605–616. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Exposure to Cadmium: A Major Public Health Concern. Available online: http://www.who.int/ipcs/features/cadmium.pdf (accessed on 13 March 2021).
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef]
- Angeletti, R.; Binato, G.; Guidotti, M.; Morelli, S.; Pastorelli, A.A.; Sagratella, E.; Ciardullo, S.; Stacchini, P. Cadmium bioaccumulation in Mediterranean spider crab (Maya squinado): Human consumption and health implications for exposure in Italian population. Chemosphere 2014, 100, 83–88. [Google Scholar] [CrossRef]
- Souza Arroyo, V.; Martínez Flores, K.; Ortiz, L.; Gómez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. Liver and Cadmium Toxicity. J. Drug Metab. Toxicol. 2012, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Nájera-Fernández, C.; Zafrilla, B.; Bonete, M.J.; Martínez-Espinosa, R.M. Role of the denitrifying Haloarchaea in the treatment of nitrite-brines. Int. Microbiol. 2012, 15, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Espinosa, R.M. Microorganisms and Their Metabolic Capabilities in the Context of the Biogeochemical Nitrogen Cycle at Extreme Environments. Int. J. Mol. Sci. 2020, 21, 4228. [Google Scholar] [CrossRef] [PubMed]
- Matarredona, L.; Camacho, M.; Zafrilla, B.; Bravo-Barrales, G.; Esclapez, J.; Bonete, M.J. The Survival of Haloferax mediterranei under Stressful Conditions. Microorganisms 2021, 9, 336. [Google Scholar] [CrossRef]
- Llorca, M.G.; Martínez-Espinosa, R.M. Assessment of Haloferax mediterranei Genome in Search of Copper-Molecular Machinery With Potential Applications for Bioremediation. Front. Microbiol. 2022, 13, 895296. [Google Scholar] [CrossRef]
- Rodríguez-Valera, F.; Ruiz-Berraquero, F.; Ramos-Cormenzana, A. Isolation of Extremely Halophilic Bacteria Able to Grow in Defined Inorganic Media with Single Carbon Sources. J. Gen. Microbiol. 1980, 119, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Espinosa, R.M. Fisiología de la Asimilación de Nitrógeno en “Haloferax Mediterranei”. Purificación y Caracterización de Nitrato y Nitrito Reductasas Asimilativas. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2003. [Google Scholar]
- Mojica, F.J.M.; Rodríguez-Valera, F. The Discovery of CRISPR in archaea and bacteria. FEBS J. 2016, 283, 3162–3169. [Google Scholar] [CrossRef] [Green Version]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Giani, M.; Montero-Lobato, Z.; Garbayo, I.; Vílchez, C.; Vega, J.M.; Martínez-Espinosa, R.M. Haloferax mediterranei cells as C50 Carotenoid Factories. Mar. Drugs 2021, 19, 100. [Google Scholar] [CrossRef]
- Rodríguez-Valera, F.; Lillo, J.A.G.; Antón, J.; Meseguer, I. Biopolymer production by Haloferax mediterranei. In General and Applied Aspects of Halophilic Microorganisms; Rodríguez-Valera, F., Ed.; Springer: Boston, MA, USA, 1991; Volume 201, pp. 373–380. [Google Scholar]
- Bonete, M.J.; Bautista, V.; Esclapez, J.; García-Bonete, M.J.; Pire, C.; Camacho, M.; Torregrosa-Crespo, J.; Martínez-Espinosa, R.M. New Uses of Haloarchaeal Species in Bioremediation Processes. In Advances in Bioremediation of Wastewater and Polluted Soil; Shiomi, N., Ed.; Intech: London, UK, 2015; pp. 23–49. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Espinosa, R.M.; Richardson, D.J.; Bonete, M.J. Characterisation of chlorate reduction in the haloarchaeon Haloferax mediterranei. Biochim. Biophy. Acta Gen. Subj. 2015, 1850, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Oren, A.; Elevi Bardavid, R.; Mana, L. Perchlorate and halophilic prokaryotes: Implications for possible halophilic life on Mars. Extremophiles 2014, 18, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Vera-Bernal, M.; Martínez-Espinosa, R.M. Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea. Microbiol. Res. 2021, 12, 354–375. [Google Scholar] [CrossRef]
- Nieto, J.J.; Ventosa, A.; Ruiz-Berraquero, F. Susceptibility of halobacteria to heavy metals. Appl. Environ. Microbiol. 1987, 53, 1199–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, G.; Dumitru, L. Biosorption of Some Heavy Metals from Media with High Salt Concentrations by Halophilic Archaea. Biotechnol. Biotechnol. Equip. 2009, 23, 791–795. [Google Scholar] [CrossRef]
- Das, D.; Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Cadmium resistance in extremely halophilic archaeon Haloferax strain BBK2. Chemosphere 2014, 112, 385–392. [Google Scholar] [CrossRef]
- Kushner, D.J. Growth and nutrition of halophilic bacteria. In The Biology of Halophilic Bacteria, 1st ed.; Vreeland, R.H., Hochstein, L.I., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 84–101. [Google Scholar]
- Arribas Jimeno, S.; Hernández Méndez, J.; Lucena Conce, F.; Burriel Marti, F. Química Analítica Cualitativa; Paraninfo: Madrid, Spain, 2002; p. 1053. [Google Scholar]
- Vido, K.; Spector, D.; Lagniel, G.; Lopez, S.; Toledano, M.B.; Labarre, J. A Proteome Analysis of the Cadmium Response in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 8469–8474. [Google Scholar] [CrossRef] [Green Version]
- Lagorce, A.; Fourçans, A.; Dutertre, M.; Bouyssiere, B.; Zivanovic, Y.; Confalonieri, F. Genome-wide transcriptional response of the Archaeon Thermococcus gammatolerans to Cadmium. PLoS ONE 2012, 7, e41935. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. EU Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union 2008, 348, 84–97. [Google Scholar]
- Voica, D.M.; Bartha, L.; Banciu, H.L.; Oren, A. Heavy metal resistance in halophilic Bacteria and Archaea. FEMS Microbiol. Lett. 2016, 363, fnw146. [Google Scholar] [CrossRef] [Green Version]
- Moopantakath, J.; Imchen, M.; Sreevalsan, A.; Siddhardha, B.; Martínez-Espinosa, R.M.; Kumavath, R. Biosynthesis of Silver Chloride Nanoparticles (AgCl-NPs) from Extreme Halophiles and Evaluation of Their Biological Applications. Curr. Microbiol. 2022, 79, 266. [Google Scholar] [CrossRef]
- Epstein, W. The Roles and Regulation of Potassium in Bacteria. Prog. Nucleic Acid Res. Mol. Biol. 2003, 75, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Greie, J.C. The extremely halophilic archaeon Halobacterium salinarum R1 responds to potassium limitation by expression of the K+-transporting KdpFABC P-type ATPase and by a decrease in intracellular K+. Extremophiles 2008, 12, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Large, A.T.; Kovacs, E.; Lund, P.A. Properties of the chaperonin complex from the halophilic archaeon Haloferax volcanii. FEBS Lett. 2002, 532, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Louis, B.G.; Fitt, P.S. Nucleic acid enzymology of extremely halophilic bacteria. Halobacterium cutirubrum ribonucleic acid-dependent ribonucleic acid polymerase. Biochem. J. 1971, 121, 629–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madern, D.; Zaccai, G. Stabilisation of halophilic malate dehydrogenase from Haloarcula marismortui by divalent cations—Effects of temperature, water isotope, cofactor and pH. Eur. J. Biochem. 1997, 249, 607–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc Proteins: Enzymes, Storage Proteins, Transcription Factors, and Replication Proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, T.V. Transition metals in control of gene expression. Science 1993, 261, 715–725. [Google Scholar] [CrossRef]
- Blencowe, D.K.; Morby, A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 291–311. [Google Scholar] [CrossRef]
- Culotta, V.C.; Daly, M.J. Manganese Complexes: Diverse Metabolic Routes to Oxidative Stress Resistance in Prokaryotes and Yeast. Antiox. Redox Signal. 2013, 19, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a first line of defence against cadmium toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Olesik, J.W. Elemental Analysis Using ICP-OES and ICP-MS. Anal. Chem. 1991, 63, 12–21. [Google Scholar] [CrossRef]
- Grindlay, G.; Gras, L.; Mora, J.; de Loos-Vollebregt, M.T.C. Carbon-related matrix Effects in inductively coupled plasma atomic emission spectrometry. Spectrochim. Acta B 2008, 63, 234. [Google Scholar] [CrossRef]
- Serrano, R.; Grindlay, G.; Gras, L.; Mora, J. Insight into the origin of carbon matrix effects on the emission signal of atomic lines in inductively coupled plasma optical emission spectrometry. Spectrochim. Acta B 2021, 177, 106070. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Fundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 December 2022).
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freedman and Co: New York, NY, USA, 1981; p. 776. [Google Scholar]
- Abdi, H.; Williams, L.J. Tukey’s honestly significant difference (HSD) test. In Encyclopedia of Research Design; SAGE Publications: Thousand Oaks, CA, USA, 2010; Volume 3, pp. 1–5. [Google Scholar]

| Pollutant | Tolerated Concentration | Description | Reference |
|---|---|---|---|
| As (V) | 20 mM | Toxicity tests carried out by agar dilution plate method in which MIC values were obtained for each metal analyzed; within all haloarchaea tested Hfx. mediterranei showed the highest tolerance to Cd (II). | [30] |
| Ag (I) | 0.5 mM | ||
| Ni (II) | 2.5 mM | ||
| Co (II) | 1 mM | ||
| Pb (II) | 20 mM | ||
| Cd (II) | 2.5 mM | ||
| Cr (VI) | 5 mM | ||
| Hg (II) | 0.01 mM | ||
| Zn (II) | 0.5 mM | ||
| Cu (II) | 1 mM | ||
| Cr (VI) | 5 mM | Both tolerance and accumulation of these metals were observed, as well as a reduction close to 100%; The organisms removed the metals more efficiently when glucose was added at a concentration of 2%; in the presence of Pb (II) and glucose at 2% the organisms were able to synthesize a greater amount of exomucopolysaccharides. | [31] |
| Pb (II) | 2.5 mM | ||
| Ni (II) | 2.5 mM | ||
| Zn (II) | 1 mM | ||
| As (V) | 20 mM | The higher the concentration of the metal in the medium, the higher the concentration of the metal in the cells. | [18] |
| Co (II) | 1.2 mM | ||
| Li (I) | >500 mM | ||
| Ni (II) | 1.6 mM | ||
| Cu (II) | NA | Through bioinformatic tools, genes coding for Cu import and export, coding for proteins involved in antioxidant mechanisms and coding for genes involved in Cu metabolism were identified, showing the potential of this organism to bioremediate Cu. | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saez-Zamacona, I.; Grindlay, G.; Martínez-Espinosa, R.M. Evaluation of Haloferax mediterranei Strain R4 Capabilities for Cadmium Removal from Brines. Mar. Drugs 2023, 21, 72. https://doi.org/10.3390/md21020072
Saez-Zamacona I, Grindlay G, Martínez-Espinosa RM. Evaluation of Haloferax mediterranei Strain R4 Capabilities for Cadmium Removal from Brines. Marine Drugs. 2023; 21(2):72. https://doi.org/10.3390/md21020072
Chicago/Turabian StyleSaez-Zamacona, Iraide, Guillermo Grindlay, and Rosa María Martínez-Espinosa. 2023. "Evaluation of Haloferax mediterranei Strain R4 Capabilities for Cadmium Removal from Brines" Marine Drugs 21, no. 2: 72. https://doi.org/10.3390/md21020072
APA StyleSaez-Zamacona, I., Grindlay, G., & Martínez-Espinosa, R. M. (2023). Evaluation of Haloferax mediterranei Strain R4 Capabilities for Cadmium Removal from Brines. Marine Drugs, 21(2), 72. https://doi.org/10.3390/md21020072









