Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications
Abstract
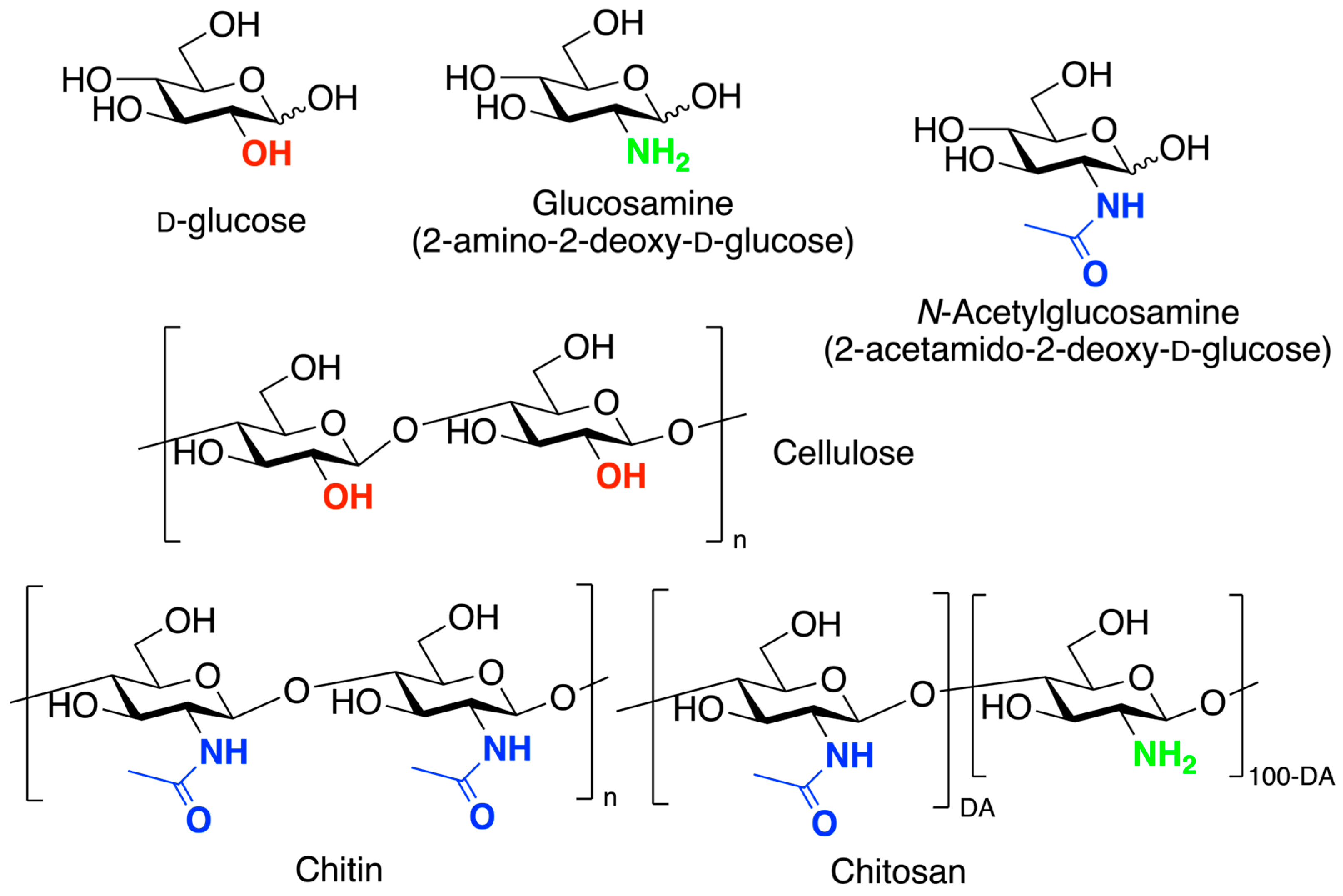
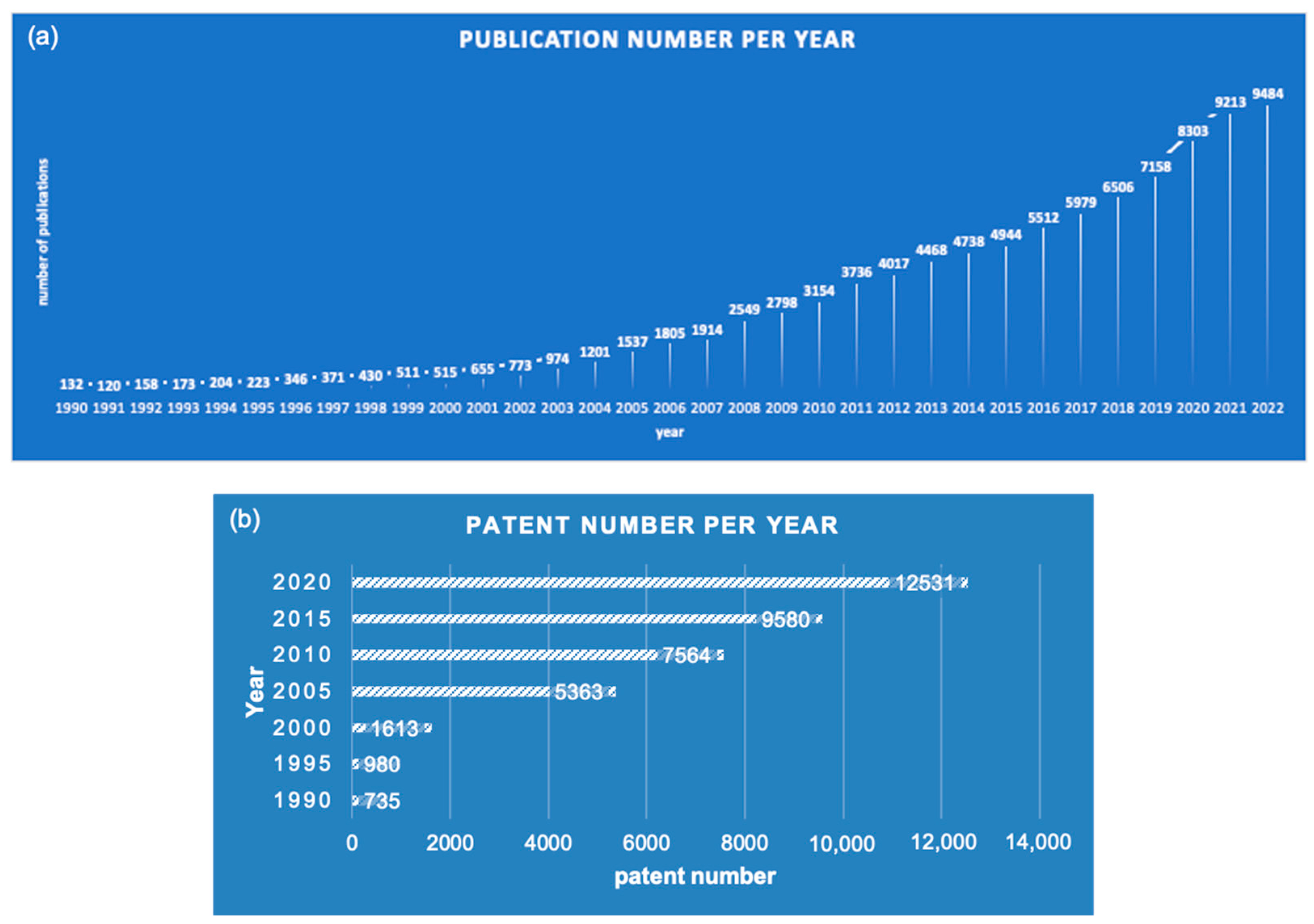
1. Introduction
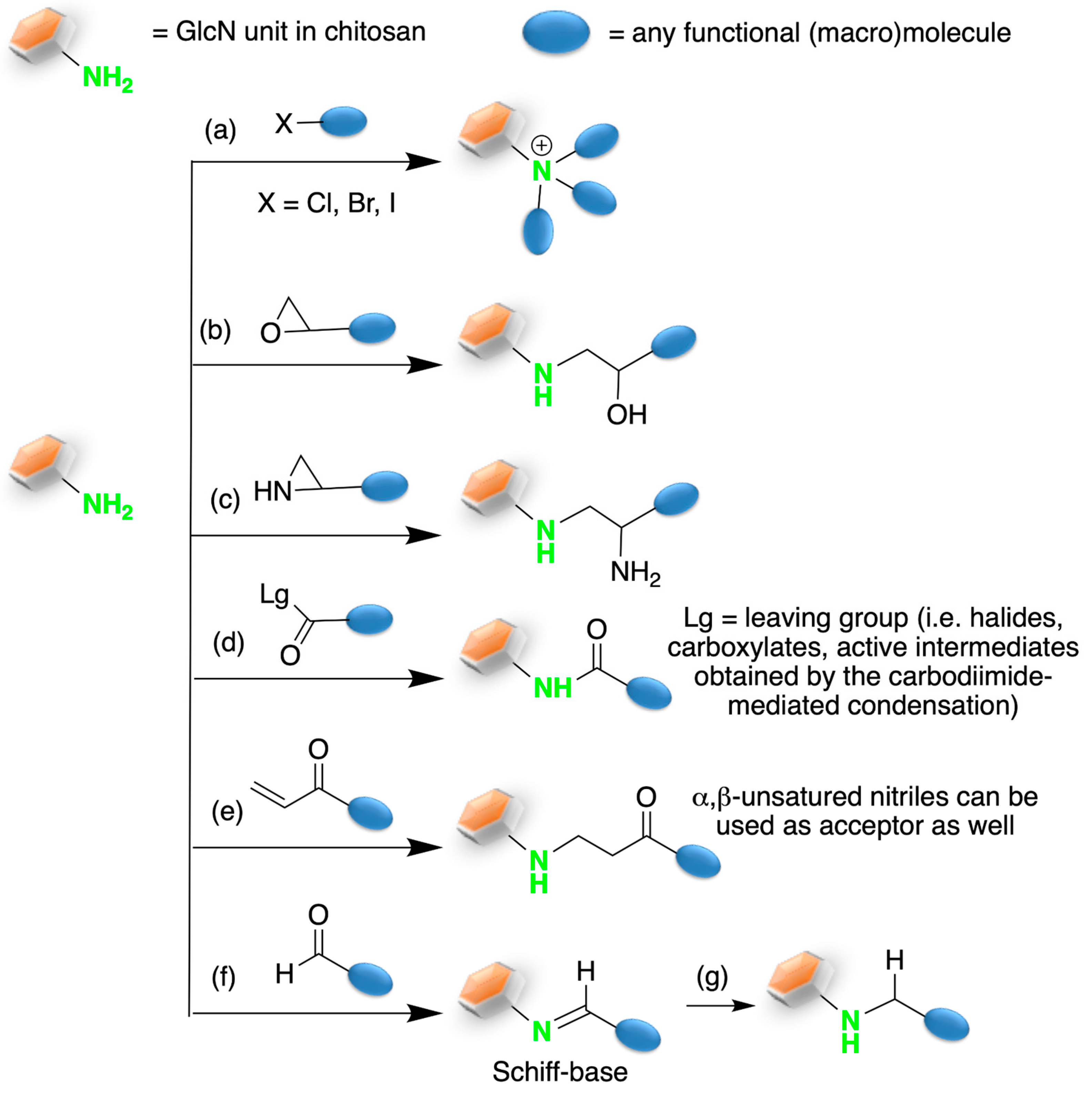
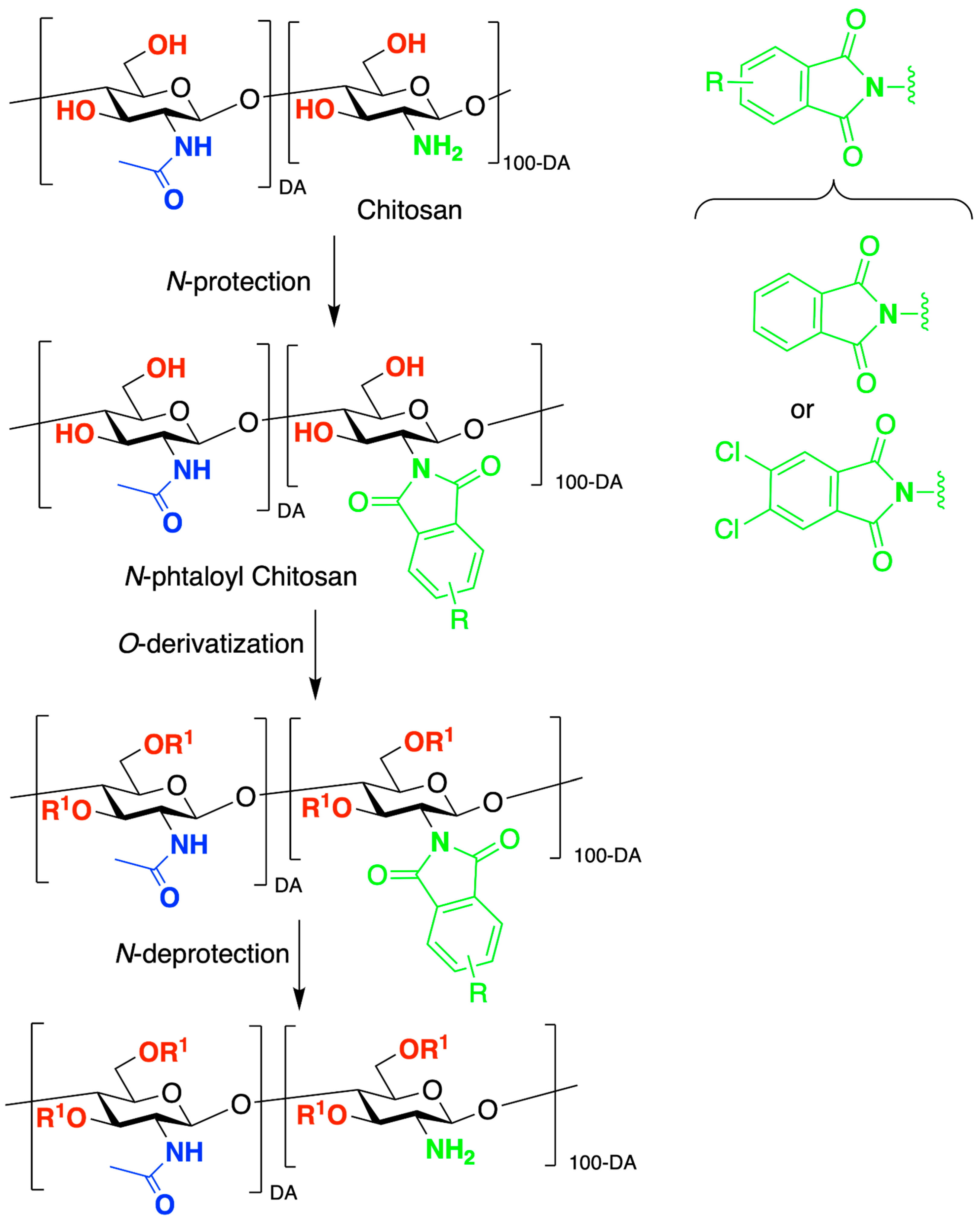
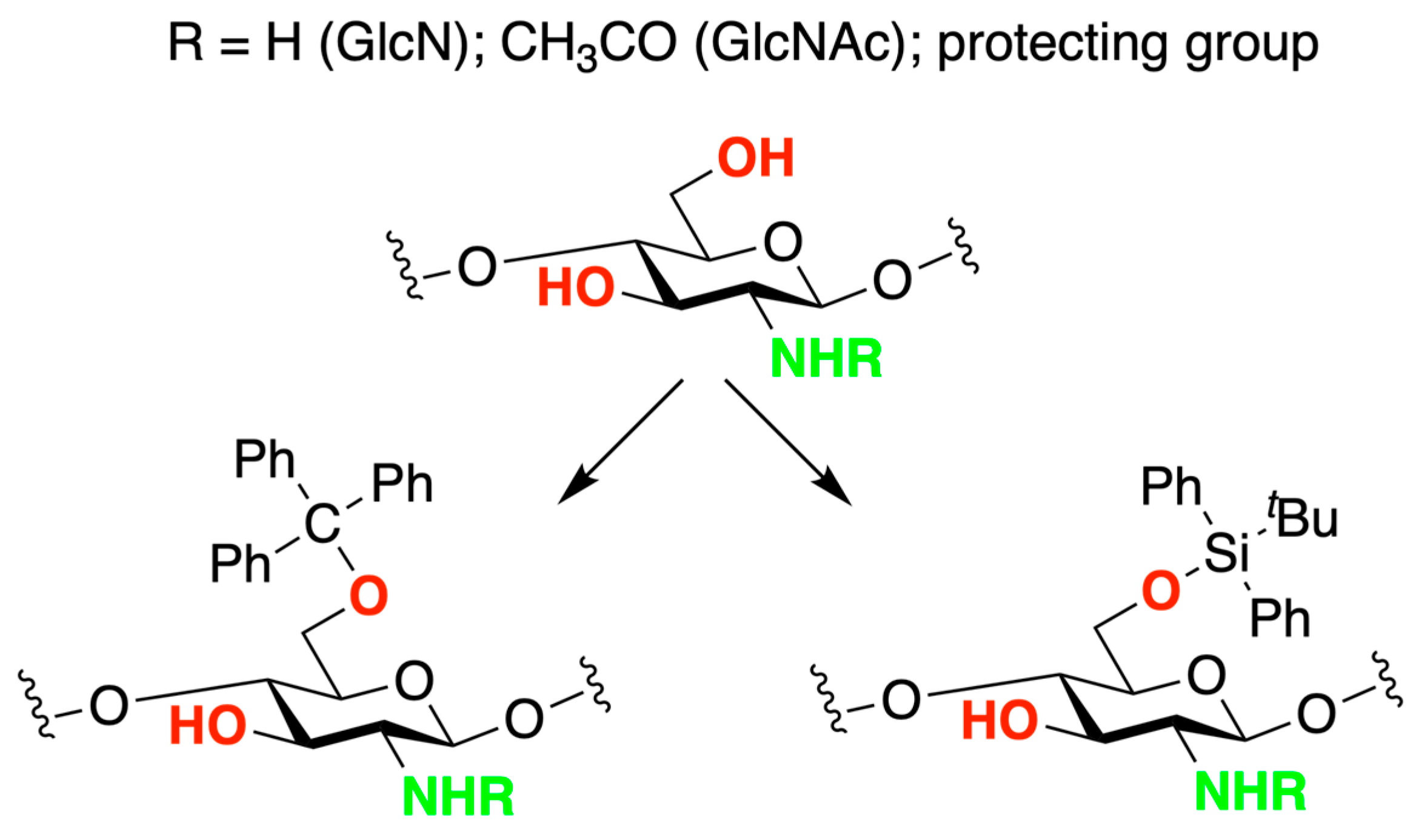
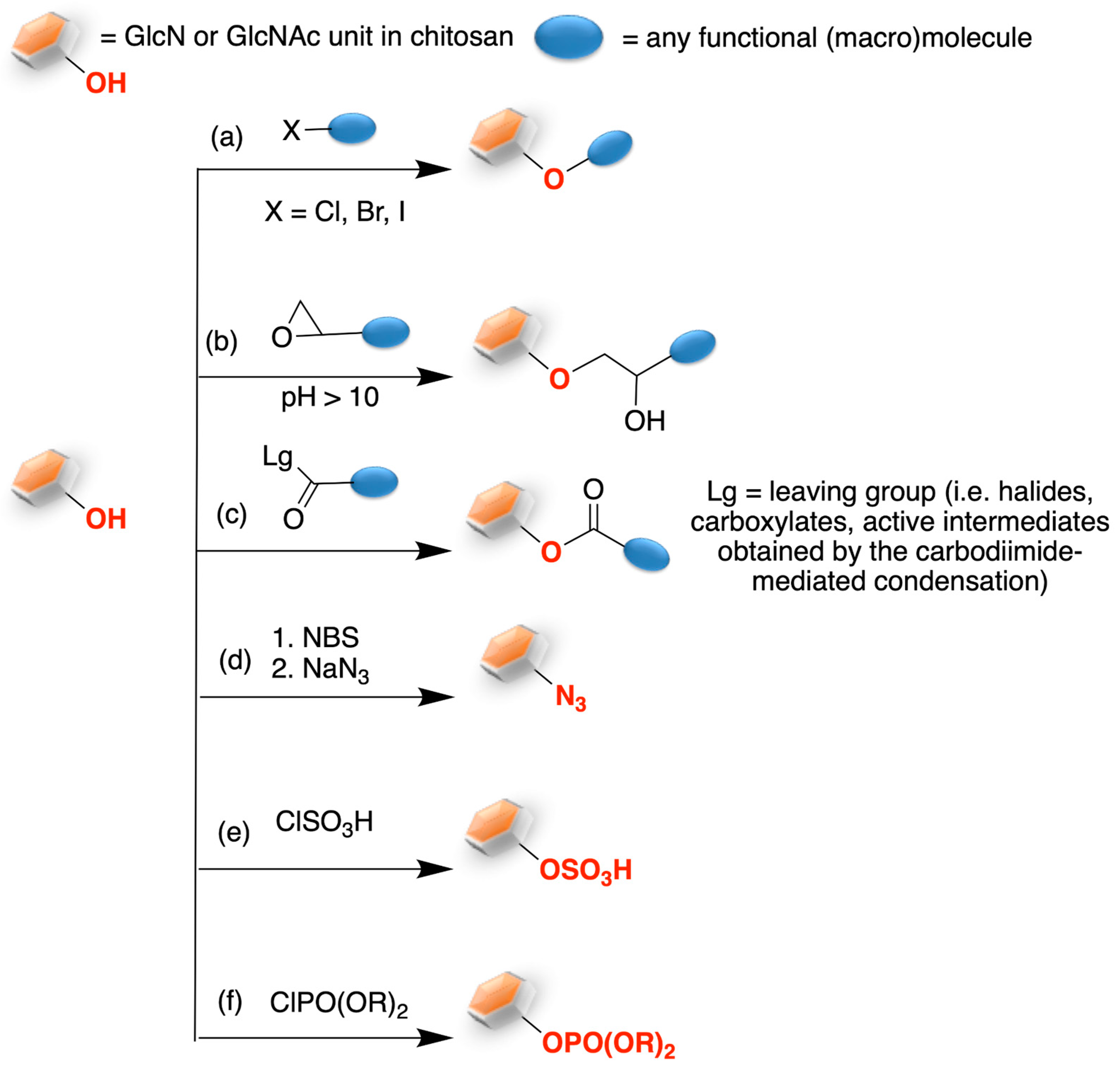
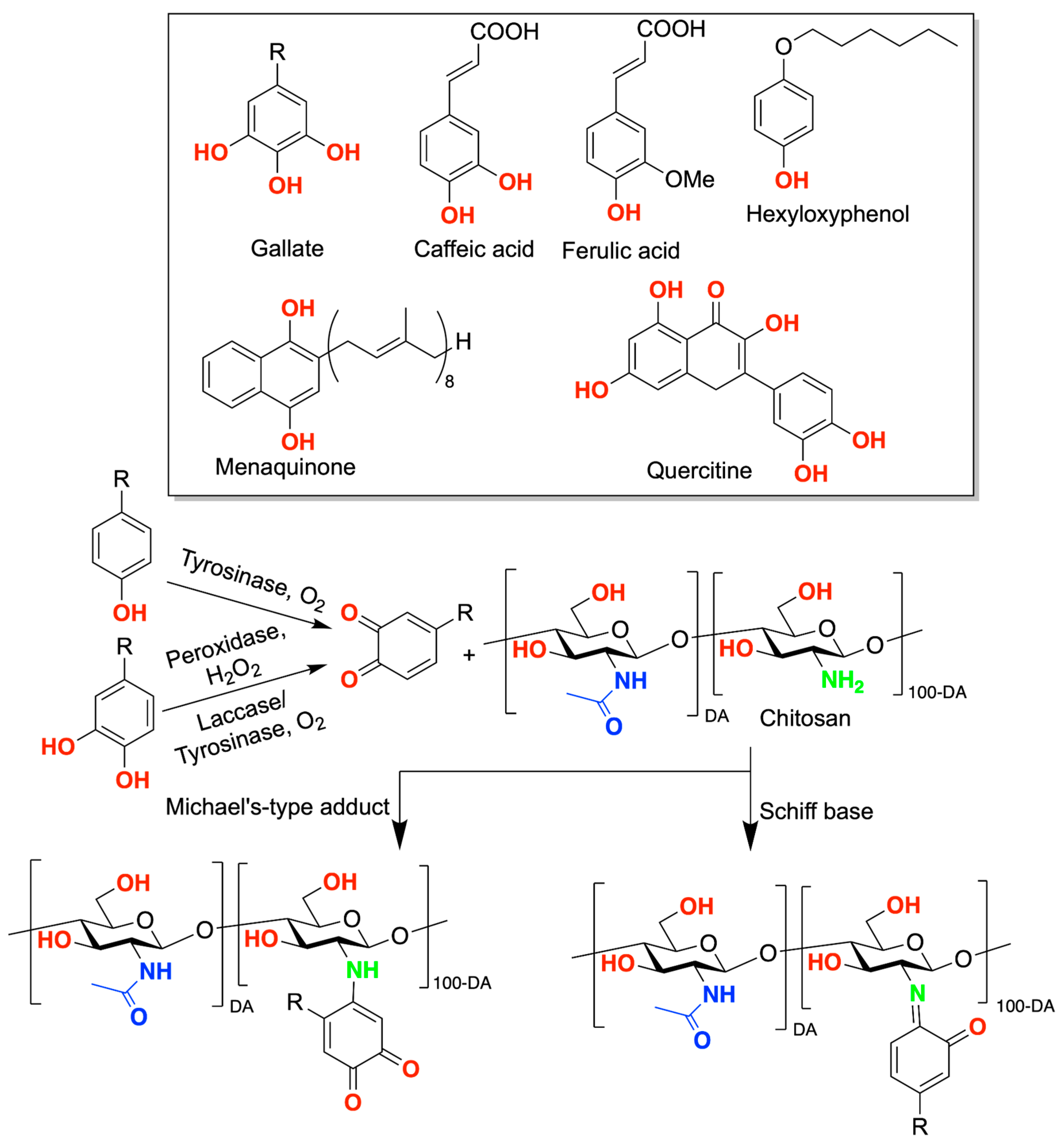
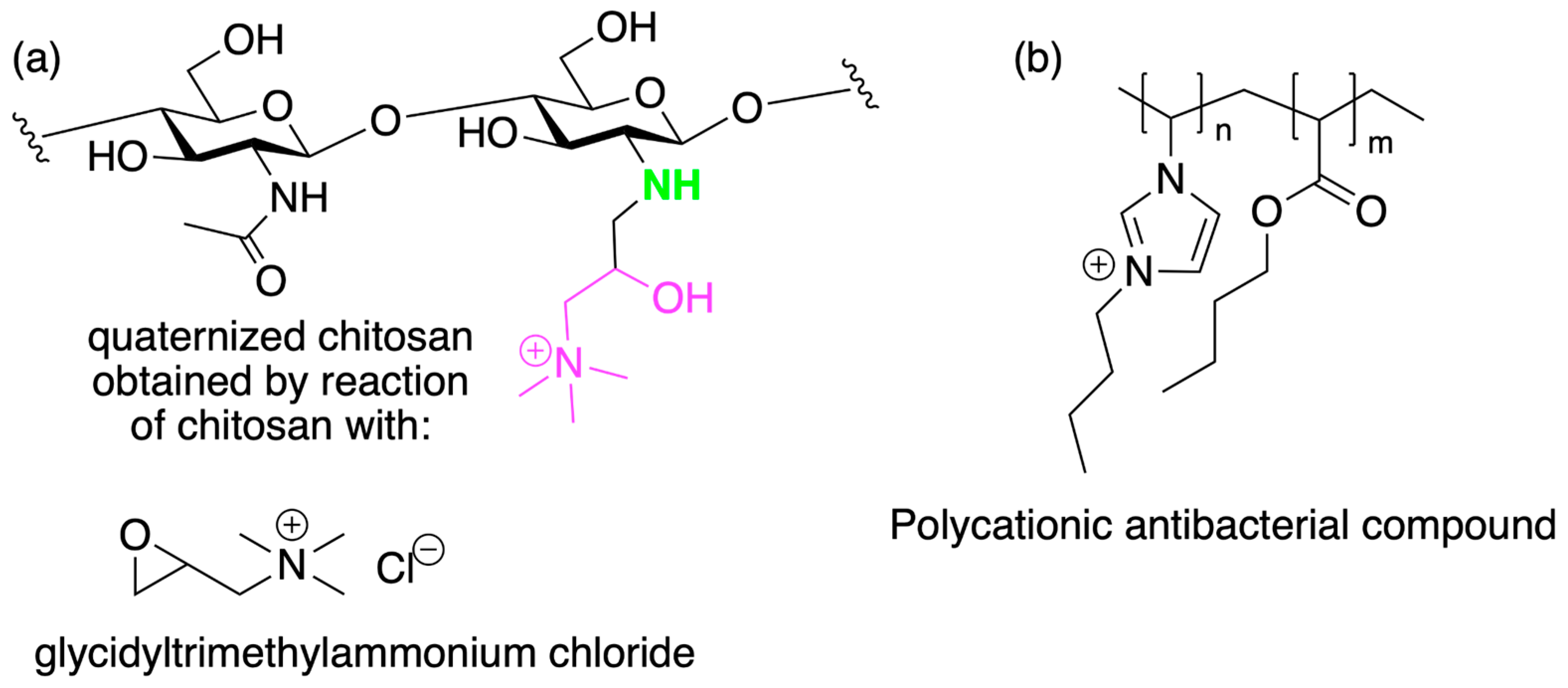
2. Chitosan Functionalities: Friend or Foe?
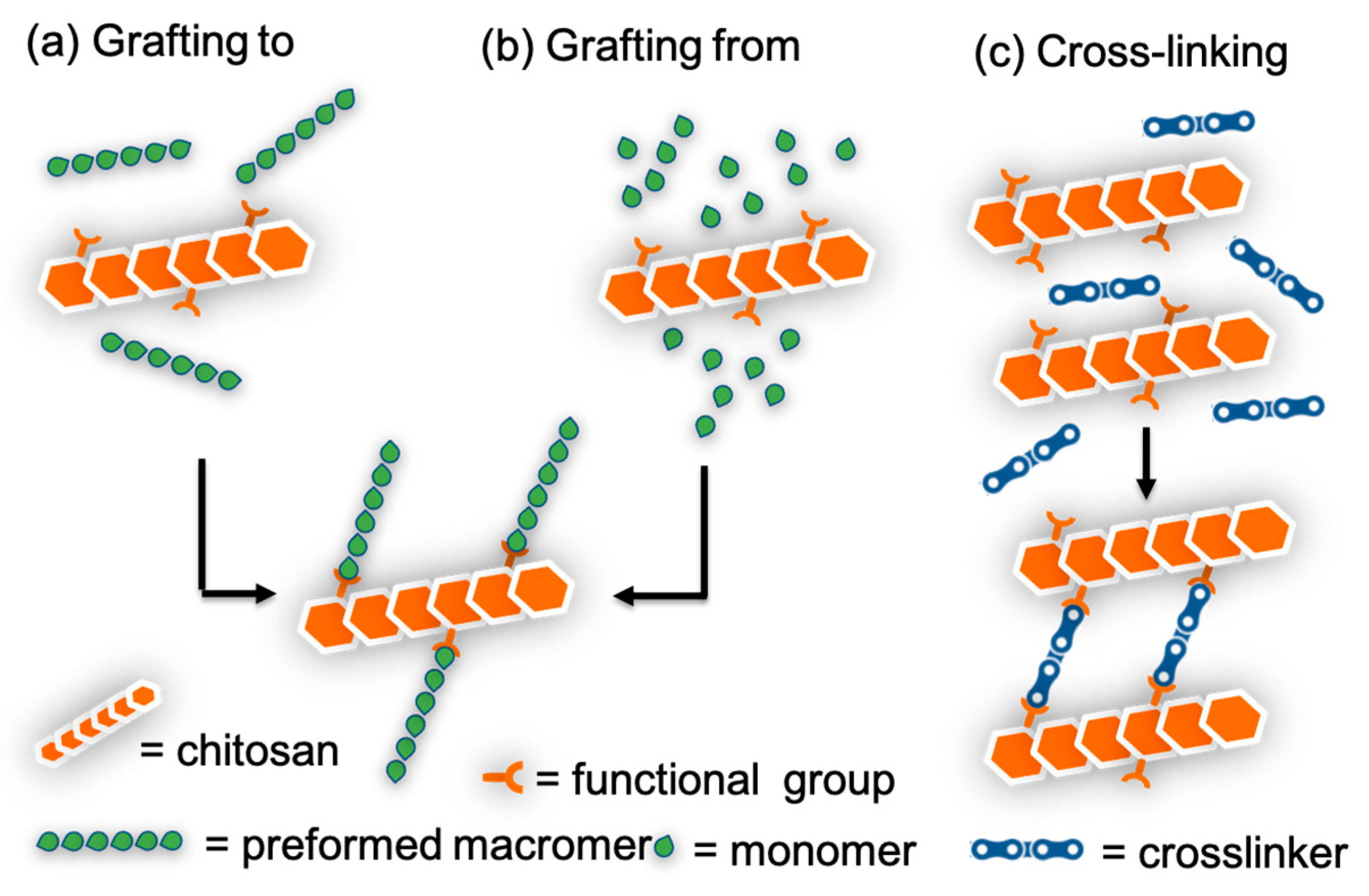
3. Modulation of Chitosan Solubility, and Potential Application in the Material Science Field
4. Chitosan-Based Hydrogels for Biomedical Applications
4.1. Chitosan-Based Hydrogels by Physical Cross-Linking
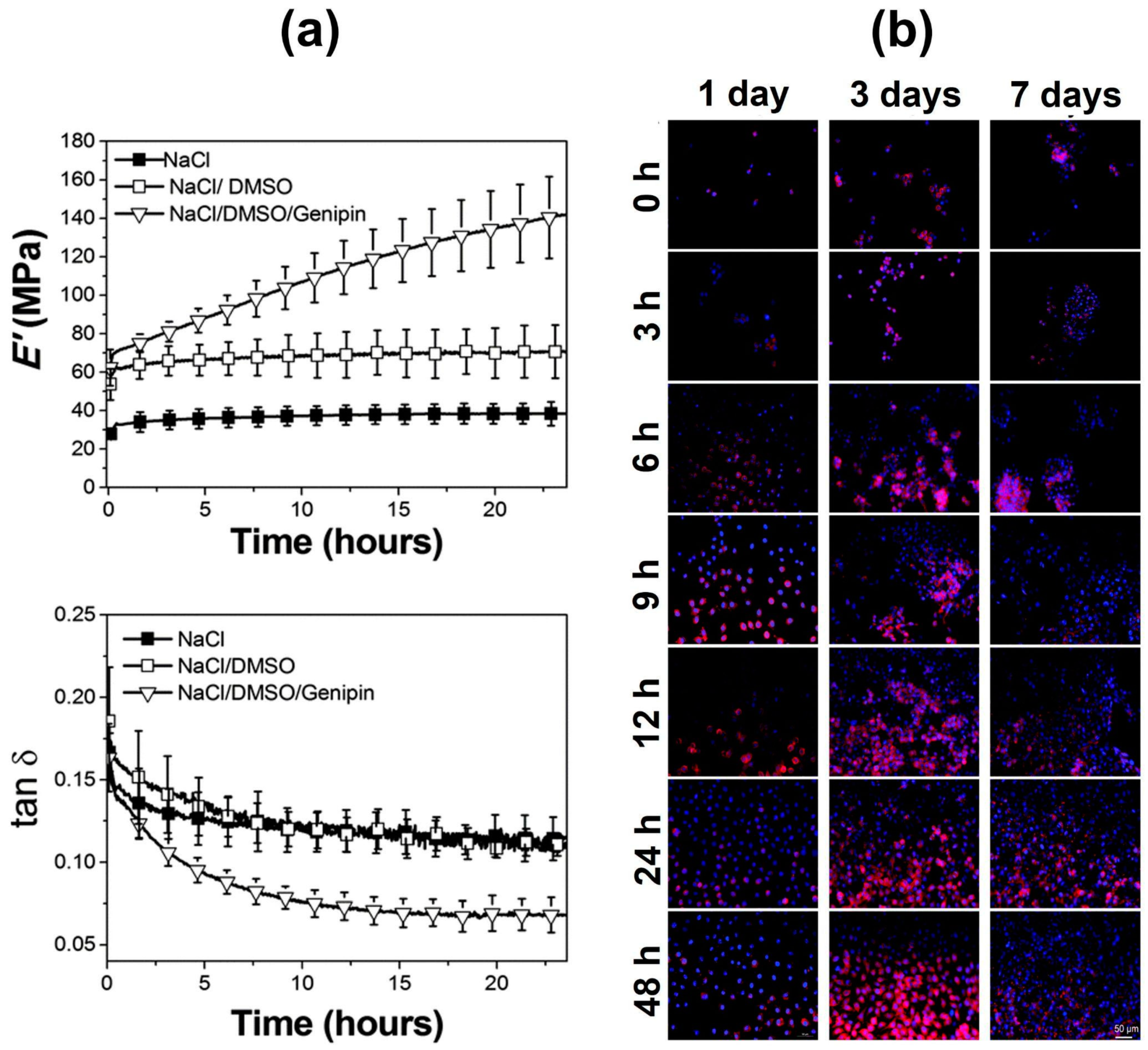
4.2. Chitosan-Based Hydrogels by Chemical Crosslinking
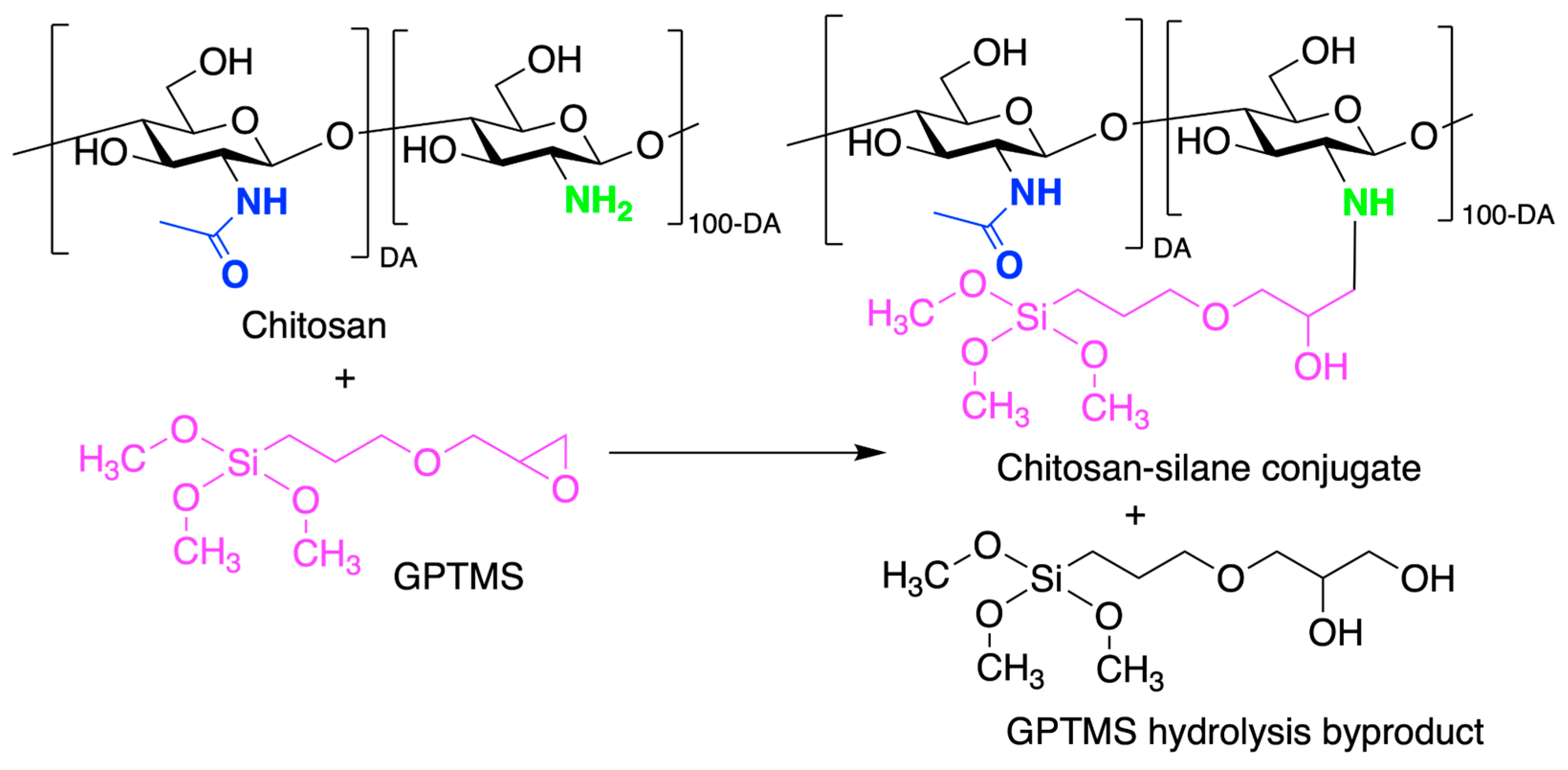
5. Chitosan-Based Organic–Inorganic Hybrids for Biomedical Applications
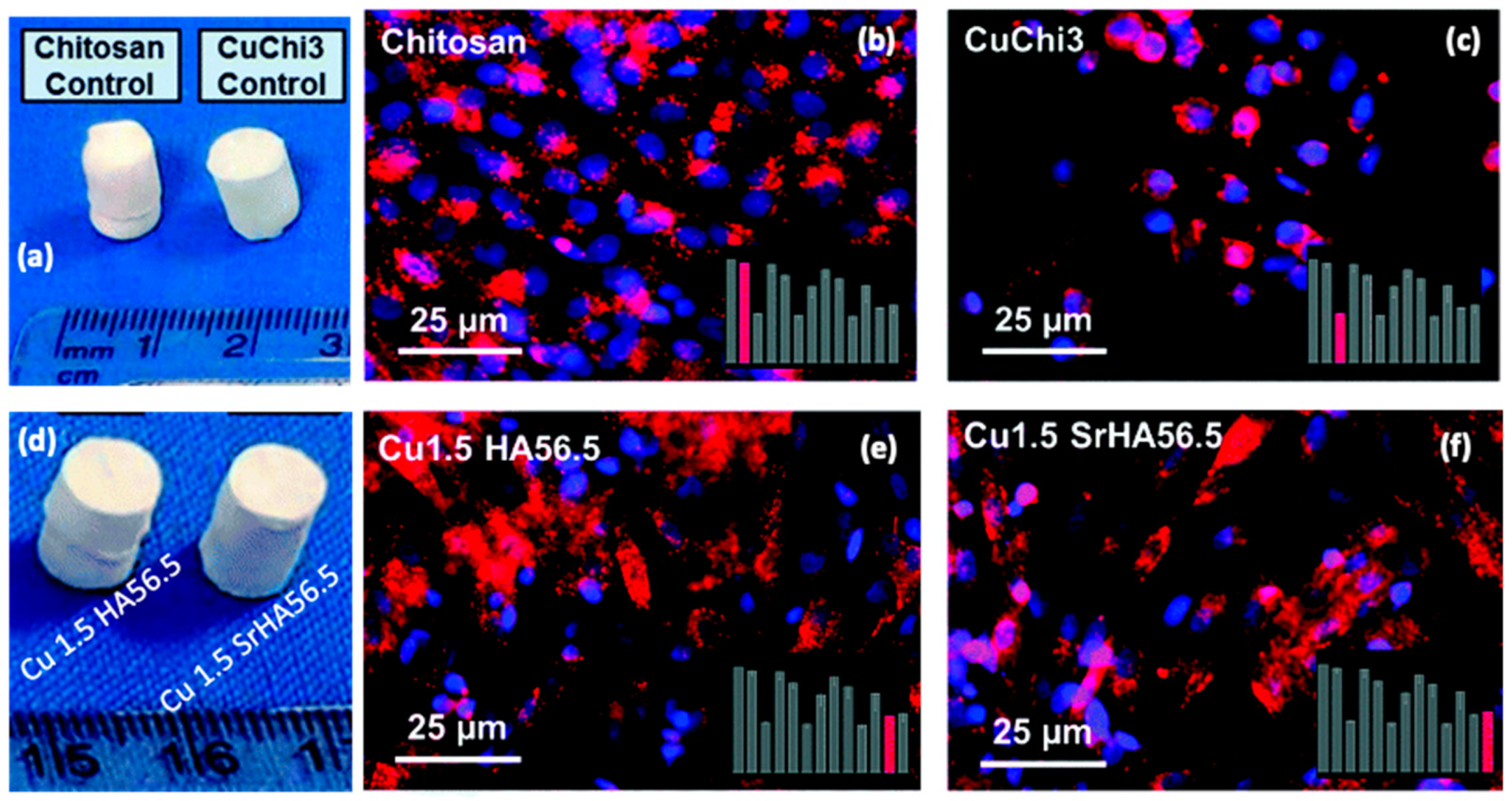
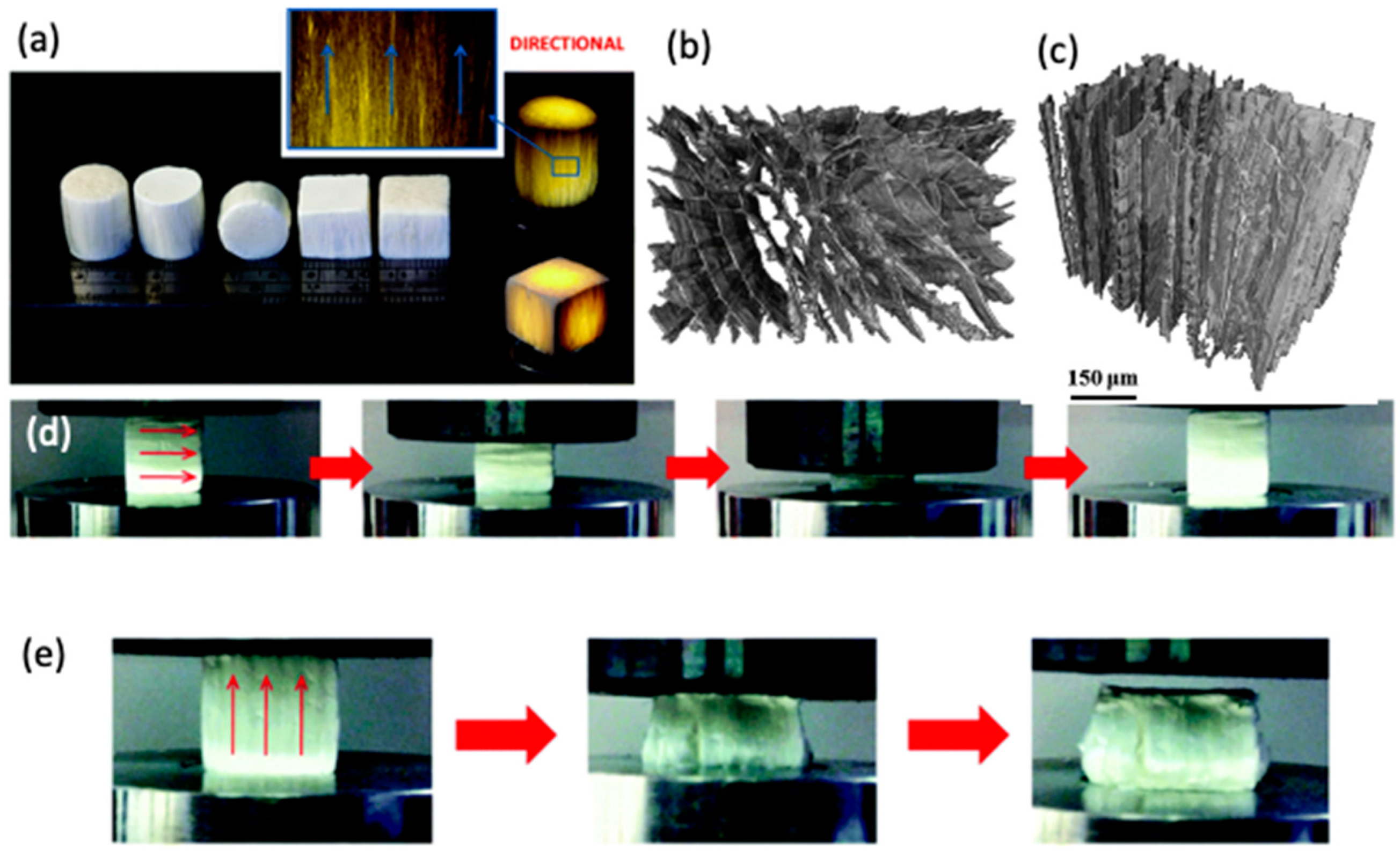
5.1. Class I Chitosan-Based Hybrids
5.2. Class II Chitosan-Based Hybrids
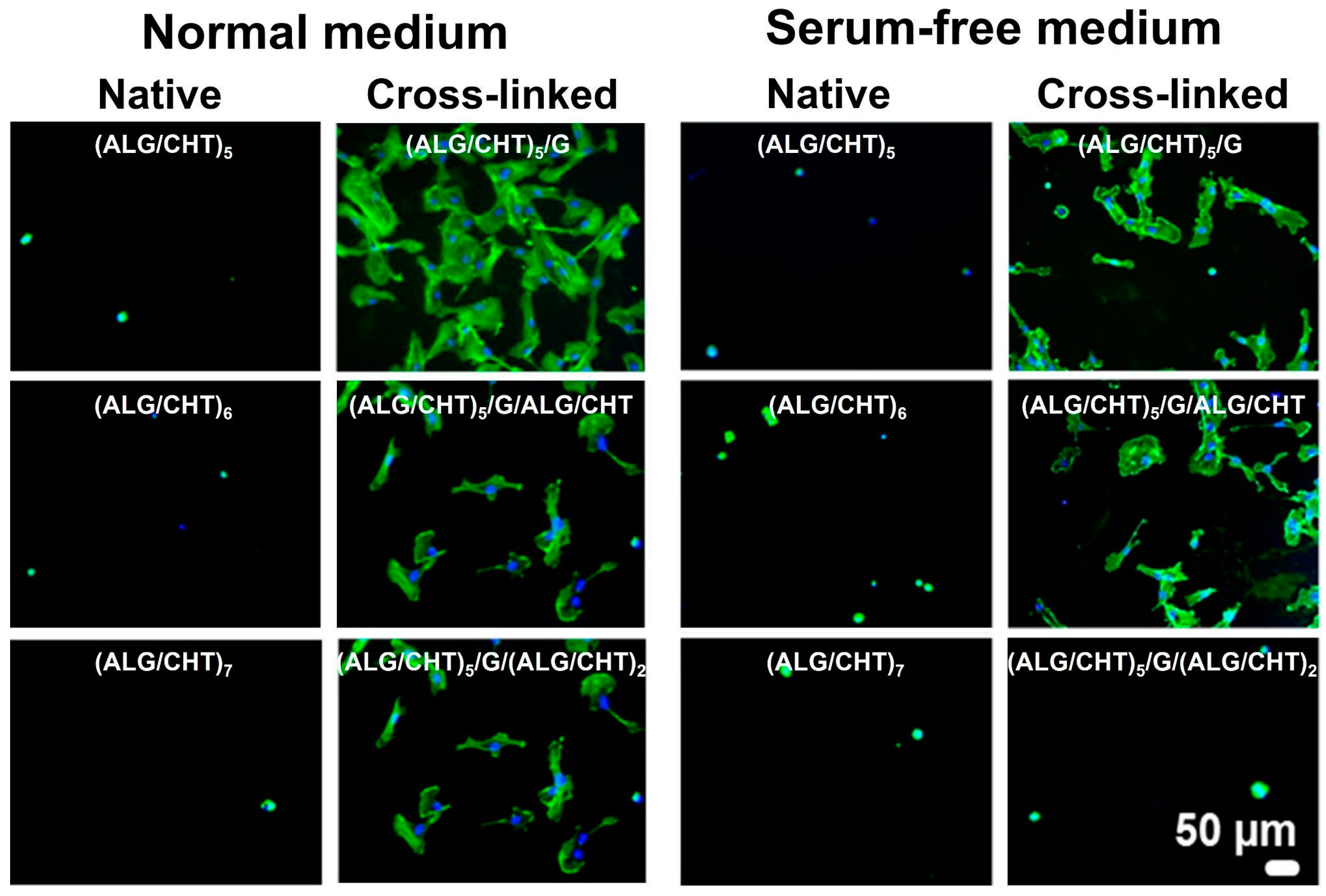
6. Chitosan-Based Layer-by-Layer Assemblies for Biomedical Applications
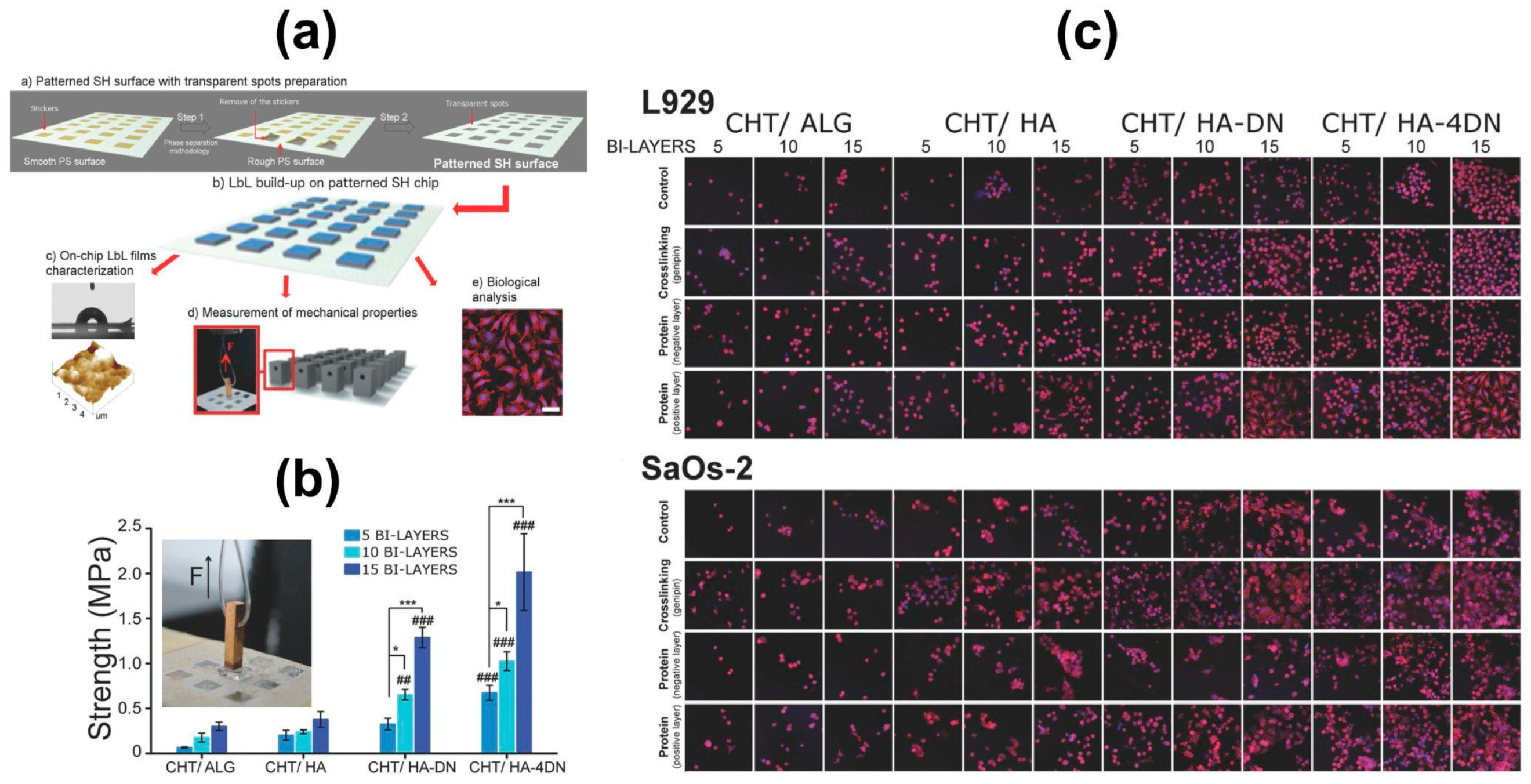
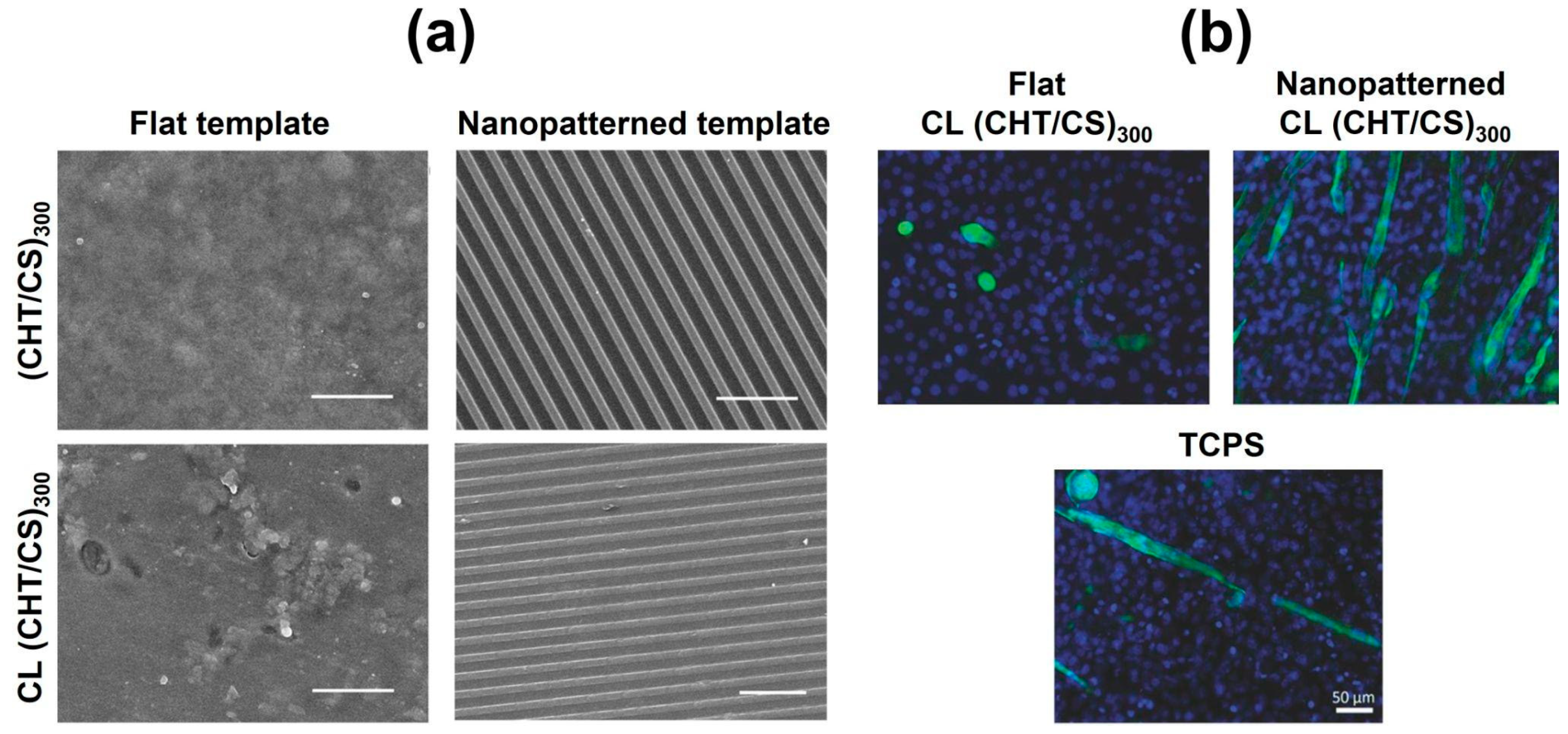
6.1. Multilayered Thin Films and Free-Standing Multilayered Membranes
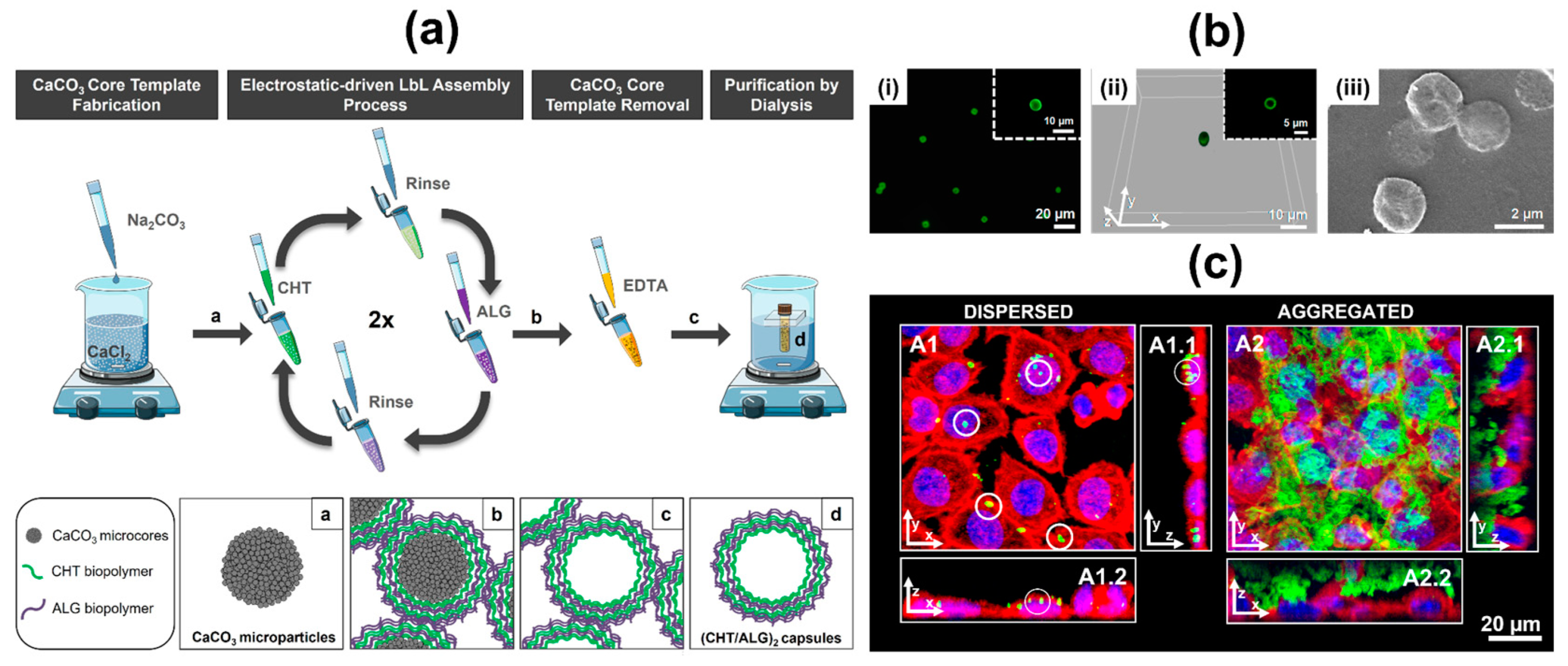
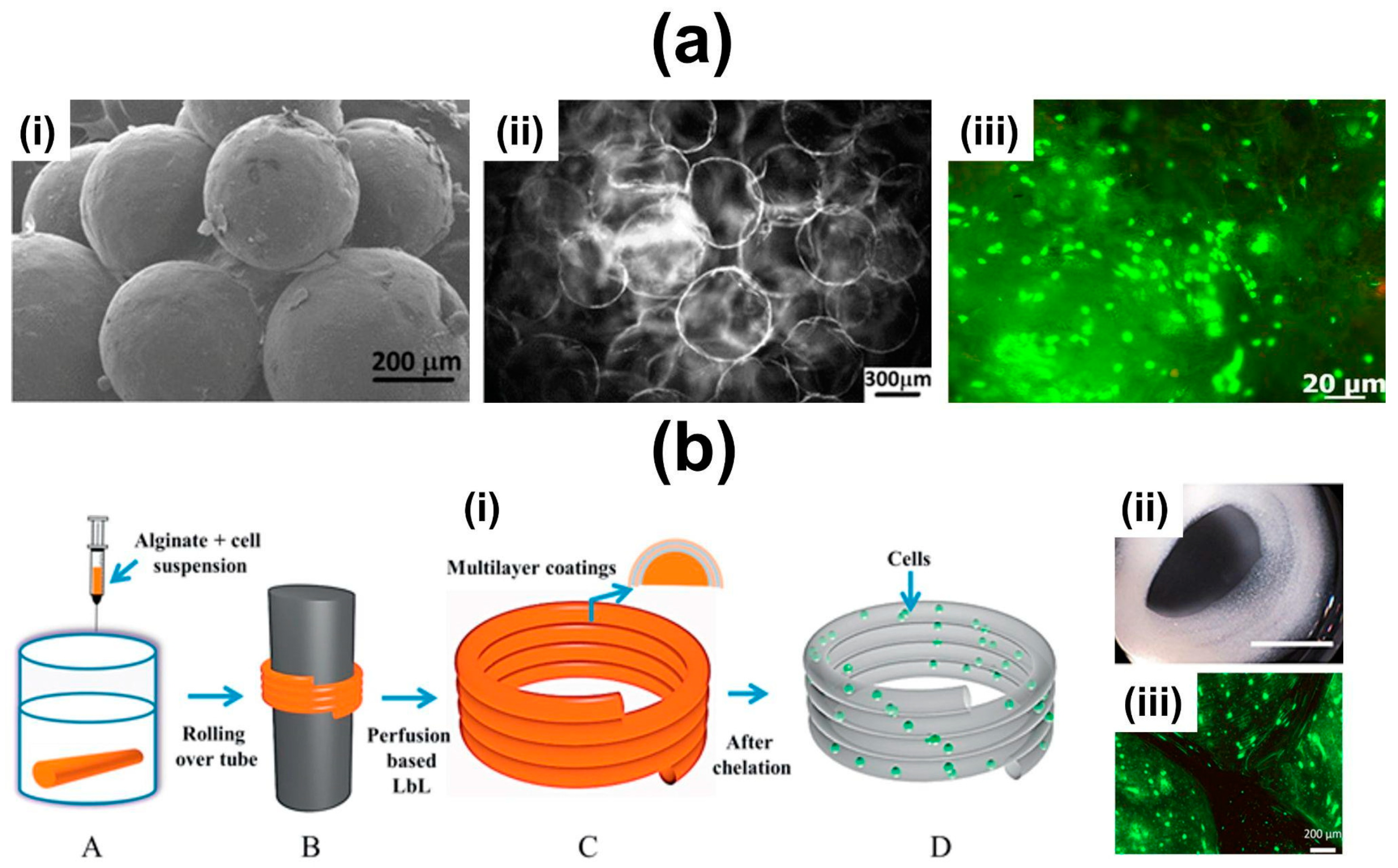
6.2. Multilayered Particles, Hollow Multilayered Capsules and Hierarchical (multi)Compartmentalized Capsules
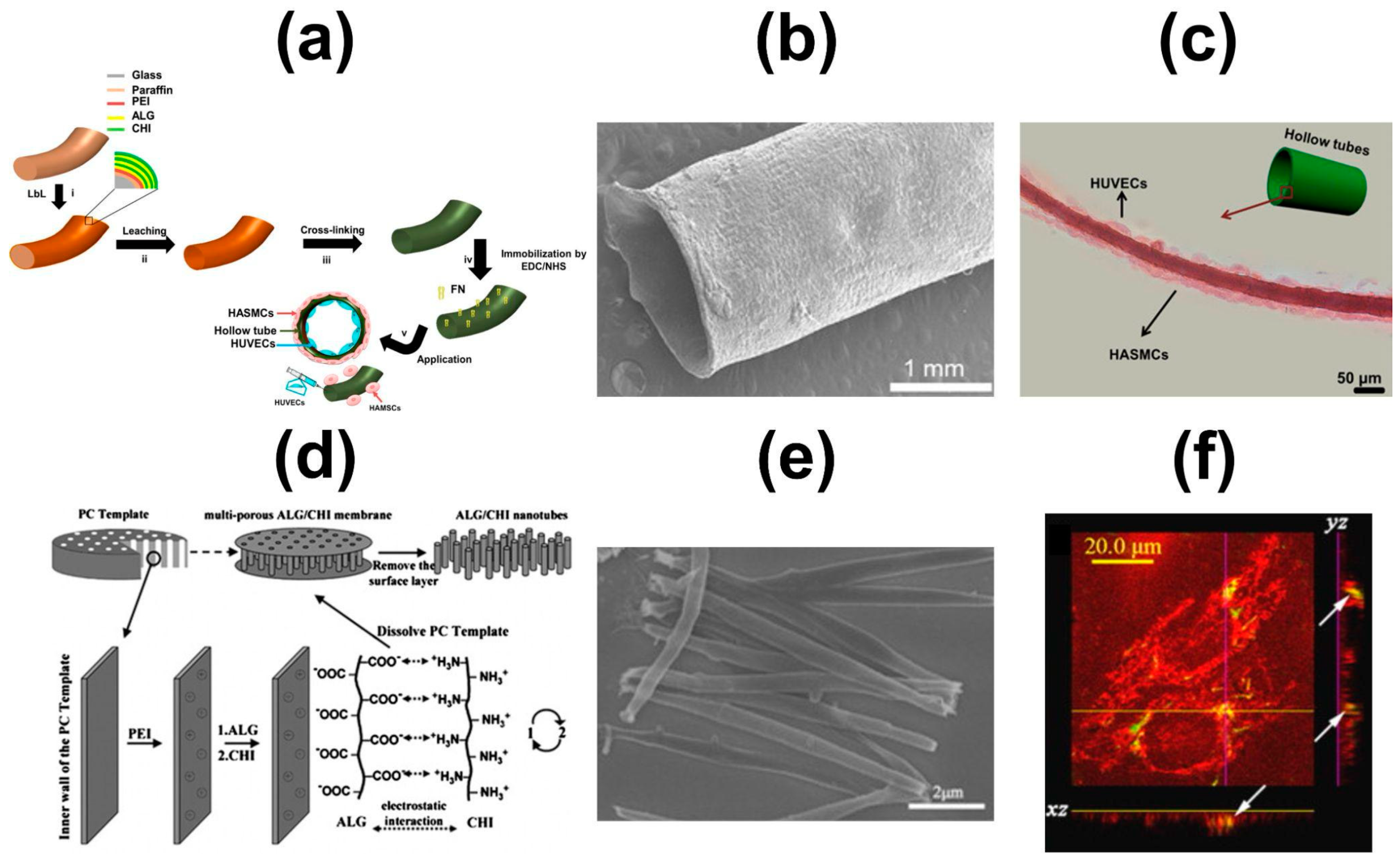
6.3. Hollow Multilayered Tubes
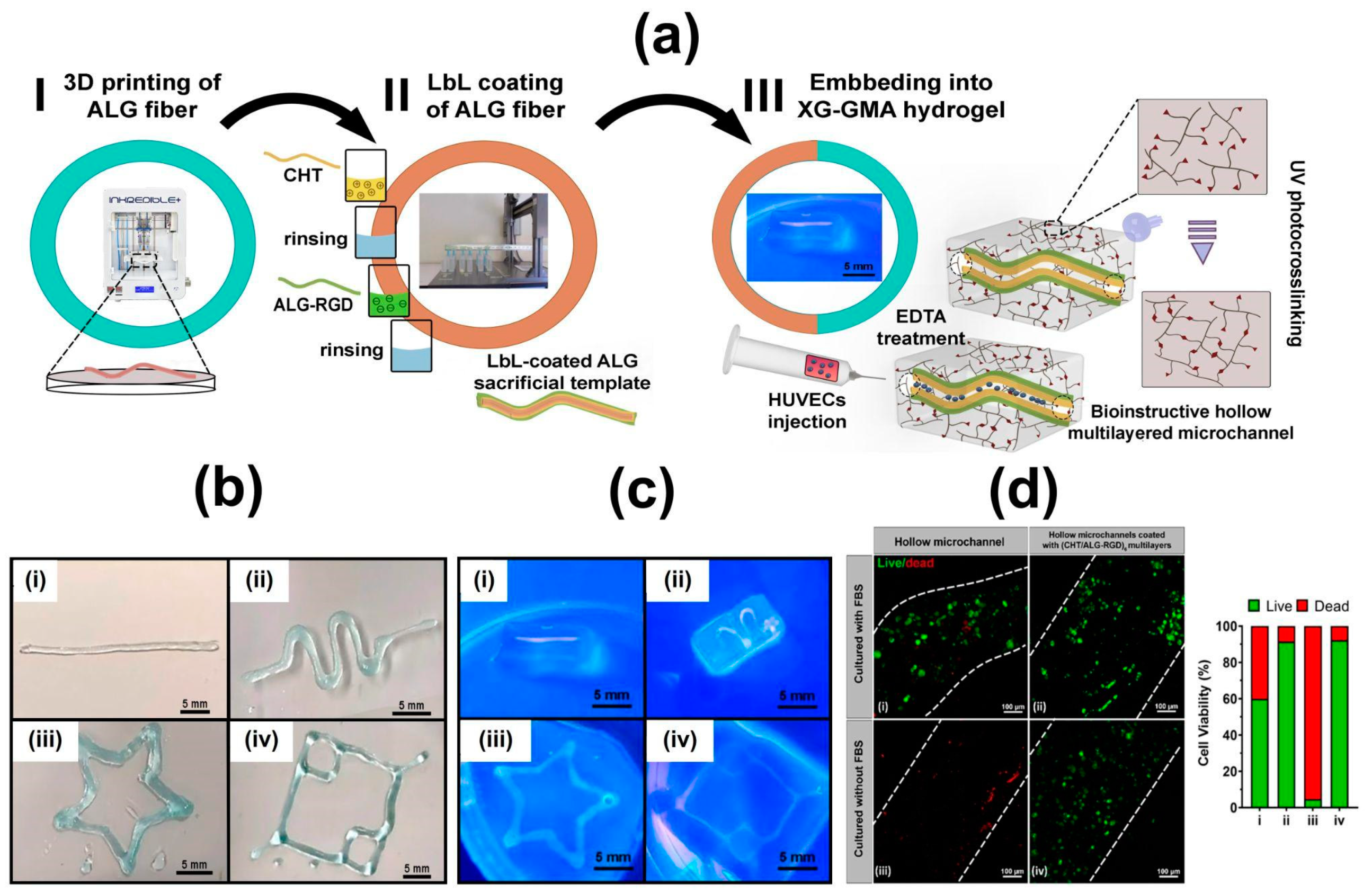
6.4. 3D Constructs
6.5. Living Cell Surfaces
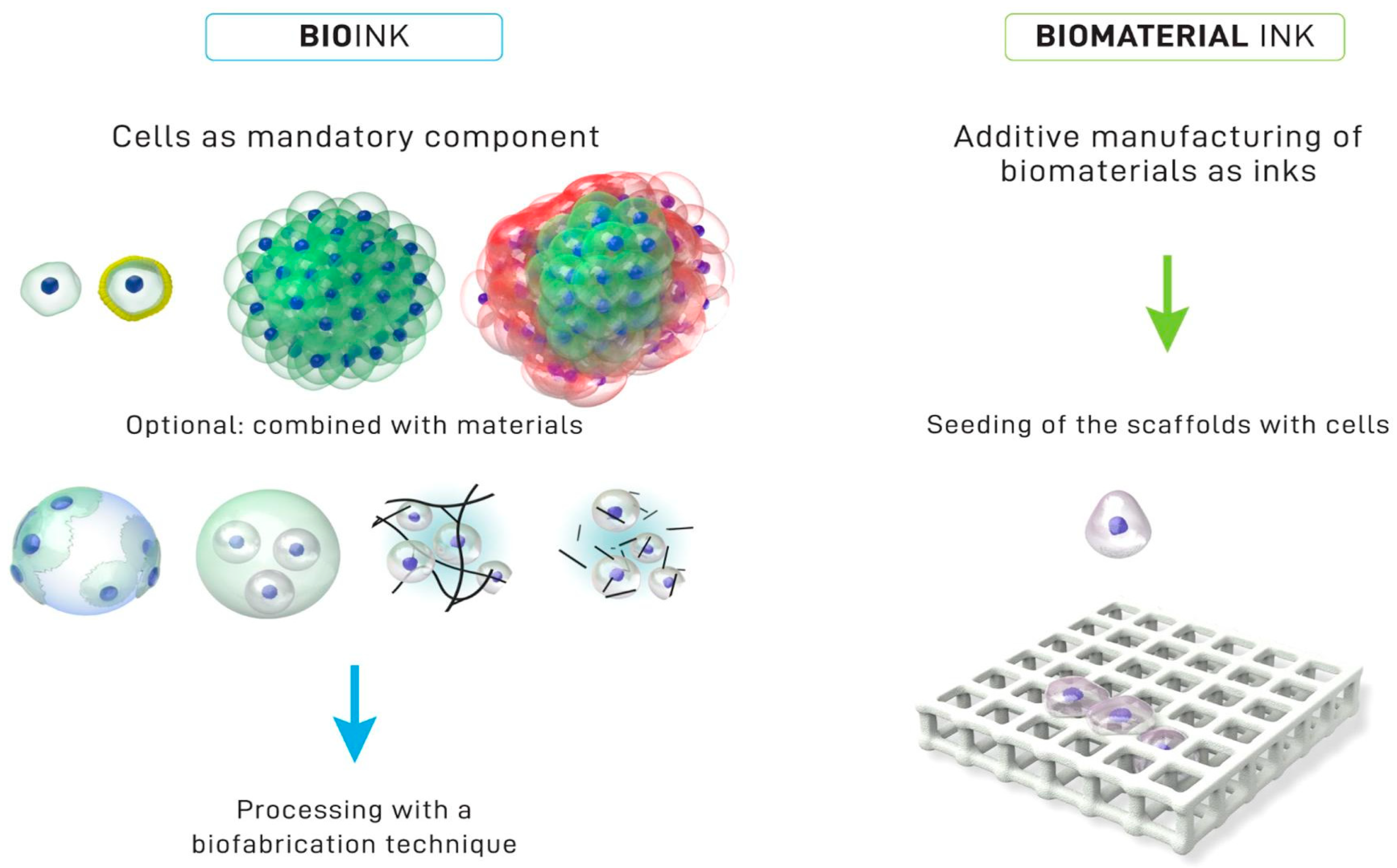
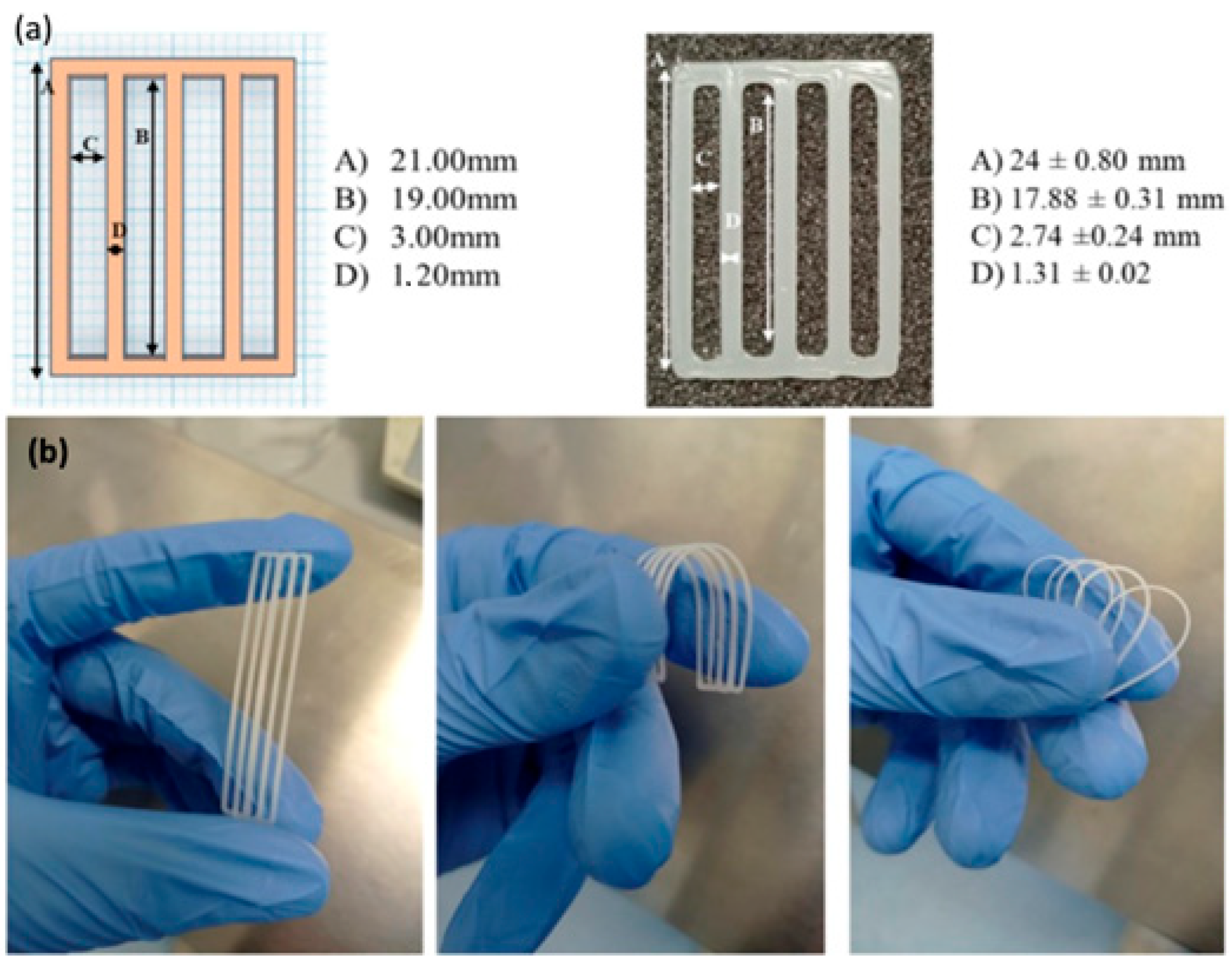
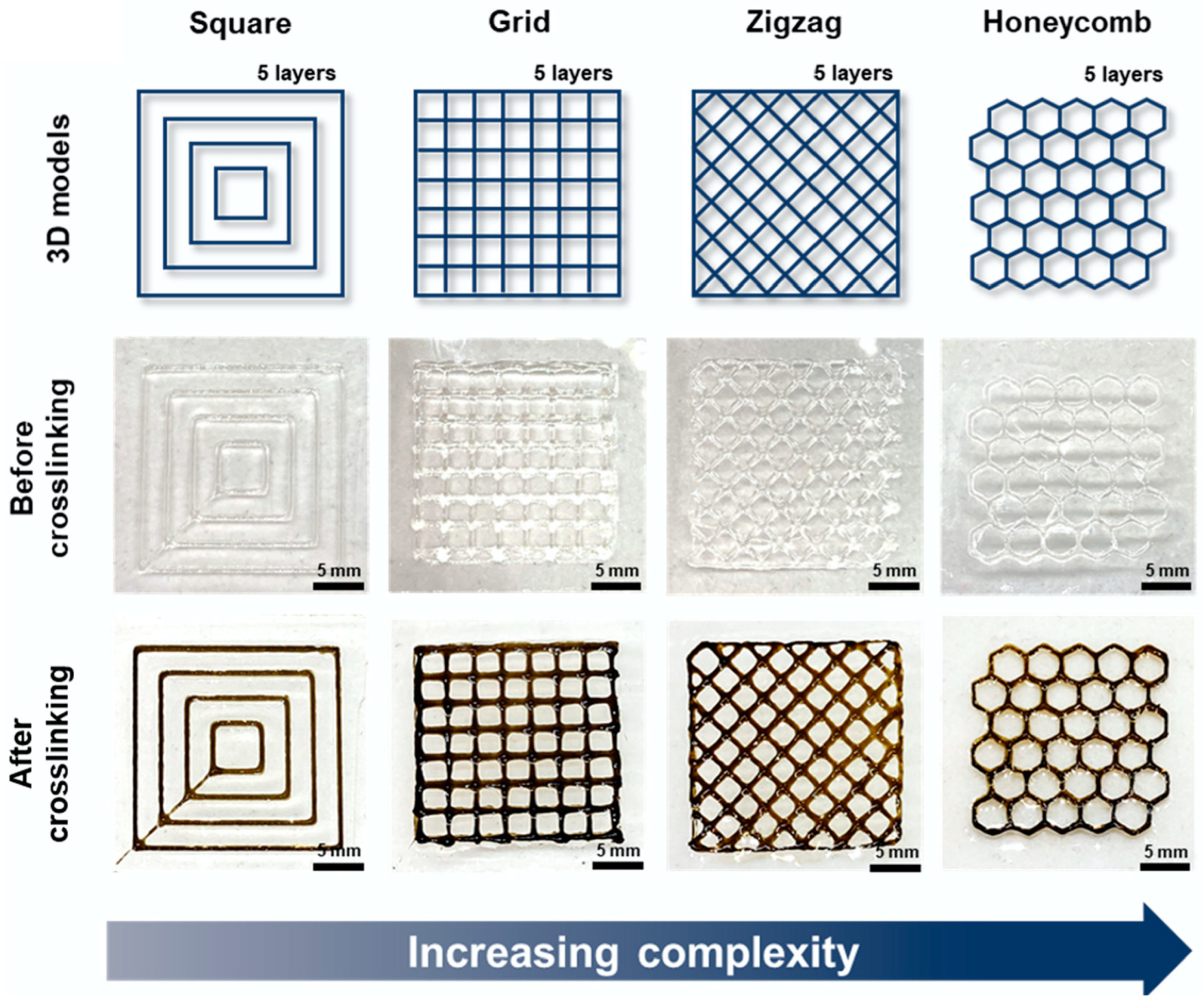
7. Chitosan-Based Inks for 3D Printing Applications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ALG | alginate |
| APTES | aminopropyl triethoxysilane |
| BCG | bacille Calmette-Guérin |
| BGNPs | bioactive glass nanoparticles |
| CAD | computer-aided drafting |
| CHT | chitosan |
| CS | chondroitin sulfate |
| DA | degree of acetylation |
| dECM | decellularized ECM |
| DMSO | dimethyl sulfoxide |
| DN | dopamine |
| DP | degree of polymerization |
| ECM | extracellular matrix |
| EDC | 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride |
| EDTA | ethylenediamine-tetraacetic acid |
| ELRs | elastin-like recombinamers |
| GlcN | 2-amino-2-deoxy-D-glucose, glucosamine |
| GlcNAc | 2-acetamido-2-deoxy-glucose N-acetylglucosamine |
| GPTMS | 3-glycidoxy propyl trimethoxysilane |
| IPN | interpenetrating polymer network |
| LbL | layer-by-layer |
| MNPs | magnetic nanoparticles |
| NHS | N-hydroxysuccinimide |
| PA | pattern of acetylation |
| PCL | poly(ε-caprolactone) |
| PEI | polyethyleneimine |
| PLA | poly(L-lactic) acid |
| PP | polypropylene |
| PPi | pyrophosphate |
| Q-CHT | quaternized chitosan |
| RGD | arginine-glycine-aspartic acid peptide |
| SIPN | semi-interpenetrating polymer network |
| TEM | transmission electron microscopy |
| TEOS | tetraethoxysilane |
| TEOS | tetraethoxysilane |
| TMX | tamoxifen |
| TPP | triple polyphosphate |
| TPS | trisodium phosphate |
| XG | xanthan gum |
| μCT | X-ray micro-computed tomography |
References
- Skarbek, K.; Milewska, M.J. Biosynthetic and Synthetic Access to Amino Sugars. Carbohydr. Res. 2016, 434, 44–71. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A “Hidden Figure” in the Fungal Cell Wall. Curr. Top. Microbiol. Immunol. 2020, 425, 83–111. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Tolaimate, A.; Rhazi, M.; Alagui, A.; Desbrieres, J.; Rinaudo, M. Valorization of Waste Products from Fishing Industry by Production of the Chitin and Chitosane. Phys. Chem. News 2008, 42, 120–127. [Google Scholar]
- Crini, G. Historical Landmarks in the Discovery of Chitin. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–47. ISBN 978-3-030-16538-3. [Google Scholar]
- Braconnot, H. Sur la nature des champignons. Ann. Chim. Phys. 1811, 79, 265–304. [Google Scholar]
- Rouget, C. Des Substances Amylaceesdans Les Tissus Des Animaux, Specialement Des Articules (Chitine). Comp. Rend. 1859, 48, 792–795. [Google Scholar]
- Hoppe-Seyler, F. Ueber Chitin Und Cellulose. Berichte Dtsch. Chem. Ges. 1894, 27, 3329–3331. [Google Scholar] [CrossRef]
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Austin, P.R. Chitin Solutions and Purification of Chitin. In Methods in Enzymology; Biomass Part B: Lignin, Pectin, and Chitin; Academic Press: New York, NY, USA, 1988; Volume 161, pp. 403–407. [Google Scholar]
- Funahashi, R.; Ono, Y.; Qi, Z.-D.; Saito, T.; Isogai, A. Molar Masses and Molar Mass Distributions of Chitin and Acid-Hydrolyzed Chitin. Biomacromolecules 2017, 18, 4357–4363. [Google Scholar] [CrossRef]
- Berezina, N. Production and Application of Chitin. Phys. Sci. Rev. 2016, 1. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Dimzon, I.K.D.; Ebert, J.; Knepper, T.P. The Interaction of Chitosan and Olive Oil: Effects of Degree of Deacetylation and Degree of Polymerization. Carbohydr. Polym. 2013, 92, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Desbrières, J.; Heux, L.; Mazeau, K.; Rinaudo, M. Overview on Structural Characterization of Chitosan Molecules in Relation with Their Behavior in Solution. Macromol. Symp. 2001, 168, 1–20. [Google Scholar] [CrossRef]
- Rusu-Balaita, L.; Desbrières, J.; Rinaudo, M. Formation of a Biocompatible Polyelectrolyte Complex: Chitosan-Hyaluronan Complex Stability. Polym. Bull. 2003, 50, 91–98. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A Validated 1H NMR Method for the Determination of the Degree of Deacetylation of Chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Roberts, G.A.F. The Road Is Long. In Proceedings of the 8th International Conference of the European Chitin Society, Antalya, Turkey, 8–11 September 2007. [Google Scholar]
- Basa, S.; Nampally, M.; Honorato, T.; Das, S.N.; Podile, A.R.; El Gueddari, N.E.; Moerschbacher, B.M. The Pattern of Acetylation Defines the Priming Activity of Chitosan Tetramers. J. Am. Chem. Soc. 2020, 142, 1975–1986. [Google Scholar] [CrossRef]
- Lopez, J.M.; Sánchez, L.F.; Nakamatsu, J.; Maruenda, H. Study of the Acetylation Pattern of Chitosan by Pure Shift NMR. Anal. Chem. 2020, 92, 12250–12256. [Google Scholar] [CrossRef]
- World Bio Mark. Insights. The Chitosan Packaging Industry Is only just Beginning. 2022. Available online: https://worldbiomarketinsights.com/the-chitosan-packaging-industry-is-only-just-beginning/ (accessed on 22 September 2022).
- Chitosan Market Size, Price Trends, Analysis & Forecast 2022–2027. Available online: https://www.imarcgroup.com/chitosan-market (accessed on 22 September 2022).
- Pappalardo, V.; Remadi, Y.; Cipolla, L.; Scotti, N.; Ravasio, N.; Zaccheria, F. Fishery Waste Valorization: Sulfated ZrO2 as a Heterogeneous Catalyst for Chitin and Chitosan Depolymerization. Front. Chem. 2022, 10, 1057461. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, M.M.A.; Borges, J.; Correia, F.J.S.; Calado, R.; Rodrigues, J.M.M.; Patrício, S.G.; Mano, J.F. Green Approaches for Extraction, Chemical Modification and Processing of Marine Polysaccharides for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 1041102. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.-L. Chitin Extraction from Shrimp Waste by Liquid Fermentation Using an Alkaline Protease-Producing Strain, Brevibacillus Parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can Fungi Compete with Marine Sources for Chitosan Production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Kurakula, M. Prospection of Recent Chitosan Biomedical Trends: Evidence from Patent Analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef]
- Cohen, E.; Poverenov, E. Hydrophilic Chitosan Derivatives: Synthesis and Applications. Chem. Eur. J. 2022, 28, e202202156. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and Sulfated Chitosan Derivatives for Biomedical Applications: A Review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Chemistry and Applications of Phosphorylated Chitin and Chitosan. E-Polym. 2006, 6. [Google Scholar] [CrossRef]
- Jothimani, B.; Sureshkumar, S.; Venkatachalapathy, B. Hydrophobic Structural Modification of Chitosan and Its Impact on Nanoparticle Synthesis—A Physicochemical Study. Carbohydr. Polym. 2017, 173, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.; Korchagina, E. Chitosan and Its Hydrophobic Derivatives: Preparation and Aggregation in Dilute Aqueous Solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Al-Sabagh, A.M. Surface Active Properties of Chitosan and Its Derivatives. Colloids Surf. B Biointerfaces 2009, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic Derivatives of Chitosan: Characterization and Rheological Behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.B.; Smith, W.; Balogh, P.; Duggan, M.J.; MacIntire, I.C.; Harris, E.; Mesar, T.; Raghavan, S.R.; King, D.R. Hydrophobically-Modified Chitosan Foam: Description and Hemostatic Efficacy. J. Surg. Res. 2015, 193, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Alkabli, J. Progress in Preparation of Thiolated, Crosslinked, and Imino-Chitosan Derivatives Targeting Specific Applications. Eur. Polym. J. 2022, 165, 110998. [Google Scholar] [CrossRef]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated Chitosans: A Multi-Talented Class of Polymers for Various Applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef] [PubMed]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated Polymeric Hydrogels for Biomedical Application: Cross-Linking Mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Wibel, R.; Braun, D.E.; Hämmerle, L.; Jörgensen, A.M.; Knoll, P.; Salvenmoser, W.; Steinbring, C.; Bernkop-Schnürch, A. In Vitro Investigation of Thiolated Chitosan Derivatives as Mucoadhesive Coating Materials for Solid Lipid Nanoparticles. Biomacromolecules 2021, 22, 3980–3991. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Z. Advances in Alkylated Chitosan and Its Applications for Hemostasis. Macromol 2022, 2, 346–360. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Durand, G.; le Foll, M.D. Functionalization of Chitosan Oligomers: From Aliphatic Epoxide to Cardanol-Grafted Oligomers for Oil-in-Water Emulsions. Biomacromolecules 2021, 22, 846–854. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric Oxide-Releasing Chitosan Oligosaccharides as Antibacterial Agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef]
- Hirano, S.; Ohe, Y.; Ono, H. Selective N-Acylation of Chitosan. Carbohydr. Res. 1976, 47, 315–320. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S. Michael Reaction of Chitosan with Various Acryl Reagents in Water. Biomacromolecules 2003, 4, 1250–1254. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A Review on Applications of Chitosan-Based Schiff Bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary Ammonium Chitosans: The Importance of the Positive Fixed Charge of the Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Sahariah, P.; Hjálmarsdóttir, M.Á.; Másson, M. Chitosan-Hydroxycinnamic Acid Conjugates: Optimization of the Synthesis and Investigation of the Structure Activity Relationship. Carbohydr. Polym. 2022, 277, 118896. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Mol. Basel Switz. 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Orsini, S.F.; Cipolla, L.; Petroni, S.; Dirè, S.; Ceccato, R.; Callone, E.; Bongiovanni, R.; Dalle Vacche, S.; Di Credico, B.; Mostoni, S.; et al. Synthesis and Characterization of Alkoxysilane-Bearing Photoreversible Cinnamic Side Groups: A Promising Building-Block for the Design of Multifunctional Silica Nanoparticles. Langmuir 2022, 38, 15662–15671. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Ikeda, H.; Shimojoh, M.; Yang, J. N-Phthaloylated Chitosan as an Essential Precursor for Controlled Chemical Modifications of Chitosan: Synthesis and Evaluation. Polym. J. 2007, 39, 945–952. [Google Scholar] [CrossRef]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective Protection of the Amino Groups of Chitosan by Controlled Phthaloylation: Facile Preparation of a Precursor Useful for Chemical Modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef]
- Torii, Y.; Ikeda, H.; Shimojoh, M.; Kurita, K. Chemoselective Protection of Chitosan by Dichlorophthaloylation: Preparation of a Key Intermediate for Chemical Modifications. Polym. Bull. 2009, 62, 749–759. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Zampano, G.; Bertoldo, M.; Ciardelli, F. Defined Chitosan-Based Networks by C-6-Azide–Alkyne “Click” Reaction. React. Funct. Polym. 2010, 70, 272–281. [Google Scholar] [CrossRef]
- Revuelta, J.; Fraile, I.; T. Monterrey, D.; Peña, N.; Benito-Arenas, R.; Bastida, A.; Fernández-Mayoralas, A.; García-Junceda, E. Heparanized Chitosans: Towards the Third Generation of Chitinous Biomaterials. Mater. Horiz. 2021, 8, 2596–2614. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.; Wang, L.; Du, J.; Dong, K.; Wang, C.; Liu, X. Study of Two Chitosan Derivatives Phosphorylated at Hydroxyl or Amino Groups for Application as Flocculants. J. Appl. Polym. Sci. 2012, 125, E299–E305. [Google Scholar] [CrossRef]
- Shokri, Z.; Seidi, F.; Saeb, M.R.; Jin, Y.; Li, C.; Xiao, H. Elucidating the Impact of Enzymatic Modifications on the Structure, Properties, and Applications of Cellulose, Chitosan, Starch and Their Derivatives: A Review. Mater. Today Chem. 2022, 24, 100780. [Google Scholar] [CrossRef]
- Linhorst, M.; Wattjes, J.; Moerschbacher, B.M. Chitin Deacetylase as a Biocatalyst for the Selective N-Acylation of Chitosan Oligo- and Polymers. ACS Catal. 2021, 11, 14456–14466. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liang, X.; McClements, D.J.; Liu, X.; Liu, F. Applications of Oxidases in Modification of Food Molecules and Colloidal Systems: Laccase, Peroxidase and Tyrosinase. Trends Food Sci. Technol. 2020, 103, 78–93. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Javvaji, V.; Kim, E.; Lee, M.E.; Raghavan, S.R.; Wang, Q.; Payne, G.F. Tyrosinase-Mediated Grafting and Crosslinking of Natural Phenols Confers Functional Properties to Chitosan. Biochem. Eng. J. 2014, 89, 21–27. [Google Scholar] [CrossRef]
- Liu, N.; Ni, S.; Gao, H.; Chang, Y.; Fu, Y.; Liu, W.; Qin, M. Laccase-Catalyzed Grafting of Lauryl Gallate on Chitosan to Improve Its Antioxidant and Hydrophobic Properties. Biomacromolecules 2021, 22, 4501–4509. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, N.; Wang, Q.; Yuan, J.; Yu, Y.; Wang, P.; Fan, X. Eco-Friendly Grafting of Chitosan as a Biopolymer onto Wool Fabrics Using Horseradish Peroxidase. Fibers Polym. 2019, 20, 261–270. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic Synthesis of Chitosan Derivatives and Their Potential Applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Berti, F.; Toffoli, G. Chitosan-Based Biocompatible Copolymers for Thermoresponsive Drug Delivery Systems: On the Development of a Standardization System. Pharmaceutics 2021, 13, 1876. [Google Scholar] [CrossRef]
- Acciaretti, F.; Vesentini, S.; Cipolla, L. Fabrication Strategies towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem. Asian J. 2022, 17, e202200797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan Grafted/Cross-Linked with Biodegradable Polymers: A Review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Dey, S.; Nayak, A.K. Chapter 12—Graft Copolymers of Chitosan in Drug Delivery Applications. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 301–322. ISBN 978-0-12-819336-5. [Google Scholar]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A Review on the Synthesis of Graft Copolymers of Chitosan and Their Potential Applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and Graft Copolymers of Chitosan and Its Pharmaceutical Applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Russo, L.; Battocchio, C.; Secchi, V.; Magnano, E.; Nappini, S.; Taraballi, F.; Gabrielli, L.; Comelli, F.; Papagni, A.; Costa, B.; et al. Thiol–Ene Mediated Neoglycosylation of Collagen Patches: A Preliminary Study. Langmuir 2014, 30, 1336–1342. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Skorik, Y.A. Click Reactions in Chitosan Chemistry. Russ. Chem. Bull. 2017, 66, 769–781. [Google Scholar] [CrossRef]
- Bini, D.; Russo, L.; Battocchio, C.; Natalello, A.; Polzonetti, G.; Doglia, S.M.; Nicotra, F.; Cipolla, L. Dendron Synthesis and Carbohydrate Immobilization on a Biomaterial Surface by a Double-Click Reaction. Org. Lett. 2014, 16, 1298–1301. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs. 2022, 20, 536. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.B.; dos Santos, A.M.; Boni, F.I.; dos Santos, K.C.; Robusti, L.M.G.; de Souza, M.P.C.; Ferreira, N.N.; Carvalho, S.G.; Cardoso, V.M.O.; Chorilli, M.; et al. Design of Chitosan-Based Particle Systems: A Review of the Physicochemical Foundations for Tailored Properties. Carbohydr. Polym. 2020, 250, 116968. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Fernando, L.D.; Dickwella Widanage, M.C.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.-P.; Wang, P.; Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls From Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 727053. [Google Scholar] [CrossRef]
- Faria, R.R.; Guerra, R.F.; de Sousa Neto, L.R.; Motta, L.F.; de Franca, E.F. Computational Study of Polymorphic Structures of α- and β- Chitin and Chitosan in Aqueous Solution. J. Mol. Graph. Model. 2016, 63, 78–84. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Moustafa, M.E.; Yang, H. Ecofriendly Method to Dissolve Chitosan in Plain Water. ACS Biomater. Sci. Eng. 2019, 5, 6355–6360. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Freitas, E.D.; Moura, C.F.; Kerwald, J.; Beppu, M.M. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers 2020, 12, 2878. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, J.; Qin, W.; Hu, Q.; Tang, B.Z. Gelation Process Visualized by Aggregation-Induced Emission Fluorogens. Nat. Commun. 2016, 7, 12033. [Google Scholar] [CrossRef]
- Ru, G.; Wu, S.; Yan, X.; Liu, B.; Gong, P.; Wang, L.; Feng, J. Inverse Solubility of Chitin/Chitosan in Aqueous Alkali Solvents at Low Temperature. Carbohydr. Polym. 2019, 206, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific Manipulations of a Rigid Polysaccharide: Syntheses of Novel Chitosan Derivatives with Excellent Solubility in Common Organic Solvents by Regioselective Chemical Modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]
- Wang, S.; Sha, J.; Wang, W.; Qin, C.; Li, W.; Qin, C. Superhydrophobic Surfaces Generated by One-Pot Spray-Coating of Chitosan-Based Nanoparticles. Carbohydr. Polym. 2018, 195, 39–44. [Google Scholar] [CrossRef] [PubMed]
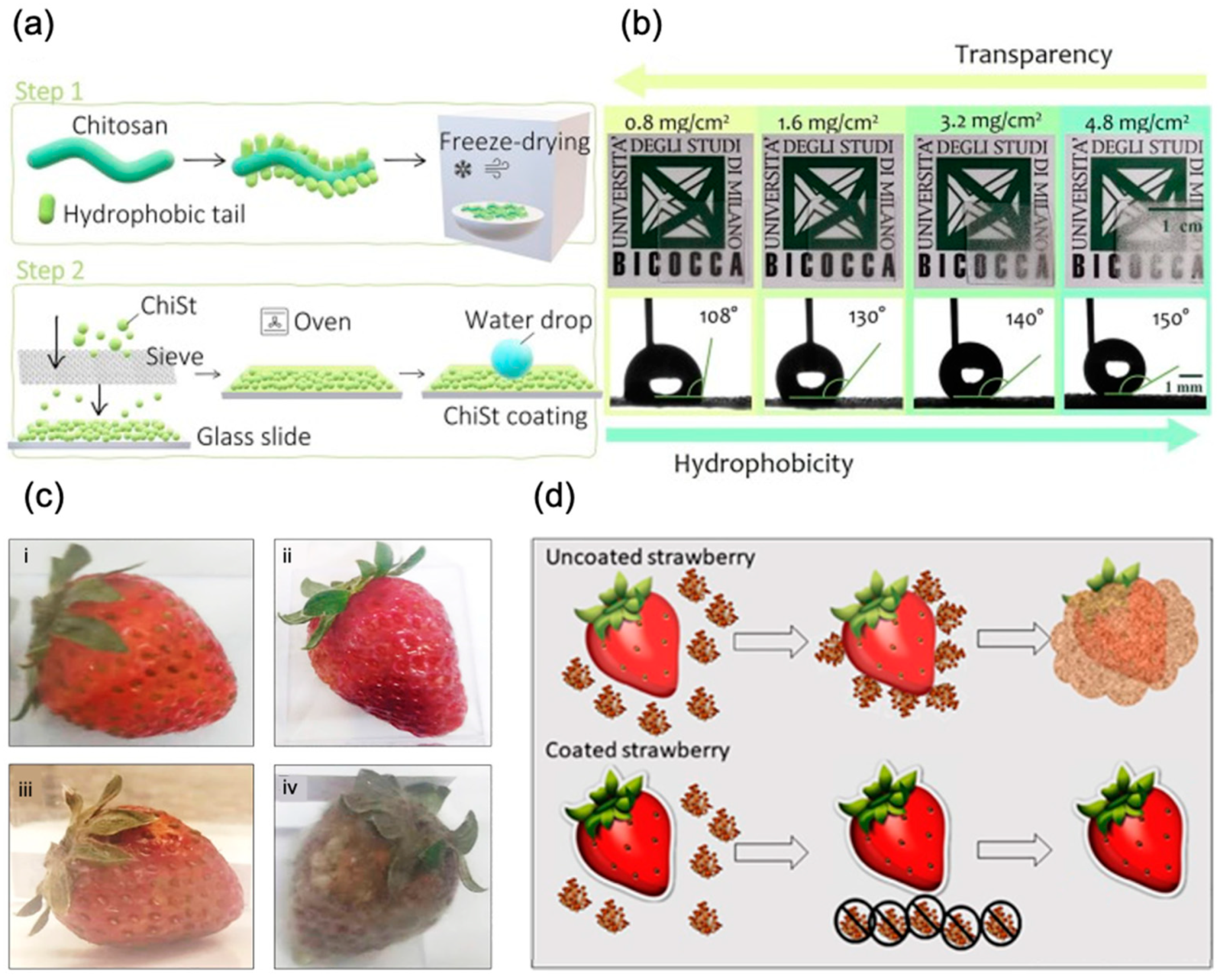
- Tagliaro, I.; Seccia, S.; Pellegrini, B.; Bertini, S.; Antonini, C. Chitosan-Based Coatings with Tunable Transparency and Superhydrophobicity: A Solvent-Free and Fluorine-Free Approach by Stearoyl Derivatization. Carbohydr. Polym. 2023, 302, 120424. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; García-Abuín, A.; Gómez-Díaz, D.; Navaza, J.M. Physicochemical Characterization of Chitosan Derivatives. CyTA J. Food 2013, 11, 190–197. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Mati-Baouche, N.; Elchinger, P.-H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an Adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-Based Pickering Emulsions and Their Applications: A Review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Heuzey, M.-C. Chitosan-Based Conventional and Pickering Emulsions with Long-Term Stability. Langmuir 2016, 32, 929–936. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2020; pp. 457–489. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Jacob, M.; Vignesh, N.S.; Varalakshmi, P. Pristine and Modified Chitosan as Solid Catalysts for Catalysis and Biodiesel Production: A Minireview. Int. J. Biol. Macromol. 2021, 167, 807–833. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.R.L.; Moraes, J.S. Removal of Organic Pollutants from Wastewater Using Chitosan: A Literature Review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into Recent Advances of Chitosan-Based Adsorbents for Sustainable Removal of Heavy Metals and Anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Annu; Raja, A.N. Recent Development in Chitosan-Based Electrochemical Sensors and Its Sensing Application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Antos, D.; Górak, A. Review on the Application of Chitin and Chitosan in Chromatography. React. Funct. Polym. 2020, 152, 104606. [Google Scholar] [CrossRef]
- Klinkesorn, U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
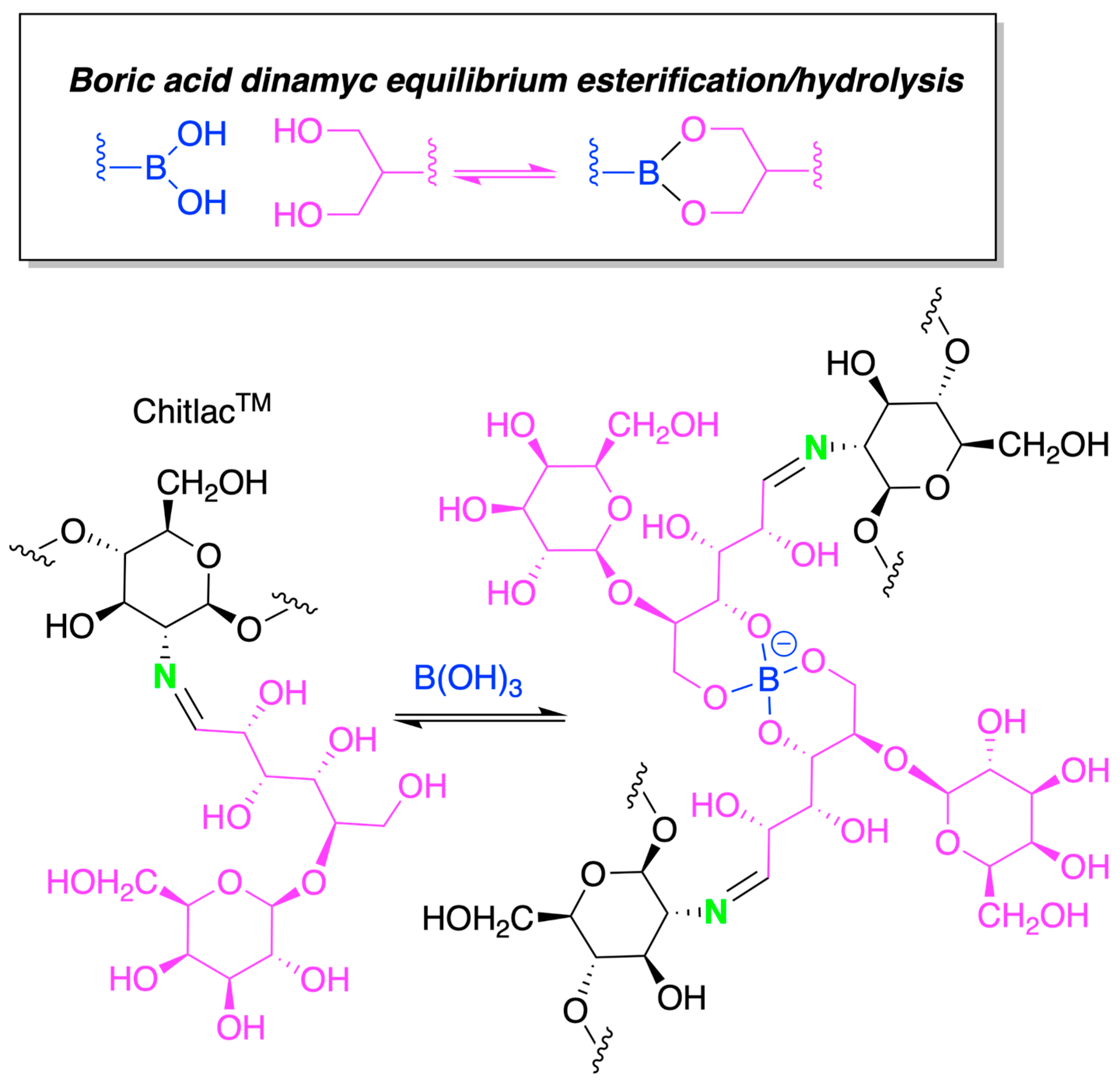
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular Structuring of Hyaluronan-Lactose-Modified Chitosan Matrix: Towards High-Performance Biopolymers with Excellent Biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.-L.; Cheroni, C.; Borbély, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From Cohorts to Molecules: Adverse Impacts of Endocrine Disrupting Mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with Chitosan-Based Edible Films for Mechanical/Biological Protection of Strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Gupta, S.; Santhosh, R.; Syed, I.; Sarkar, P. 3—Biopolymer-Based Edible Films and Coatings for Food Applications. In Food, Medical, and Environmental Applications of Polysaccharides; Pal, K., Banerjee, I., Sarkar, P., Bit, A., Kim, D., Anis, A., Maji, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–107. ISBN 978-0-12-819239-9. [Google Scholar]
- Vu, K.D.; Hollingsworth, R.G.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of Edible Bioactive Coating Based on Modified Chitosan for Increasing the Shelf Life of Strawberries. Food Res. Int. 2011, 44, 198–203. [Google Scholar] [CrossRef]
- Quintana, S.E.; Llalla, O.; García-Zapateiro, L.A.; García-Risco, M.R.; Fornari, T. Preparation and Characterization of Licorice-Chitosan Coatings for Postharvest Treatment of Fresh Strawberries. Appl. Sci. 2020, 10, 8431. [Google Scholar] [CrossRef]
- Salvati Manni, L.; Assenza, S.; Duss, M.; Vallooran, J.J.; Juranyi, F.; Jurt, S.; Zerbe, O.; Landau, E.M.; Mezzenga, R. Soft Biomimetic Nanoconfinement Promotes Amorphous Water over Ice. Nat. Nanotechnol. 2019, 14, 609–615. [Google Scholar] [CrossRef]
- Kocherbitov, V. The Nature of Nonfreezing Water in Carbohydrate Polymers. Carbohydr. Polym. 2016, 150, 353–358. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Kawai, K.; Suzuki, T. Impacts of Freezing and Molecular Size on Structure, Mechanical Properties and Recrystallization of Freeze-Thawed Polysaccharide Gels. LWT Food Sci. Technol. 2016, 68, 190–201. [Google Scholar] [CrossRef]
- Maity, T.; Saxena, A.; Raju, P.S. Use of Hydrocolloids as Cryoprotectant for Frozen Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 420–435. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Mohammed, A.M.; Saleem, I.; Alsharidah, M.; Al Rugaie, O.; Ahmed, F.; Osman, S.K. Smart Injectable Chitosan Hydrogels Loaded with 5-Fluorouracil for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 661. [Google Scholar] [CrossRef]
- Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.S.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Kurakula, M.; Raghavendra Naveen, N. Electrospraying: A Facile Technology Unfolding the Chitosan Based Drug Delivery and Biomedical Applications. Eur. Polymer J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Patel, B.; Manne, R.; Patel, D.B.; Gorityala, S.; Palaniappan, A.; Kurakula, M. Chitosan as Functional Biomaterial for Designing Delivery Systems in Cardiac Therapies. Gels 2021, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Y.; Wu, Y. Advances in Chitosan-Based Drug Delivery Vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Kurakula, M.; Gorityala, S.; Patel, D.B.; Basim, P.; Patel, B.; Kumar Jha, S. Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries. Polysaccharides 2021, 2, 519–537. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-Based Nanoparticles as Drug Delivery Systems: A Review on Two Decades of Research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Self-Associating Polymers Chitosan and Hyaluronan for Constructing Composite Membranes as Skin-Wound Dressings Carrying Therapeutics. Mol. Basel Switz. 2021, 26, 2535. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The Chitosan Hydrogels: From Structure to Function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels Basel Switz. 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and Interactions in Chitosan Hydrogels Formed by Complexation or Aggregation for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and Interactions in Covalently and Ionically Crosslinked Chitosan Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Sheng, Y.; Cao, C.; Liang, Z.; Yin, Z.-Z.; Gao, J.; Cai, W.; Kong, Y. Construction of a Dual-Drug Delivery System Based on Oxidized Alginate and Carboxymethyl Chitosan for Chemo-Photothermal Synergistic Therapy of Osteosarcoma. Eur. Polym. J. 2022, 174, 111331. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of PH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable Antibacterial Conductive Hydrogels with Dual Response to an Electric Field and PH for Localized “Smart” Drug Release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels 2016, 2, 28. [Google Scholar] [CrossRef]
- Sgambato, A.; Pastori, V.; Russo, L.; Vesentini, S.; Lecchi, M.; Cipolla, L. Neoglycosylated Collagen: Effect on Neuroblastoma F-11 Cell Lines. Molecules 2020, 25, 4361. [Google Scholar] [CrossRef]
- Russo, L.; Cipolla, L. Glycomics: New Challenges and Opportunities in Regenerative Medicine. Chem. Eur. J. 2016, 22, 13380–13388. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Sgambato, A.; Lecchi, M.; Pastori, V.; Raspanti, M.; Natalello, A.; Doglia, S.M.; Nicotra, F.; Cipolla, L. Neoglucosylated Collagen Matrices Drive Neuronal Cells to Differentiate. ACS Chem. Neurosci. 2014, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Morrish, C.; Whitehead, F.; Istivan, T.; Kasapis, S. The Effect of Trisodium Phosphate Crosslinking on the Diffusion Kinetics of Caffeine from Chitosan Networks. Food Chem. 2022, 381, 132272. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the Correlation between the Microscopic Structure and Properties of Phosphate-Cross-Linked Chitosan Gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic Hydrogel Based on Chitosan Cross-Linked with 6-Phosphogluconic Trisodium Salt as a Drug Delivery System. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-Based Networks from Homogeneous Chitosan-Tripolyphosphate Hydrogels: Synthesis and Characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
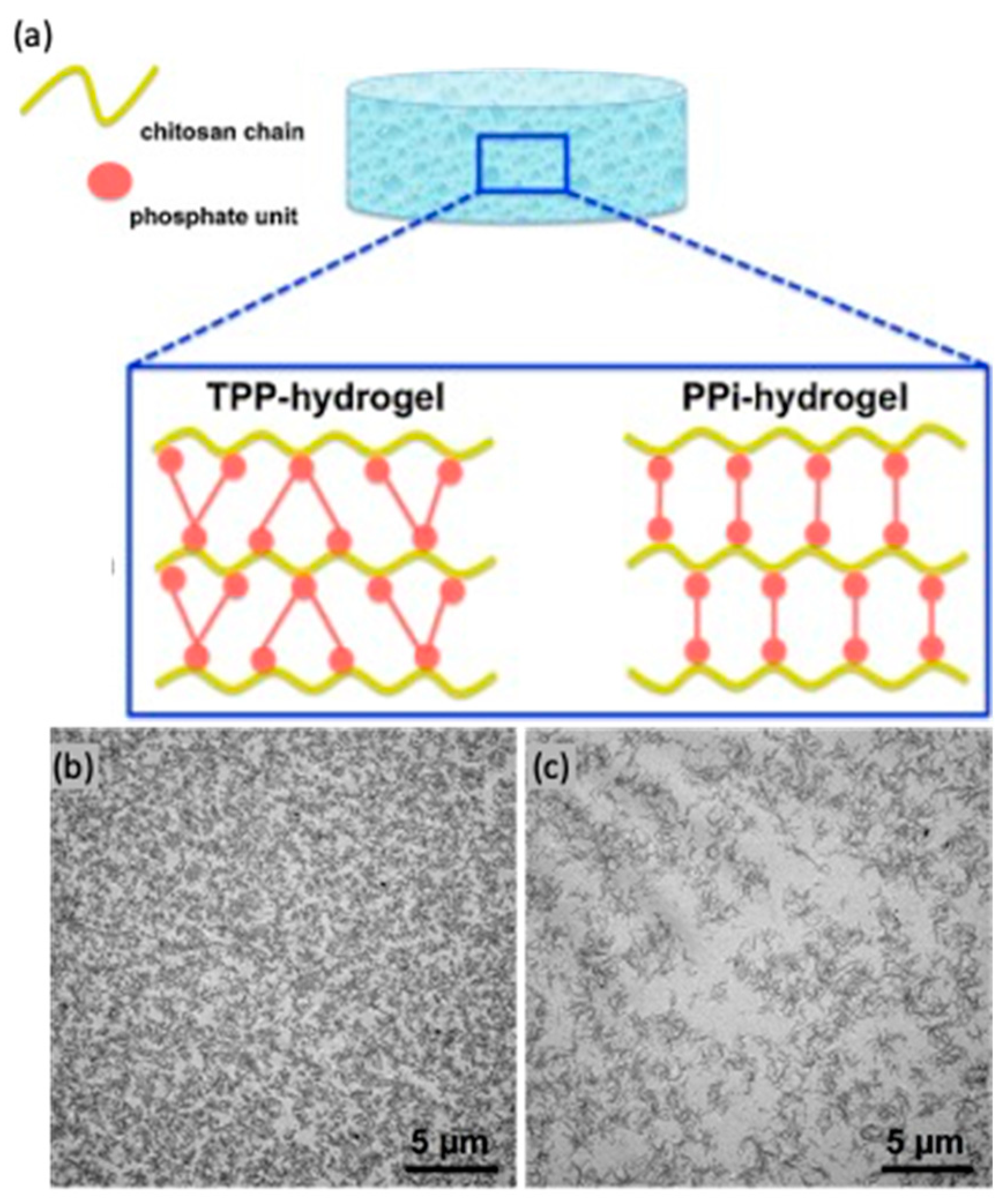
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the Ionotropic Gelation of Chitosan Using Tripolyphosphate and Pyrophosphate as Cross-Linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological Study of Chitosan/Polyol-Phosphate Systems: Influence of the Polyol Part on the Thermo-Induced Gelation Mechanism. Langmuir ACS J. Surf. Colloids 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive Chitosan/Glycerophosphate-Based Hydrogel and Its Derivatives in Pharmaceutical and Biomedical Applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A Review on Injectable Chitosan/Beta Glycerophosphate Hydrogels for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Boisclair, J.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Chitosan/Glucose 1-Phosphate as New Stable in Situ Forming Depot System for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2014, 88, 361–373. [Google Scholar] [CrossRef] [PubMed]
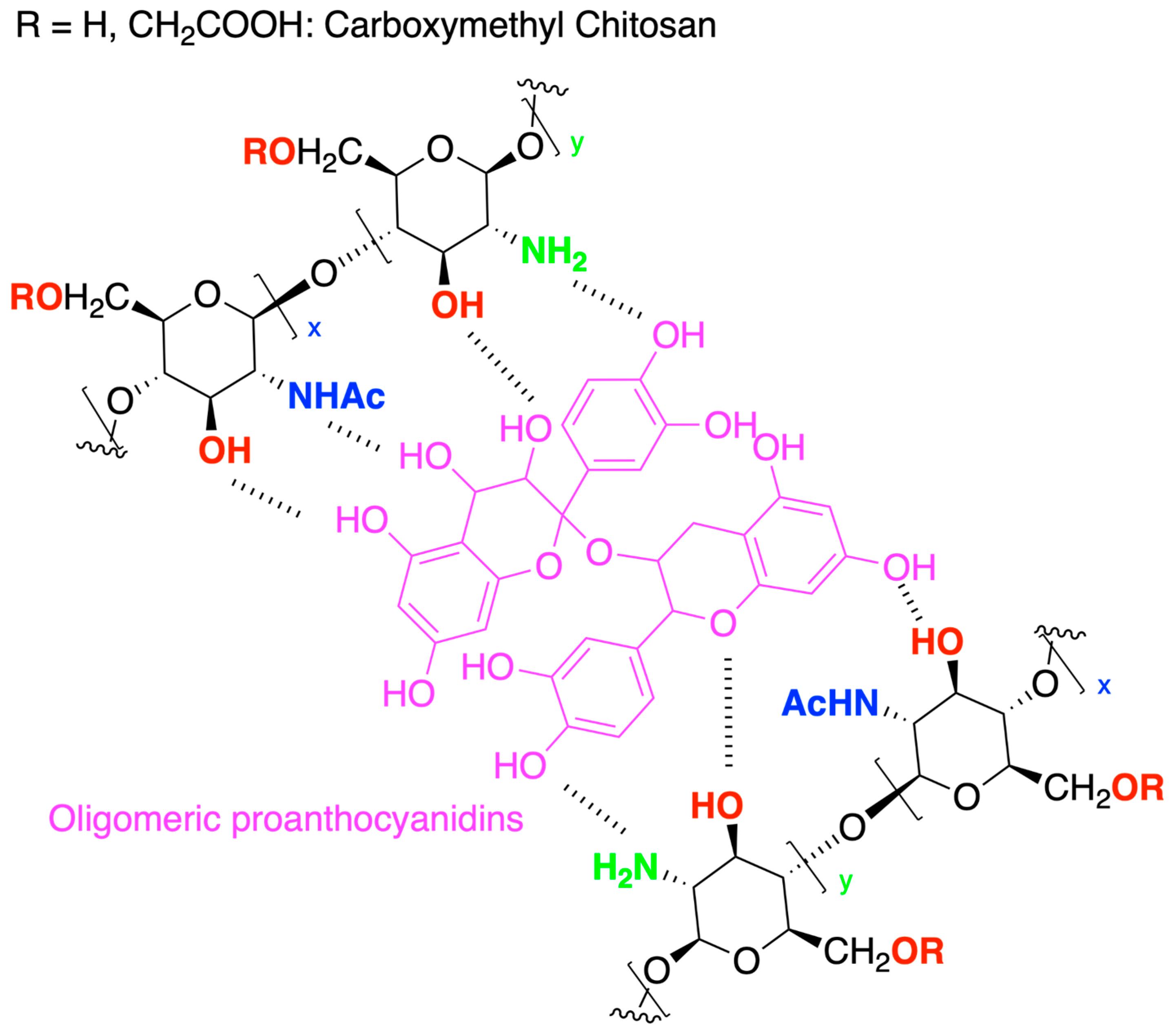
- He, Y.; Guo, S.; Chang, R.; Zhang, D.; Ren, Y.; Guan, F.; Yao, M. Facile Preparation of Antibacterial Hydrogel with Multi-Functions Based on Carboxymethyl Chitosan and Oligomeric Procyanidin. RSC Adv. 2022, 12, 20897–20905. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of Hydrogels Based on Chitosan/Alginate for the Delivery of Lysozyme and Their Antibacterial Activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, L.; Bianco-Peled, H. Pectin-Chitosan Physical Hydrogels as Potential Drug Delivery Vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, Â.M.; Coimbra, I.B. Chondrogenesis of Human Amniotic Fluid Stem Cells in Chitosan-Xanthan Scaffold for Cartilage Tissue Engineering. Sci. Rep. 2021, 11, 3063. [Google Scholar] [CrossRef]
- Vieira de Souza, T.; Malmonge, S.M.; Santos, A.R. Development of a Chitosan and Hyaluronic Acid Hydrogel with Potential for Bioprinting Utilization: A Preliminary Study. J. Biomater. Appl. 2021, 36, 358–371. [Google Scholar] [CrossRef]
- Quadrado, R.F.N.; Fajardo, A.R. Vapor-Induced Polyelectrolyte Complexation of Chitosan/Pectin: A Promising Strategy for the Preparation of Hydrogels for Controlled Drug Delivery. J. Mol. Liq. 2022, 361, 119604. [Google Scholar] [CrossRef]
- Zarandona, I.; Bengoechea, C.; Álvarez-Castillo, E.; de la Caba, K.; Guerrero, A.; Guerrero, P. 3D Printed Chitosan-Pectin Hydrogels: From Rheological Characterization to Scaffold Development and Assessment. Gels 2021, 7, 175. [Google Scholar] [CrossRef]
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative Study of Collagen and Gelatin in Chitosan-Based Hydrogels for Effective Wound Dressing: Physical Properties and Fibroblastic Cell Behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, Y.N.; Huang, T.; Felix, O.; Jung, L.; Tropel, P.; Viville, S.; Decher, G. What Is Really Driving Cell-Surface Interactions? Layer-by-Layer Assembled Films May Help to Answer Questions Concerning Cell Attachment and Response to Biomaterials. Biointerphases 2016, 11, 019009. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional Polymer Surfaces for Controlling Cell Behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
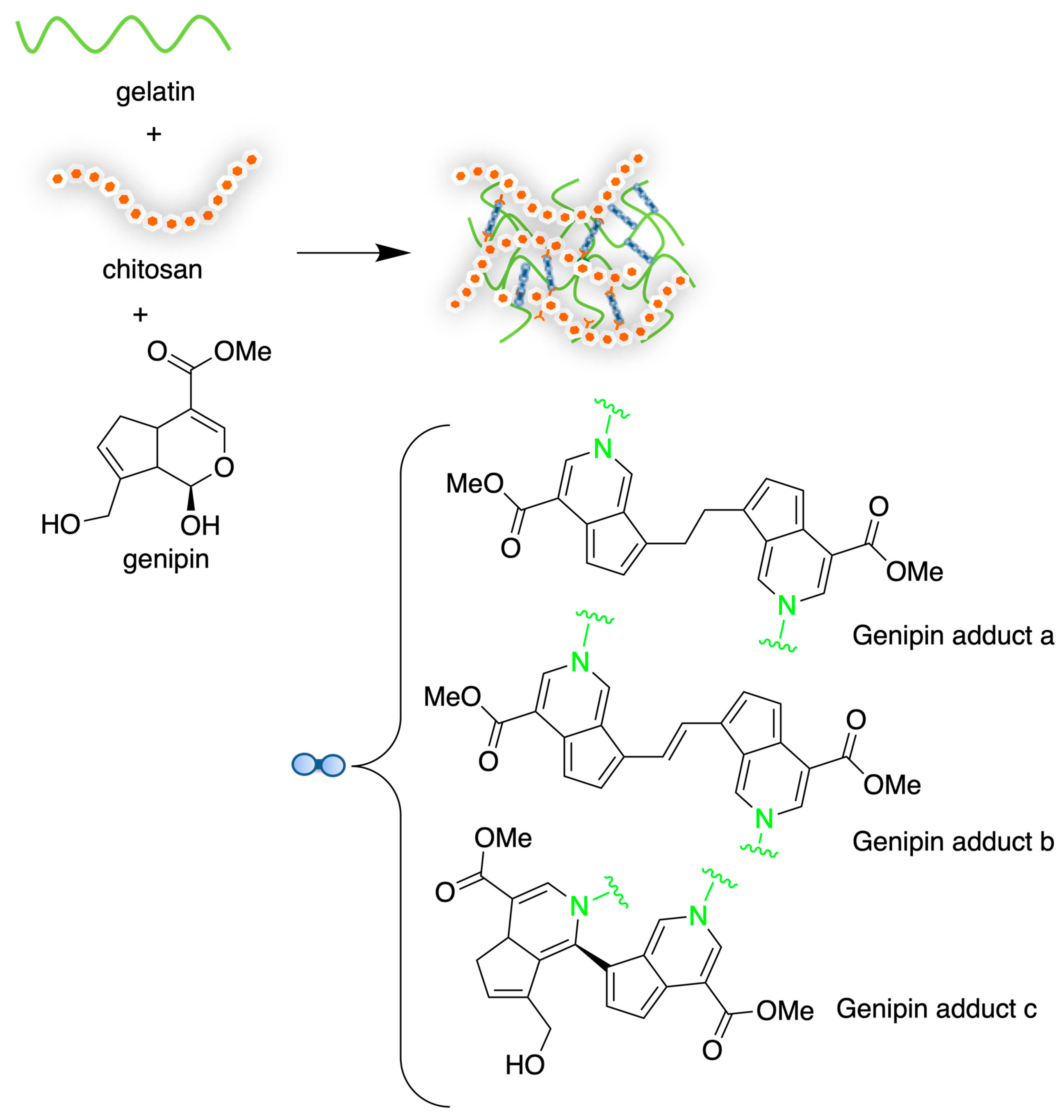
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and Characterization of IPN Hydrogels Composed of Chitosan and Gelatin Cross-Linked by Genipin. Carbohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef]
- Wahid, F.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Xie, Y.-Y.; Zhong, C. Development of Bacterial Cellulose/Chitosan Based Semi-Interpenetrating Hydrogels with Improved Mechanical and Antibacterial Properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- Dash, M.; Ferri, M.; Chiellini, F. Synthesis and Characterization of Semi-Interpenetrating Polymer Network Hydrogel Based on Chitosan and Poly(Methacryloylglycylglycine). Mater. Chem. Phys. 2012, 135, 1070–1076. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural Hydrogels R&D Process: Technical and Regulatory Aspects for Industrial Implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef]
- Song, J.; Zhang, C.; Kong, S.; Liu, F.; Hu, W.; Su, F.; Li, S. Novel Chitosan Based Metal-Organic Polyhedrons/Enzyme Hybrid Hydrogel with Antibacterial Activity to Promote Wound Healing. Carbohydr. Polym. 2022, 291, 119522. [Google Scholar] [CrossRef]
- Mio, L.; Sacco, P.; Donati, I. Influence of Temperature and Polymer Concentration on the Nonlinear Response of Highly Acetylated Chitosan–Genipin Hydrogels. Gels 2022, 8, 194. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez-Martínez, V.; Benito-Cid, S.; Moreno-Benítez, I.; Bengoa-Larrauri, M.; Pérez-González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Self-Healing, Antibacterial and Anti-Inflammatory Chitosan-PEG Hydrogels for Ulcerated Skin Wound Healing and Drug Delivery. Biomater. Adv. 2022, 139, 212992. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
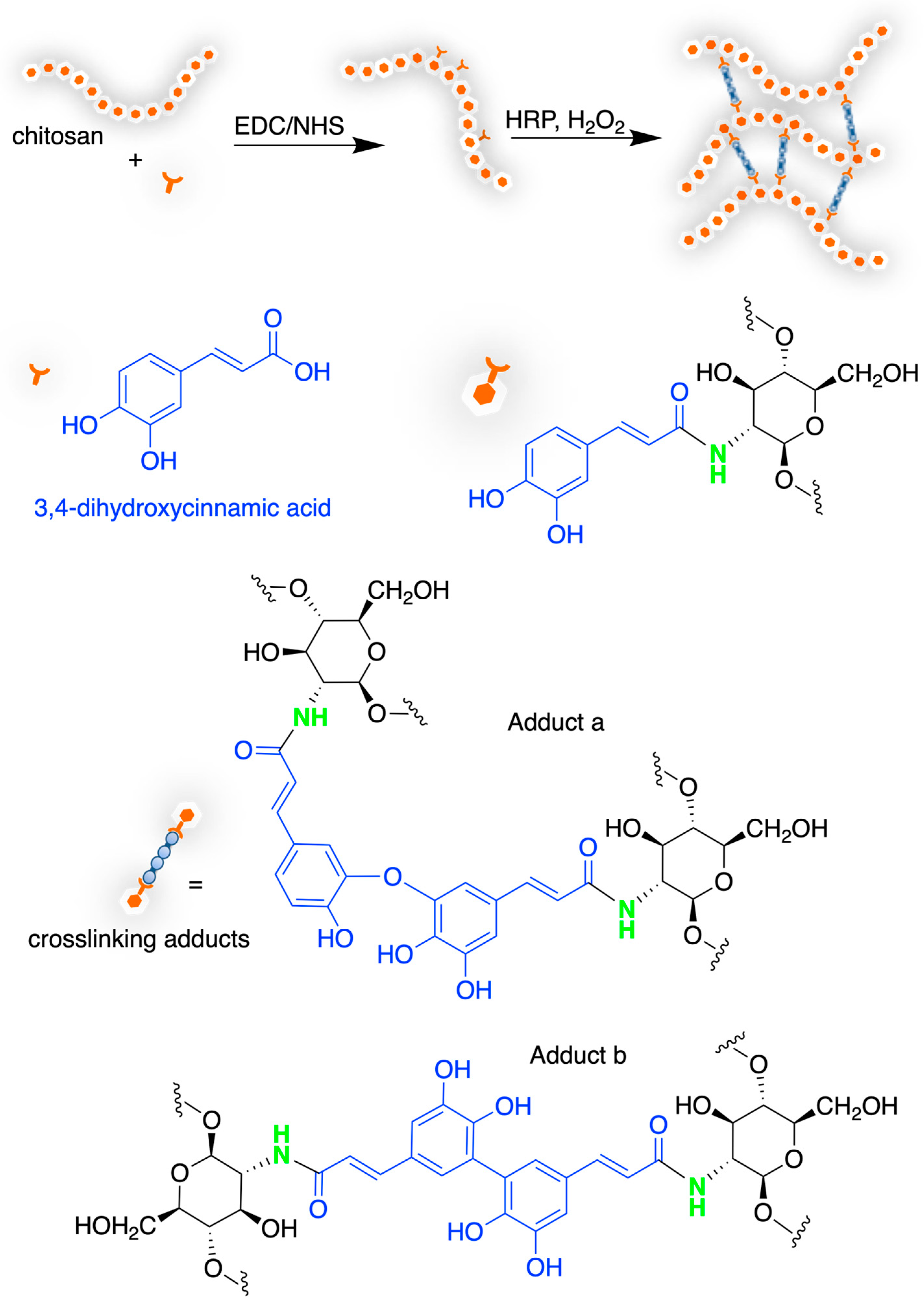
- Zhou, S.; Bei, Z.; Wei, J.; Yan, X.; Wen, H.; Cao, Y.; Li, H. Mussel-Inspired Injectable Chitosan Hydrogel Modified with Catechol for Cell Adhesion and Cartilage Defect Repair. J. Mater. Chem. B 2022, 10, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Francis Suh, J.-K.; Matthew, H.W.T. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
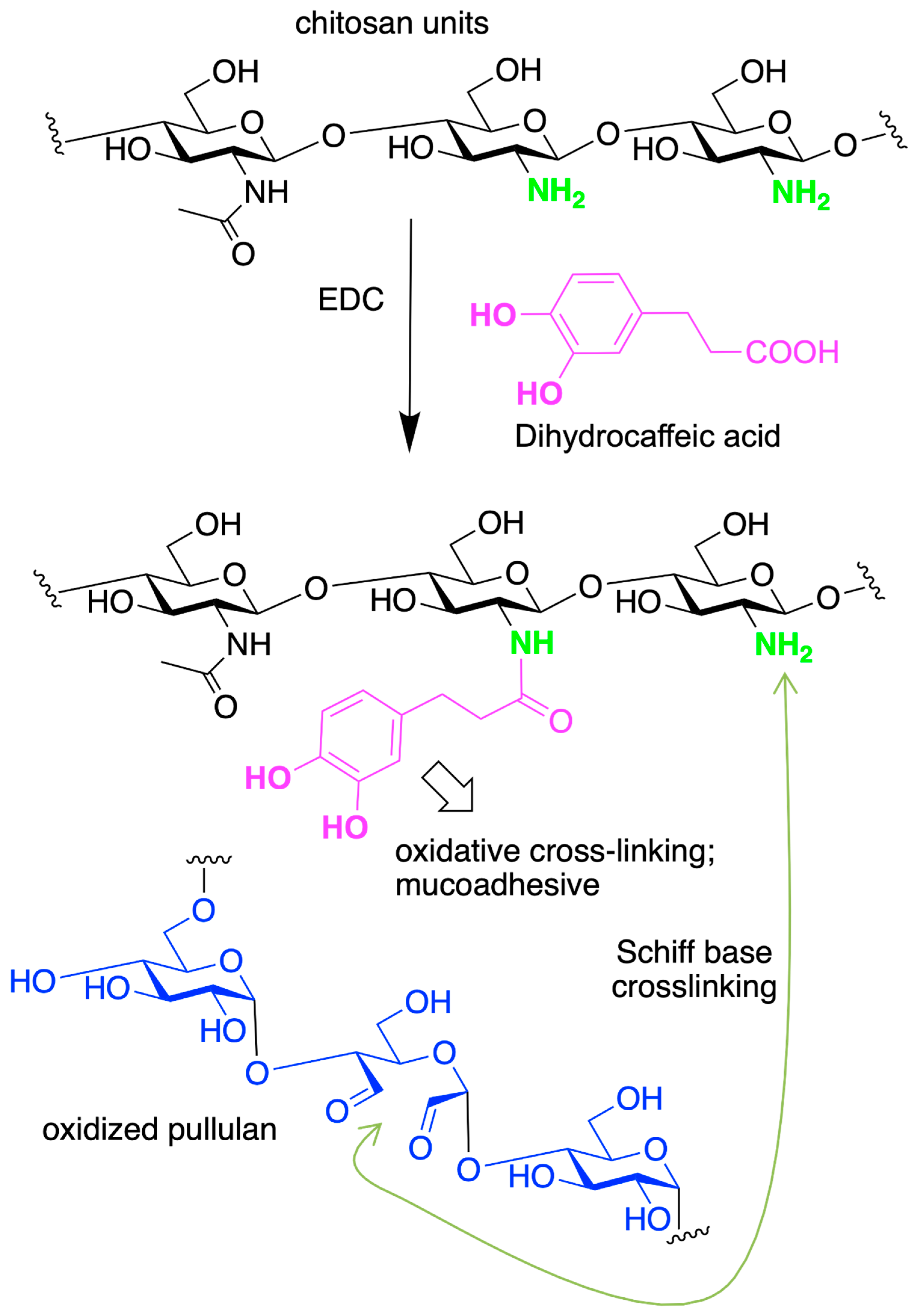
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. PH-Responsive Injectable Hydrogels with Mucosal Adhesiveness Based on Chitosan-Grafted-Dihydrocaffeic Acid and Oxidized Pullulan for Localized Drug Delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-Inspired Adhesive Catechol-Conjugated Chitosan for Biomedical Applications: A Mini Review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Donati, I.; Stredanska, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The Aggregation of Pig Articular Chondrocyte and Synthesis of Extracellular Matrix by a Lactose-Modified Chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Scognamiglio, F.; Asaro, F.; Travan, A.; Borgogna, M.; Marsich, E.; Cok, M.; Paoletti, S.; Donati, I. Nucleation, Reorganization and Disassembly of an Active Network from Lactose-Modified Chitosan Mimicking Biological Matrices. Carbohydr. Polym. 2019, 208, 451–456. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Donati, I.; Borgogna, M.; Marsich, E. A Hydrogel System Based on a Lactose-Modified Chitosan for Viscosupplementation in Osteoarthritis. Carbohydr. Polym. 2020, 248, 116787. [Google Scholar] [CrossRef]
- Yeh, Y.Y.; Tsai, Y.T.; Wu, C.Y.; Tu, L.H.; Bai, M.Y.; Yeh, Y.C. The Role of Aldehyde-Functionalized Crosslinkers on the Property of Chitosan Hydrogels. Macromol. Biosci. 2022, 22, 2100477, Erratum in: Macromol. Biosci. 2022, 22, e2200366. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-Imine-Chitosan Hydrogels: Supramolecular Architecturing as a Crosslinking Method toward Multifunctional Hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Mititelu Tartau, L.; Marin, L. New Formulations Based on Salicyl-Imine-Chitosan Hydrogels for Prolonged Drug Release. Int. J. Biol. Macromol. 2020, 160, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Moraru, S.; Popescu, M.-C.; Nicolescu, A.; Zgardan, C.; Simionescu, B.C.; Barboiu, M. Out-of-Water Constitutional Self-Organization of Chitosan-Cinnamaldehyde Dynagels. Chem. Weinh. Bergstr. Ger. 2014, 20, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Rosca, I.; Sandu, A.-I.; Marin, L. Chitosan Crosslinking with a Vanillin Isomer toward Self-Healing Hydrogels with Antifungal Activity. Int. J. Biol. Macromol. 2022, 205, 574–586. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Jones, J.R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Tallia, F.; Russo, L.; Li, S.; Orrin, A.L.H.; Shi, X.; Chen, S.; Steele, J.A.M.; Meille, S.; Chevalier, J.; Lee, P.D.; et al. Bouncing and 3D Printable Hybrids with Self-Healing Properties. Mater. Horiz. 2018, 5, 849–860. [Google Scholar] [CrossRef]
- Connell, L.S.; Gabrielli, L.; Mahony, O.; Russo, L.; Cipolla, L.; Jones, J.R. Functionalizing Natural Polymers with Alkoxysilane Coupling Agents: Reacting 3-Glycidoxypropyl Trimethoxysilane with Poly(γ-Glutamic Acid) and Gelatin. Polym. Chem. 2017, 8, 1095–1103. [Google Scholar] [CrossRef]
- Russo, L.; Landi, E.; Tampieri, A.; Natalello, A.; Doglia, S.M.; Gabrielli, L.; Cipolla, L.; Nicotra, F. Sugar-Decorated Hydroxyapatite: An Inorganic Material Bioactivated with Carbohydrates. Carbohydr. Res. 2011, 346, 1564–1568. [Google Scholar] [CrossRef]
- Sandri, M.; Natalello, A.; Bini, D.; Gabrielli, L.; Cipolla, L.; Nicotra, F. Sweet and Salted: Sugars Meet Hydroxyapatite. Synlett 2011, 2011, 1845–1848. [Google Scholar] [CrossRef]
- Russo, L.; Gabrielli, L.; Valliant, E.M.; Nicotra, F.; Jiménez-Barbero, J.; Cipolla, L.; Jones, J.R. Novel Silica/Bis(3-Aminopropyl) Polyethylene Glycol Inorganic/Organic Hybrids by Sol–Gel Chemistry. Mater. Chem. Phys. 2013, 140, 168–175. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M. Chitosan Bio-Based Organic–Inorganic Hybrid Aerogel Microspheres. Chem. Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-N.; Yan, L.-P.; Dong, Y.-F.; Liu, X.; Wu, G.; Zhao, N.-R. Robust and Nanostructured Chitosan–Silica Hybrids for Bone Repair Application. J. Mater. Chem. B 2020, 8, 5042–5051. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone Morphogenetic Proteins in Tissue Engineering: The Road from Laboratory to Clinic, Part II (BMP Delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/Hydroxyapatite Composite Bone Tissue Engineering Scaffolds with Dual and Decoupled Therapeutic Ion Delivery: Copper and Strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic Ions as Therapeutic Agents in Tissue Engineering Scaffolds: An Overview of Their Biological Applications and Strategies for New Developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Russo, L.; Taraballi, F.; Lupo, C.; Poveda, A.; Jiménez-Barbero, J.; Sandri, M.; Tampieri, A.; Nicotra, F.; Cipolla, L. Carbonate Hydroxyapatite Functionalization: A Comparative Study towards (Bio)Molecules Fixation. Interface Focus 2014, 4, 20130040. [Google Scholar] [CrossRef]
- Gabrielli, L.; Connell, L.; Russo, L.; Jiménez-Barbero, J.; Nicotra, F.; Cipolla, L.; Jones, J.R. Exploring GPTMS Reactivity against Simple Nucleophiles: Chemistry beyond Hybrid Materials Fabrication. RSC Adv. 2013, 4, 1841–1848. [Google Scholar] [CrossRef]
- Gabrielli, L.; Russo, L.; Poveda, A.; Jones, J.R.; Nicotra, F.; Jiménez-Barbero, J.; Cipolla, L. Epoxide Opening versus Silica Condensation during Sol–Gel Hybrid Biomaterial Synthesis. Chem. Eur. J. 2013, 19, 7856–7864. [Google Scholar] [CrossRef]
- Wang, D.; Romer, F.; Connell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; McPhail, D.S.; Hanna, J.V.; Jones, J.R. Highly Flexible Silica/Chitosan Hybrid Scaffolds with Oriented Pores for Tissue Regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar] [CrossRef] [PubMed]
- Jayash, S.N.; Cooper, P.R.; Shelton, R.M.; Kuehne, S.A.; Poologasundarampillai, G. Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery. Int. J. Mol. Sci. 2021, 22, 12267. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of Colloidal Particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process, 1 Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles on Charged Surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Berichte Bunsenges. Für Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, J. Layer-by-Layer Assembly for Rapid Fabrication of Thick Polymeric Films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Andres, C.M.; Kotov, N.A. Inkjet Deposition of Layer-by-Layer Assembled Films. J. Am. Chem. Soc. 2010, 132, 14496–14502. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, C.; Jiang, C.; Shao, L.; Zhang, Y.; Shi, F. Rapid Multilayer Construction on a Non-Planar Substrate by Layer-by-Layer Self-Assembly under High Gravity. RSC Adv. 2014, 4, 59528–59534. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, M.; Jia, G.; Wang, Y.; An, Q.; Zeng, X.; Shen, Z.; Zhang, Y.; Shi, F. Layer-by-Layer Self-Assembly under High Gravity Field. Langmuir 2012, 28, 9849–9856. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Fujiwara, T.; Akashi, M. Inkjet Printing of Layer-by-Layer Assembled Poly(Lactide) Stereocomplex with Encapsulated Proteins. Langmuir 2014, 30, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, R.; Shchepelina, O.; Choi, I.; Tsukruk, V.V. Inkjet-Assisted Layer-by-Layer Printing of Encapsulated Arrays. ACS Appl. Mater. Interfaces 2012, 4, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Reis, R.L.; Mano, J.F. Multilayered Hierarchical Capsules Providing Cell Adhesion Sites. Biomacromolecules 2013, 14, 743–751. [Google Scholar] [CrossRef]
- Correia, C.R.; Sher, P.; Reis, R.L.; Mano, J.F. Liquified Chitosan–Alginate Multilayer Capsules Incorporating Poly(L-Lactic Acid) Microparticles as Cell Carriers. Soft Matter 2013, 9, 2125–2130. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-Layer Assembly Methods and Their Biomedical Applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Mijangos, C.; Hernández, R. Polyelectrolyte Multilayer Films Based on Natural Polymers: From Fundamentals to Bio-Applications. Polymers 2021, 13, 2254. [Google Scholar] [CrossRef]
- Alkekhia, D.; Hammond, P.T.; Shukla, A. Layer-by-Layer Biomaterials for Drug Delivery. Annu. Rev. Biomed. Eng. 2020, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Hu, M.; Zhang, H.; Li, B.; Lei, W.; Chen, J.; Chang, H.; Wang, L.; Ji, J. Layer-by-Layer Assembly as a Robust Method to Construct Extracellular Matrix Mimic Surfaces to Modulate Cell Behavior. Prog. Polym. Sci. 2019, 92, 1–34. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Mano, J.F. Biomimetic Extracellular Environment Based on Natural Origin Polyelectrolyte Multilayers. Small 2016, 12, 4308–4342. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Almodóvar, J.; Boudou, T.; Picart, C. Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D. Adv. Healthc. Mater. 2015, 4, 811–830. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Mano, J.F. Polyelectrolyte Multilayered Assemblies in Biomedical Technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.T. Building Biomedical Materials Layer-by-Layer. Mater. Today 2012, 15, 196–206. [Google Scholar] [CrossRef]
- Sousa, M.P.; Arab-Tehrany, E.; Cleymand, F.; Mano, J.F. Surface Micro- and Nanoengineering: Applications of Layer-by-Layer Technology as a Versatile Tool to Control Cellular Behavior. Small 2019, 15, 1901228. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Fernandez-Gutierrez, M.; San Roman, J.; Mijangos, C.; Hernández, R. Local and Controlled Release of Tamoxifen from Multi (Layer-by-Layer) Alginate/Chitosan Complex Systems. Carbohydr. Polym. 2019, 206, 428–434. [Google Scholar] [CrossRef]
- Silva, J.M.; García, J.R.; Reis, R.L.; García, A.J.; Mano, J.F. Tuning Cell Adhesive Properties via Layer-by-Layer Assembly of Chitosan and Alginate. Acta Biomater. 2017, 51, 279–293. [Google Scholar] [CrossRef]
- Wu, L.; Wu, C.; Liu, G.; Liao, N.; Zhao, F.; Yang, X.; Qu, H.; Peng, B.; Chen, L.; Yang, G. A Surface-Mediated SiRNA Delivery System Developed with Chitosan/Hyaluronic Acid-SiRNA Multilayer Films through Layer-by-Layer Self-Assembly. Appl. Surf. Sci. 2016, 389, 395–403. [Google Scholar] [CrossRef]
- Couto, D.S.; Alves, N.M.; Mano, J.F. Nanostructured Multilayer Coatings Combining Chitosan with Bioactive Glass Nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, D.; Jeong, H.; Heo, J.; Hong, J. Drug Loading and Release Behavior Depending on the Induced Porosity of Chitosan/Cellulose Multilayer Nanofilms. Mol. Pharm. 2017, 14, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Rechtenbach, A.; Hao, J.; Bossert, J.; Jandt, K.D. Polysaccharide-Protein Surface Modification of Titanium via a Layer-by-Layer Technique: Characterization and Cell Behaviour Aspects. Biomaterials 2005, 26, 5960–5971. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Moghaddam, S.Z.; Thormann, E. Chitosan/Alginate Dialdehyde Multilayer Films with Modulated PH-Responsiveness and Swelling. Macromol. Chem. Phys. 2020, 221, 1900499. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridade, S.G.; Costa, R.R.; Alves, N.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.F. PH Responsiveness of Multilayered Films and Membranes Made of Polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured Polymeric Coatings Based on Chitosan and Dopamine-Modified Hyaluronic Acid for Biomedical Applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef]
- Costa, R.R.; Neto, A.I.; Calgeris, I.; Correia, C.R.; Pinho, A.C.M.; Fonseca, J.; Öner, E.T.; Mano, J.F. Adhesive Nanostructured Multilayer Films Using a Bacterial Exopolysaccharide for Biomedical Applications. J. Mater. Chem. B 2013, 1, 2367–2374. [Google Scholar] [CrossRef]
- Boddohi, S.; Almodóvar, J.; Zhang, H.; Johnson, P.A.; Kipper, M.J. Layer-by-Layer Assembly of Polysaccharide-Based Nanostructured Surfaces Containing Polyelectrolyte Complex Nanoparticles. Colloids Surf. B Biointerfaces 2010, 77, 60–68. [Google Scholar] [CrossRef]
- Alves, N.M.; Picart, C.; Mano, J.F. Self Assembling and Crosslinking of Polyelectrolyte Multilayer Films of Chitosan and Alginate Studied by QCM and IR Spectroscopy. Macromol. Biosci. 2009, 9, 776–785. [Google Scholar] [CrossRef]
- Urbaniak, T.; García-Briones, G.S.; Zhigunov, A.; Hladysh, S.; Adrian, E.; Lobaz, V.; Krunclová, T.; Janoušková, O.; Pop-Georgievski, O.; Kubies, D. Quaternized Chitosan/Heparin Polyelectrolyte Multilayer Films for Protein Delivery. Biomacromolecules 2022, 23, 4734–4748. [Google Scholar] [CrossRef]
- Neto, A.I.; Vasconcelos, N.L.; Oliveira, S.M.; Ruiz-Molina, D.; Mano, J.F. High-Throughput Topographic, Mechanical, and Biological Screening of Multilayer Films Containing Mussel-Inspired Biopolymers. Adv. Funct. Mater. 2016, 26, 2745–2755. [Google Scholar] [CrossRef]
- Sousa, M.P.; Cleymand, F.; Mano, J.F. Elastic Chitosan/Chondroitin Sulfate Multilayer Membranes. Biomed. Mater. 2016, 11, 035008. [Google Scholar] [CrossRef] [PubMed]
- Caridade, S.G.; Monge, C.; Gilde, F.; Boudou, T.; Mano, J.F.; Picart, C. Free-Standing Polyelectrolyte Membranes Made of Chitosan and Alginate. Biomacromolecules 2013, 14, 1653–1660. [Google Scholar] [CrossRef]
- Silva, J.M.; Duarte, A.R.C.; Caridade, S.G.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored Freestanding Multilayered Membranes Based on Chitosan and Alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridade, S.G.; Oliveira, N.M.; Reis, R.L.; Mano, J.F. Chitosan–Alginate Multilayered Films with Gradients of Physicochemical Cues. J. Mater. Chem. B 2015, 3, 4555–4568. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, A.; Kedilaya, D.; Stojanović, S.; Radenković, M.; Marx, C.K.; Najman, S.; Pietzsch, M.; Mano, J.F.; Groth, T. Free-Standing Multilayer Films as Growth Factor Reservoirs for Future Wound Dressing Applications. Biomater. Adv. 2022, 142, 213166. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Caridade, S.G.; Reis, R.L.; Mano, J.F. Polysaccharide-Based Freestanding Multilayered Membranes Exhibiting Reversible Switchable Properties. Soft Matter 2016, 12, 1200–1209. [Google Scholar] [CrossRef]
- Borges, J.; Caridade, S.G.; Silva, J.M.; Mano, J.F. Unraveling the Effect of the Hydration Level on the Molecular Mobility of Nanolayered Polymeric Systems. Macromol. Rapid Communed. 2015, 36, 405–412. [Google Scholar] [CrossRef]
- Gil, S.; Silva, J.M.; Mano, J.F. Magnetically Multilayer Polysaccharide Membranes for Biomedical Applications. ACS Biomater. Sci. Eng. 2015, 1, 1016–1025. [Google Scholar] [CrossRef]
- Sousa, M.P.; Mano, J.F. Cell-Adhesive Bioinspired and Catechol-Based Multilayer Freestanding Membranes for Bone Tissue Engineering. Biomimetics 2017, 2, 19. [Google Scholar] [CrossRef]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired Multilayer Membranes as Potential Adhesive Patches for Skin Wound Healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.R.; Alves, N.M.; Mano, J.F. Biomimetic Polysaccharide/Bioactive Glass Nanoparticles Multilayer Membranes for Guided Tissue Regeneration. RSC Adv. 2016, 6, 75988–75999. [Google Scholar] [CrossRef]
- Sousa, M.P.; Caridade, S.G.; Mano, J.F. Control of Cell Alignment and Morphology by Redesigning ECM-Mimetic Nanotopography on Multilayer Membranes. Adv. Healthc. Mater. 2017, 6, 1601462. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.I.; Sousa, M.P.; Custódio, C.A.; Pinto, V.C.; Sousa, P.J.; Minas, G.; Cleymand, F.; Mano, J.F. Multilayered Membranes with Tuned Well Arrays to Be Used as Regenerative Patches. Acta Biomater. 2017, 57, 313–323. [Google Scholar] [CrossRef]
- Ribeiro, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.D.Z.; Mano, J.F. Preparation of Well-Dispersed Chitosan/Alginate Hollow Multilayered Microcapsules for Enhanced Cellular Internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef]
- Costa, R.R.; Girotti, A.; Santos, M.; Arias, F.J.; Mano, J.F.; Rodríguez-Cabello, J.C. Cellular Uptake of Multilayered Capsules Produced with Natural and Genetically Engineered Biomimetic Macromolecules. Acta Biomater. 2014, 10, 2653–2662. [Google Scholar] [CrossRef]
- Costa, R.R.; Custódio, C.A.; Arias, F.J.; Rodríguez-Cabello, J.C.; Mano, J.F. Nanostructured and Thermoresponsive Recombinant Biopolymer-Based Microcapsules for the Delivery of Active Molecules. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 895–902. [Google Scholar] [CrossRef]
- Correia, C.R.; Nadine, S.; Mano, J.F. Cell Encapsulation Systems Toward Modular Tissue Regeneration: From Immunoisolation to Multifunctional Devices. Adv. Funct. Mater. 2020, 30, 1908061. [Google Scholar] [CrossRef]
- Correia, C.R.; Pirraco, R.P.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Mano, J.F. Semipermeable Capsules Wrapping a Multifunctional and Self-Regulated Co-Culture Microenvironment for Osteogenic Differentiation. Sci. Rep. 2016, 6, 21883. [Google Scholar] [CrossRef]
- Correia, C.R.; Gil, S.; Reis, R.L.; Mano, J.F. A Closed Chondromimetic Environment within Magnetic-Responsive Liquified Capsules Encapsulating Stem Cells and Collagen II/TGF-Β3 Microparticles. Adv. Healthc. Mater. 2016, 5, 1346–1355. [Google Scholar] [CrossRef]
- Correia, C.R.; Santos, T.C.; Pirraco, R.P.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Mano, J.F. In Vivo Osteogenic Differentiation of Stem Cells inside Compartmentalized Capsules Loaded with Co-Cultured Endothelial Cells. Acta Biomater. 2017, 53, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Bjørge, I.M.; Zeng, J.; Matsusaki, M.; Mano, J.F. Liquefied Microcapsules as Dual-Microcarriers for 3D+3D Bottom-Up Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1901221. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Castro, E.; Arias, F.J.; Rodríguez-Cabello, J.C.; Mano, J.F. Multifunctional Compartmentalized Capsules with a Hierarchical Organization from the Nano to the Macro Scales. Biomacromolecules 2013, 14, 2403–2410. [Google Scholar] [CrossRef]
- Silva, J.M.; Duarte, A.R.C.; Custódio, C.A.; Sher, P.; Neto, A.I.; Pinho, A.C.M.; Fonseca, J.; Reis, R.L.; Mano, J.F. Nanostructured Hollow Tubes Based on Chitosan and Alginate Multilayers. Adv. Healthc. Mater. 2014, 3, 433–440. [Google Scholar] [CrossRef]
- Silva, J.M.; Custódio, C.A.; Reis, R.L.; Mano, J.F. Multilayered Hollow Tubes as Blood Vessel Substitutes. ACS Biomater. Sci. Eng. 2016, 2, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, Q.; Duan, L.; Cui, Y.; Li, J. Assembled Alginate/Chitosan Nanotubes for Biological Application. Biomaterials 2007, 28, 3083–3090. [Google Scholar] [CrossRef]
- Sher, P.; Custódio, C.A.; Mano, J.F. Layer-By-Layer Technique for Producing Porous Nanostructured 3D Constructs Using Moldable Freeform Assembly of Spherical Templates. Small 2010, 6, 2644–2648. [Google Scholar] [CrossRef]
- Silva, J.M.; Georgi, N.; Costa, R.; Sher, P.; Reis, R.L.; Blitterswijk, C.A.V.; Karperien, M.; Mano, J.F. Nanostructured 3D Constructs Based on Chitosan and Chondroitin Sulphate Multilayers for Cartilage Tissue Engineering. PLoS ONE 2013, 8, e55451. [Google Scholar] [CrossRef]
- Sher, P.; Oliveira, S.M.; Borges, J.; Mano, J.F. Assembly of Cell-Laden Hydrogel Fiber into Non-Liquefied and Liquefied 3D Spiral Constructs by Perfusion-Based Layer-by-Layer Technique. Biofabrication 2015, 7, 011001. [Google Scholar] [CrossRef]
- Sher, P.; Correia, C.R.; Costa, R.R.; Mano, J.F. Compartmentalized Bioencapsulated Liquefied 3D Macro-Construct by Perfusion-Based Layer-by-Layer Technique. RSC Adv. 2014, 5, 2511–2516. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Reis, R.L.; Mano, J.F. Assembling Human Platelet Lysate into Multiscale 3D Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jiang, N.; Moore, J.; McCoy, C.P.; Ziminska, M.; Rafferty, C.; Sarri, G.; Hamilton, A.R.; Li, Y.; Zhang, L.; et al. Nanoscale Hybrid Coating Enables Multifunctional Tissue Scaffold for Potential Multimodal Therapeutic Applications. ACS Appl. Mater. Interfaces 2019, 11, 27269–27278. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D Printed Scaffolds for Promoting Bone Regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.F.V.; Saraiva, C.A.; Correia, T.R.; Pesqueira, T.; Patrício, S.G.; Rial-Hermida, M.I.; Borges, J.; Mano, J.F. Bioinstructive Layer-by-Layer-Coated Customizable 3D Printed Perfusable Microchannels Embedded in Photocrosslinkable Hydrogels for Vascular Tissue Engineering. Biomolecules 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Hatami, J.; Mano, J.F. Coating Strategies Using Layer-by-Layer Deposition for Cell Encapsulation. Chem. Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, 297. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Healthc. Mater. 2019, 8, 1800939. [Google Scholar] [CrossRef]
- Geng, W.; Wang, L.; Jiang, N.; Cao, J.; Xiao, Y.-X.; Wei, H.; Yetisen, A.K.; Yang, X.-Y.; Su, B.-L. Single Cells in Nanoshells for the Functionalization of Living Cells. Nanoscale 2018, 10, 3112–3129. [Google Scholar] [CrossRef]
- Hong, D.; Yang, S.H. Cationic Polymers for Coating Living Cells. Macromol. Res. 2018, 26, 1185–1192. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef]
- Jonas, A.M.; Glinel, K.; Behrens, A.; Anselmo, A.C.; Langer, R.S.; Jaklenec, A. Controlling the Growth of Staphylococcus Epidermidis by Layer-By-Layer Encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 16250–16259. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Belbekhouche, S. Controlling the Growth of Escherichia Coli by Layer-by-Layer Encapsulation. Colloids Surf. B Biointerfaces 2021, 206, 111950. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Griffiths, G. Layer-by-Layer Nanocoating of Live Bacille-Calmette-Guérin Mycobacteria with Poly(I:C) and Chitosan Enhances pro-Inflammatory Activation and Bactericidal Capacity in Murine Macrophages. Biomaterials 2016, 111, 1–12. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the Definition of an Evolving Field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Maia, J.R.; Sobreiro-Almeida, R.; Cleymand, F.; Mano, J. Biomaterials of Human Source for 3D Printing Strategies. J. Phys. Mater. 2023, 6, 012002. [Google Scholar] [CrossRef]
- Lobo, D.A.; Ginestra, P.; Ceretti, E.; Miquel, T.P.; Ciurana, J. Cancer Cell Direct Bioprinting: A Focused Review. Micromachines 2021, 12, 764. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Parhi, R. A Review of Three-Dimensional Printing for Pharmaceutical Applications: Quality Control, Risk Assessment and Future Perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102571. [Google Scholar] [CrossRef]
- Di Luca, M.; Hoskins, C.; Corduas, F.; Onchuru, R.; Oluwasanmi, A.; Mariotti, D.; Conti, B.; Lamprou, D.A. 3D Printed Biodegradable Multifunctional Implants for Effective Breast Cancer Treatment. Int. J. Pharm. 2022, 629, 122363. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Su, Y.; Kang, Y.; Xu, D.; Cheng, Y.Y.; Nie, Y.; Wang, H.; Liu, T.; Song, K. A 3D Bioprinted Decellularized Extracellular Matrix/Gelatin/Quaternized Chitosan Scaffold Assembling with Poly(Ionic Liquid)s for Skin Tissue Engineering. Int. J. Biol. Macromol. 2022, 220, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Yu, K.-F.; Lu, T.-Y.; Li, Y.-C.E.; Teng, K.-C.; Chen, Y.-C.; Wei, Y.; Lin, T.-E.; Cheng, N.-C.; Yu, J. Design and Synthesis of Stem Cell-Laden Keratin/Glycol Chitosan Methacrylate Bioinks for 3D Bioprinting. Biomacromolecules 2022, 23, 2814–2826. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, S.; Akbulut, S.O.; Sarıkaya, B.; Çakmak, S.; Gümüşderelioğlu, M. Formulation of Chitosan and Chitosan-NanoHAp Bioinks and Investigation of Printability with Optimized Bioprinting Parameters. Int. J. Biol. Macromol. 2022, 222, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Gwak, M.A.; Lee, S.J.; Lee, D.; Park, S.A.; Park, W.H. Highly Gallol-Substituted, Rapidly Self-Crosslinkable, and Robust Chitosan Hydrogel for 3D Bioprinting. Int. J. Biol. Macromol. 2022, 227, 493–504. [Google Scholar] [CrossRef]
- Condi Mainardi, J.; Rezwan, K.; Maas, M. Genipin-Crosslinked Chitosan/Alginate/Alumina Nanocomposite Gels for 3D Bioprinting. Bioprocess Biosyst. Eng. 2022, 45, 171–185. [Google Scholar] [CrossRef]
- Riofrio, A.; Alcivar, T.; Baykara, H. Environmental and Economic Viability of Chitosan Production in Guayas-Ecuador: A Robust Investment and Life Cycle Analysis. ACS Omega 2021, 6, 23038–23051. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroni, S.; Tagliaro, I.; Antonini, C.; D’Arienzo, M.; Orsini, S.F.; Mano, J.F.; Brancato, V.; Borges, J.; Cipolla, L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Mar. Drugs 2023, 21, 147. https://doi.org/10.3390/md21030147
Petroni S, Tagliaro I, Antonini C, D’Arienzo M, Orsini SF, Mano JF, Brancato V, Borges J, Cipolla L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Marine Drugs. 2023; 21(3):147. https://doi.org/10.3390/md21030147
Chicago/Turabian StylePetroni, Simona, Irene Tagliaro, Carlo Antonini, Massimiliano D’Arienzo, Sara Fernanda Orsini, João F. Mano, Virginia Brancato, João Borges, and Laura Cipolla. 2023. "Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications" Marine Drugs 21, no. 3: 147. https://doi.org/10.3390/md21030147
APA StylePetroni, S., Tagliaro, I., Antonini, C., D’Arienzo, M., Orsini, S. F., Mano, J. F., Brancato, V., Borges, J., & Cipolla, L. (2023). Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Marine Drugs, 21(3), 147. https://doi.org/10.3390/md21030147










