Morphological, Physiological and Mechanical Features of the Mutable Collagenous Tissues Associated with Autotomy and Evisceration in Dendrochirotid Holothuroids
Abstract
:1. Introduction
2. Behavioural Events
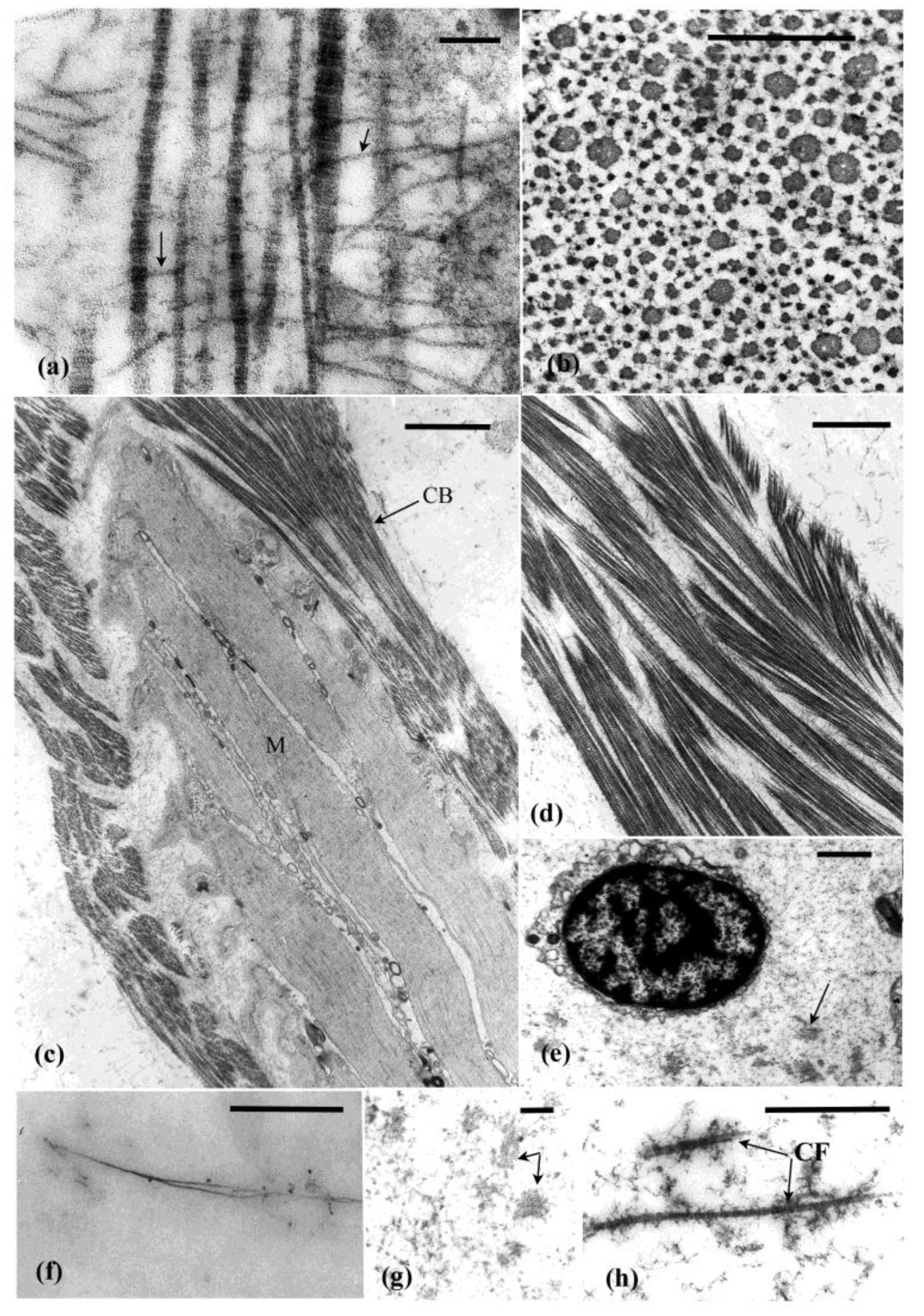
3. Histology and Tissue Organisation of the Autotomy Structures
3.1. Introvert
3.2. Pharyngeal Retractor Muscle Tendon
3.3. Intestine-Cloacal Junction
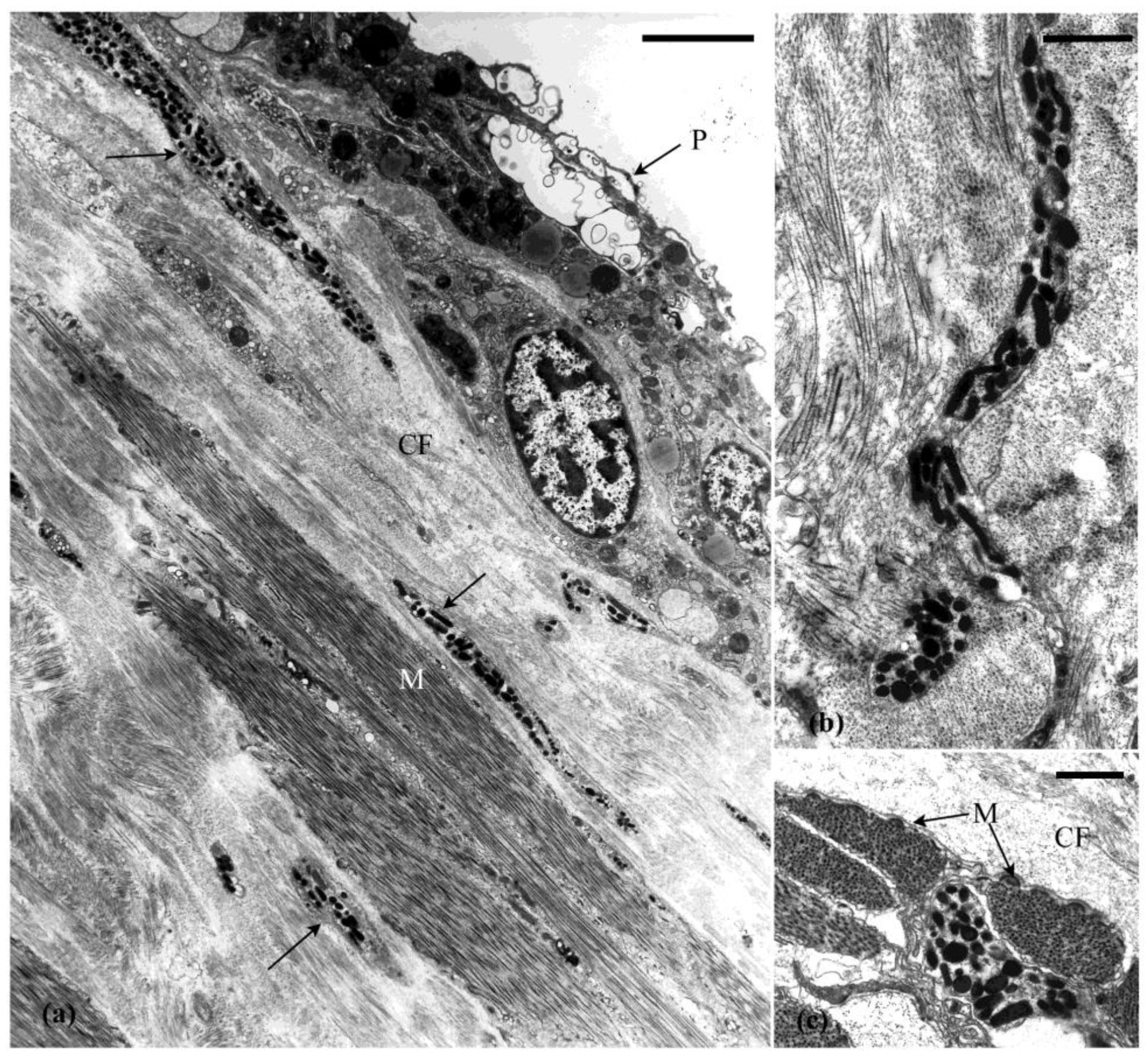
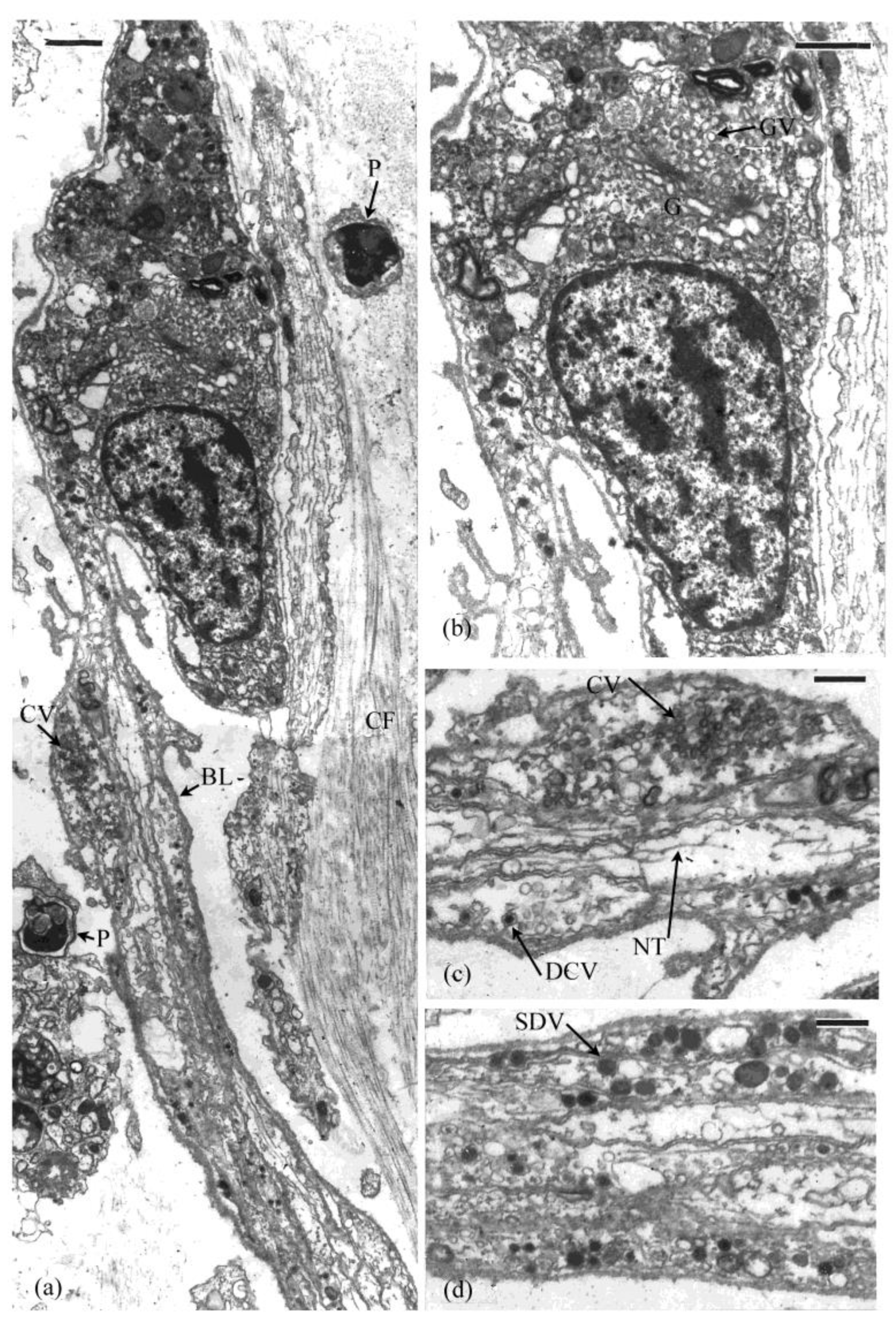
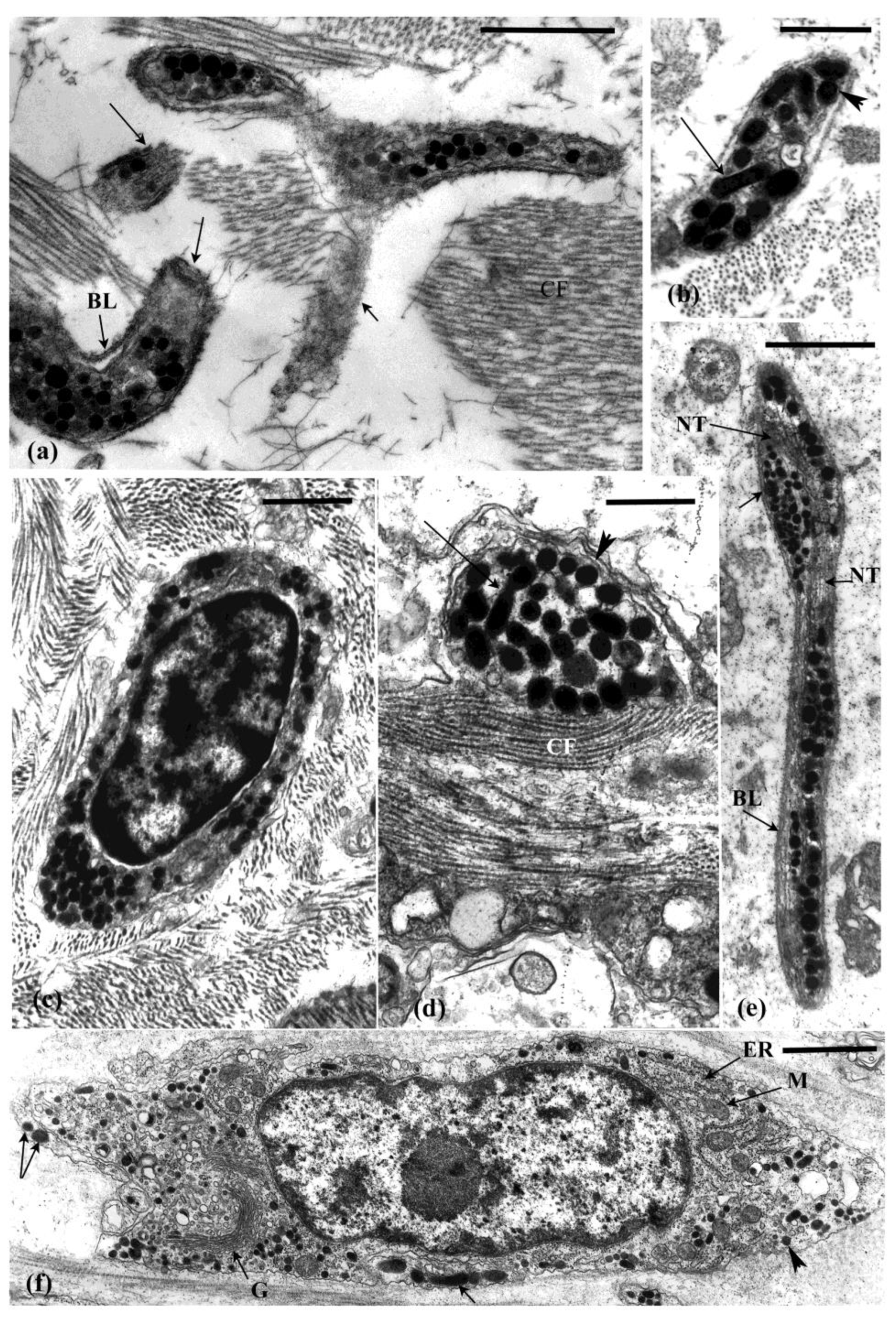
4. Cells Associated with the MCT
4.1. Neuronal Profiles
4.2. Other Cells in the Connective Tissue
5. Cell and Tissue Changes That Occur during Autotomy
6. Mechanical Properties of the Autotomy Structures
7. Induction and Control of Evisceration and the Endogenous Evisceration Factor
8. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkie, I. Variable tensility in echinoderm collagenous tissues: A review. Mar. Freshw. Behav. Physiol. 1984, 11, 1–34. [Google Scholar] [CrossRef]
- Wilkie, I.C. Design for disaster: The ophiuroid intervertebral ligament as a typical mutable collagenous structures. In Echinoderm Biology; Burke, R.D., Mladenov, P.V., Lambert, P., Parsley, R.L., Eds.; Balkema: Rotterdam, The Netherlands, 1988; pp. 25–38. [Google Scholar]
- Wilkie, I.C. Mutable collagenous tissues: Extracellular matrix as mechano-effector. In Echinoderm Studies; Jangoux, M., Lawrence, J.M., Eds.; Balkema: Rotterdam, The Netherlands, 1996; Volume 5, pp. 61–102. [Google Scholar]
- Motokawa, T. Connective tissue catch in echinoderms. Biol. Rev. 1984, 59, 255–270. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Sugni, M.; Gupta, H.S.; Candia Carnevali, M.D.; Elphick, M.R. The mutable collagenous tissue of echinoderms: From biology to biomedical applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: London, UK, 2021; pp. 3–33. [Google Scholar]
- Motokawa, T. Cholinergic control of the mechanical properties of the catch connective tissue in the holothurian body wall. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 86, 333–337. [Google Scholar] [CrossRef]
- Wilkie, I.; Emson, R. The tendons of Ophiocomina nigra and their role in autotomy (Echinodermata, Ophiuroida). Zoomorphology. 1987, 107, 33–44. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Emson, R.H.; Mladenov, P.V. Autotomy mechanism and its control in the starfish Pycnopodia helianthoides (Brandt). In Echinoderm Research; Emson, R.H., Smith, A.B., Cambell, A.C., Eds.; Balkema: Rotterdam, The Netherlands, 1995; pp. 137–146. [Google Scholar]
- Wilkie, I.C.; Griffiths, G.V.; Glennie, S.F. Morphological and physiological aspects of the autotomy plane in the aboral integument of Asterias rubens (Echinodermata). In Echinoderm Research; De Ridder, C., Dubois, P., Lahaye, M.C., Jangoux, M., Eds.; Balkema: Rotterdam, The Netherlands, 1990; pp. 301–313. [Google Scholar]
- Wilkie, I. Autotomy as a prelude to regeneration in echinoderms. Microsc. Res. Technol. 2001, 55, 369–396. [Google Scholar] [CrossRef]
- Wilkie, I. Nervously mediated change in the mechanical properties of a brittlestar ligament. Mar. Freshw. Behav. Physiol. 1978, 5, 289–306. [Google Scholar] [CrossRef]
- Wilkie, I. Functional morphology of the autotomy plane of the brittlestar Ophiocomina nigra (Abildgaard) (Ophiuroidea, Echinodermata). Zoomorphologie 1978, 91, 289–305. [Google Scholar] [CrossRef]
- Wilkie, I. The juxtaligamental cells of Ophiocomina nigra (Abildgaard) (Echinodermata: Ophiuroidea) and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res. 1979, 197, 515–530. [Google Scholar] [CrossRef]
- Byrne, M. Functional morphology of a holothurian autotomy plane and its role in evisceration. In International Echinoderms Conference Tampa Bay; Lawrence, J.M., Ed.; Balkema: Rotterdam, The Netherlands, 1982; pp. 65–68. [Google Scholar]
- Byrne, M. Ultrastructural changes in the autotomy tissues of Eupentacta quinquesemita (Echinodermata: Holothuroidea) during evisceration. In Proceedings of the Fifth International Echinoderms Conference; Keegan, B.F., O’Connor, B.D.S., Eds.; Balkema: Rotterdam, The Netherlands, 1985; pp. 413–420. [Google Scholar]
- Byrne, M. The morphology of autotomy structures in the sea cucumber Eupentacta quinquesemita before and during evisceration. J. Exp. Biol. 2001, 204, 849–863. [Google Scholar] [CrossRef]
- Emson, R.H.; Wilkie, I.C. Fission and autotomy in echinoderms. Oceanogr. Mar. Biol. Ann. Rev. 1980, 18, 155–250. [Google Scholar]
- Byrne, M.; Mazzone, F.; Elphick, M.R.; Thorndyke, M.C.; Cisternas, P. Expression of the neuropeptide SALMFamide-1 during regeneration of the seastar radial nerve cord following arm autotomy. Proc. Roy. Soc. B. 2019, 286, 20182701. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M. The link between autotomy and CNS regeneration: Echinoderms as non-model species for regenerative biology. BioEssays 2020, 42, 1900219. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, M.D. Regeneration in echinoderms: Repair, regrowth, cloning. Invertebr. Surviv. J. 2006, 3, 64–76. [Google Scholar]
- Holland, N.D.; Grimmer, J.C. Fine structure of syzygial articulations before and after arm autotomy in Florometra serratissima (Echinodermata: Crinoidea). Zoomorphology 1981, 98, 169–183. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Barbaglio, A.; Maclaren, W.M.; Candia Carnevali, M.D. Physiological and immunocytochemical evidence that glutamatergic neurotransmission is involved in the activation of arm autotomy in the featherstar Antedon mediterranea (Echinodermata: Crinoidea). J. Exp. Biol. 2010, 213, 2104–2115. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M. Induction of evisceration in the holothurian Eupentacta quinquesemita and evidence for the existence of an endogenous evisceration factor. J. Exp. Biol. 1986, 120, 25–39. [Google Scholar] [CrossRef]
- Smith, G.N.; Greenberg, M.J. Chemical control of the evisceration process in Thyone briareus. Biol. Bull. 1973, 144, 421–436. [Google Scholar] [CrossRef]
- Mladenov, P.V.; Igdoura, S.; Asotra, S.; Burke, R.D. Purification and partial characterization of an autotomy-promoting factor from the sea star Pycnopodia helianthoides. Biol. Bull. 1989, 176, 169–175. [Google Scholar] [CrossRef]
- Bobrovskaya, N.V.; Dolmatov, I.Y. Autotomy of the visceral mass in the feather star Himerometra robustipinna (Crinoidea, Comatulida). Biol. Bull. 2014, 226, 81–91. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Kalacheva, N.V.; Mekhova, E.S.; Frolova, L.T. Autotomy and regeneration of the visceral mass in feather stars. Zoomorphology 2020, 139, 171–187. [Google Scholar] [CrossRef]
- Welsch, U.; Lange, A.; Bals, R.; Heinzeller, T. Juxtaligamental cells in feather stars and isocrinids. In Echinoderm Research 1995; Emson, R., Smith, A., Campbell, A., Eds.; Balkema: Rotterdam, The Netherlands, 1995; pp. 129–135. [Google Scholar]
- Byrne, M. Evisceration behaviour and the seasonal incidence of evisceration in the holothurian Eupentacta quinquesemita (Selenka). Ophelia 1985, 24, 75–90. [Google Scholar] [CrossRef]
- Byrne, M. Autotomy, evisceration and regeneration in dendrochirotid holothuroids. In The World of Sea Cucumbers; Mercier, A., Hamel, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; in press. [Google Scholar]
- Dolmatov, I. Regeneration of the aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Monogr. Dev. Biol. 1992, 23, 40–50. [Google Scholar]
- Dolmatov, I.Y. Molecular aspects of regeneration mechanisms in holothurians. Genes 2021, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Dawbin, W.H. Auto-evisceration and the regeneration of viscera in the holothurian Stichopus mollis (Hutton). Trans. Roy. Soc. N. Z. 1949, 77, 497–523. [Google Scholar]
- García-Arrarás, J.E.; Estrada-Rodgers, L.; Santiago, R.; Torres, I.I.; Díaz-Miranda, L.; Torres-Avillán, I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima (Selenka) (Holothuroidea: Echinodermata). J. Exp. Zool. 1998, 281, 288–304. [Google Scholar] [CrossRef]
- Mashanov, V.; García-Arrarás, J. Gut regeneration in holothurians: A snapshot of recent developments. Biol. Bull. 2011, 221, 93–109. [Google Scholar] [CrossRef]
- Kille, F.R. Regeneration in Sclerodactyla briareus Leseur following induced autotomy. Biol. Bull. 1935, 69, 82–108. [Google Scholar] [CrossRef]
- Tracey, D.J. Evisceration and regeneration in Thyone okeni (Bell, 1884). Proc. Linn. Soc. NSW 1972, 97, 72–81. [Google Scholar]
- Pearse, A. Autotomy in holothurians. Biol. Bull. 1909, 18, 42–49. [Google Scholar] [CrossRef]
- Rowe, F.W.E.; O’Hara, T.D.; Bardsley, T.M. Class Holothuroidea. In Australian Echinoderms: Biology Ecology and Evolution; Byrne, M., O’Hara., T.D., Eds.; CSIRO Press: Clayton, Australia, 2017; pp. 447–490. [Google Scholar]
- Costello, J.; Keegan, B.F. Feeding and related morphological structures in the dendrochirote Aslia lefevrei (Holothuroidea: Echinodermata). Mar. Biol. 1984, 84, 135–142. [Google Scholar] [CrossRef]
- Byrne, M. The mechanical properties of the autotomy tissues of the holothurian Eupentacta quinquesemita and the effects of certain physico-chemical agents. J. Exp. Biol. 1985, 117, 69–86. [Google Scholar] [CrossRef]
- Byrne, M. Evisceration and Autotomy in the Holothurian Eupentacta quinquesemita (Selenka). Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 1983. [Google Scholar]
- Byrne, M. The ultrastructure of the morula cells of Eupentacta quinquesemita (Echinodermata: Holothuroidea) and their role in the maintenance of the extracellular matrix. J. Morphol. 1986, 188, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T. Rapid change in mechanical properties of echinoderm tissue caused by coelomic fluid. Comp. Biochem. Physiol. 1982, 73, 223–229. [Google Scholar] [CrossRef]
- Dobson, W.E.; Turner, R. Morphology and histology of the disc autotomy plane in Ophiophragmus filograneus (Echinodermata, Ophiurida). Zoomorphology 1989, 108, 323–332. [Google Scholar] [CrossRef]
- Bonneel, M.; Hennebert, E.; Byrne, M. Flammang, Mutable collagenous tissue in sea cucumbers. In The World of Sea Cucumbers; Mercier, A., Hamel, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; in press. [Google Scholar]
- Bonneel, M.; Hennebert, E.; Sesilja Aranko, A.; Hwang, D.S.; Lefevre, M.; Pommier, V.; Wattiez, R.; Delroisse, J.; Flammang, P. Molecular mechanisms mediating the mechanically adaptable connective tissues of sea cucumbers. Matrix Biol. 2022, 108, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Demeuldre, M.; Hennebert, E.; Bonneel, M.; Lengerer, B.; Van Dyck, S.; Wattiez, R.; Ladurner, P.L.; Flammang, P. Mechanical adaptability of sea cucumber Cuvierian tubules involves mutable collagenous tissue. J. Exp. Biol. 2017, 220, 2108–2119. [Google Scholar] [CrossRef] [Green Version]
- Trotter, J.; Koob, T. Evidence that calcium-dependent cellular processes are involved in the stiffening response of holothurian dermis and that dermal cells contain an organic stiffening factor. J. Exp. Biol. 1995, 198, 1951–1961. [Google Scholar] [CrossRef]
- Koob, T.J.; Koob-Emunds, M.M.; Trotter, J.A. Cell-derived stiffening and plasticizing factors in sea cucumber (Cucumaria frondosa) dermis. J. Exp. Biol. 1999, 202, 2291–2301. [Google Scholar] [CrossRef]
- Tipper, J.P.; Lyons-Levy, M.A.; Atkinson, J.A.; Trotter, J.A. Purification, characterization and cloning of tensilin, the collagen-fibril binding and tissue stiffening factor from Cucumaria frondosa dermis. Matrix Biol. 2002, 21, 625–635. [Google Scholar] [CrossRef]
- Sugi, H.; Suzuki, S.; Tsuchiya, T.; Gomi, S.; Fujieda, N. Physiological and ultrastructural studies on the longitudinal retractor muscle of a sea cucumber Stichopus japonicus: I. Factors influencing the mechanical response. J. Exp. Biol. 1982, 97, 101–111. [Google Scholar] [CrossRef]
- Motokawa, T. Factors regulating the mechanical properties of the holothurian dermis. J. Exp Biol. 1982, 99, 29–41. [Google Scholar] [CrossRef]
- Motokawa, T. The viscosity change of the body-wall dermis of the sea cucumber Stichopus chloronotus, caused by mechanical and chemical stimulation. Comp. Biochem. Physiol. 1984, 77, 419–423. [Google Scholar]
- Birenheide, R.; Tamori, M.; Motokawa, T.; Ohtani, M.; Iwakoshi, E.; Muneoka, Y.; Fujita, T.; Minakata, H.; Nomoto, K. Peptides controlling stiffness of connective tissue in sea cucumbers. Biol. Bull. 1998, 194, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Montes, G.S.; Mourão, P.A.S.; Carneiro, J.; Salles, L.M.M.; Bonetti, S.S. Collagen-proteoglycans interaction during autonomy in the sea cucumber, Stichopus badionotus. Rev. Can. Biol. 1980, 39, 157–164. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, M. Morphological, Physiological and Mechanical Features of the Mutable Collagenous Tissues Associated with Autotomy and Evisceration in Dendrochirotid Holothuroids. Mar. Drugs 2023, 21, 134. https://doi.org/10.3390/md21030134
Byrne M. Morphological, Physiological and Mechanical Features of the Mutable Collagenous Tissues Associated with Autotomy and Evisceration in Dendrochirotid Holothuroids. Marine Drugs. 2023; 21(3):134. https://doi.org/10.3390/md21030134
Chicago/Turabian StyleByrne, Maria. 2023. "Morphological, Physiological and Mechanical Features of the Mutable Collagenous Tissues Associated with Autotomy and Evisceration in Dendrochirotid Holothuroids" Marine Drugs 21, no. 3: 134. https://doi.org/10.3390/md21030134





