Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies
Abstract
:1. Introduction
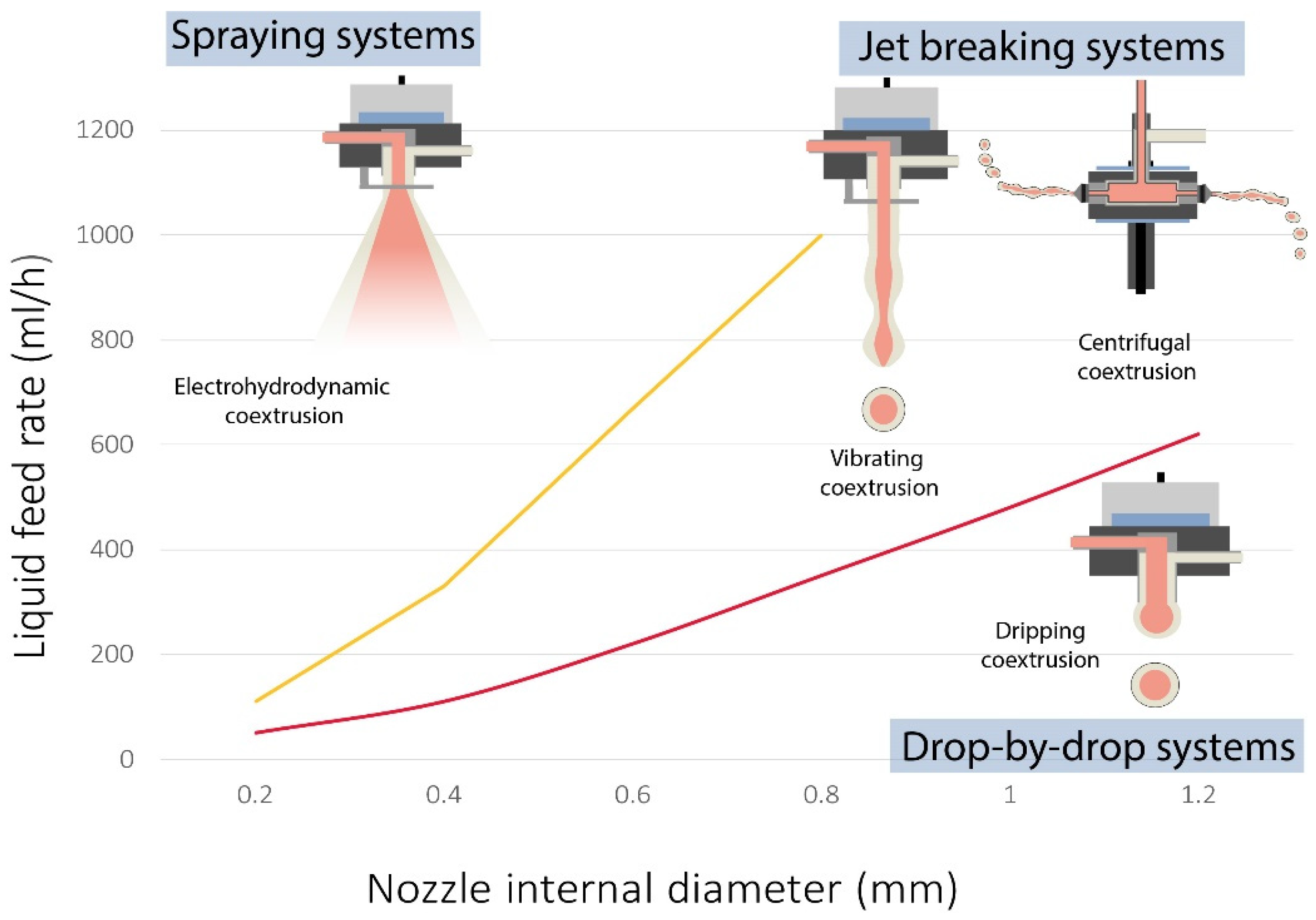
2. Principle of Coextrusion Process
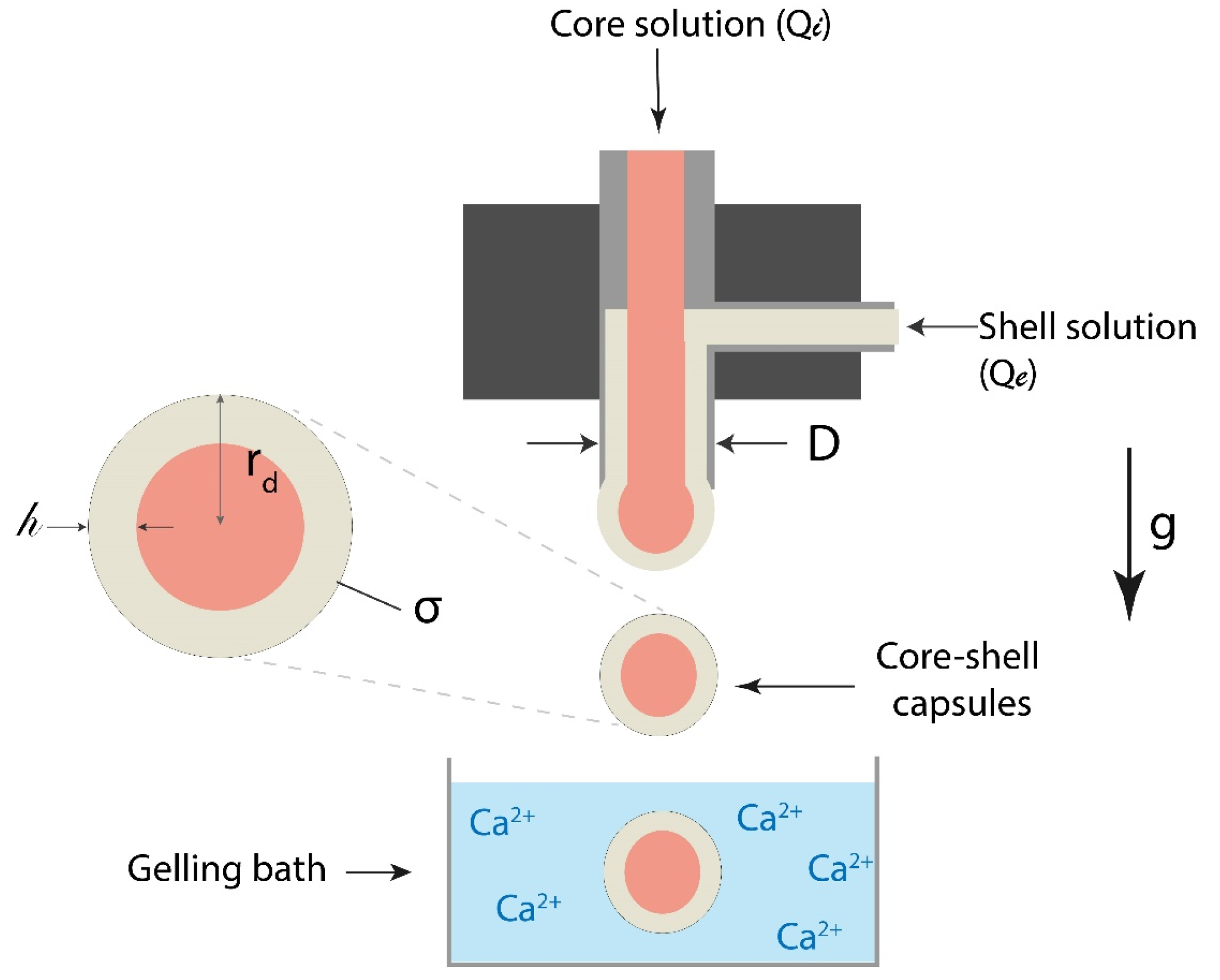
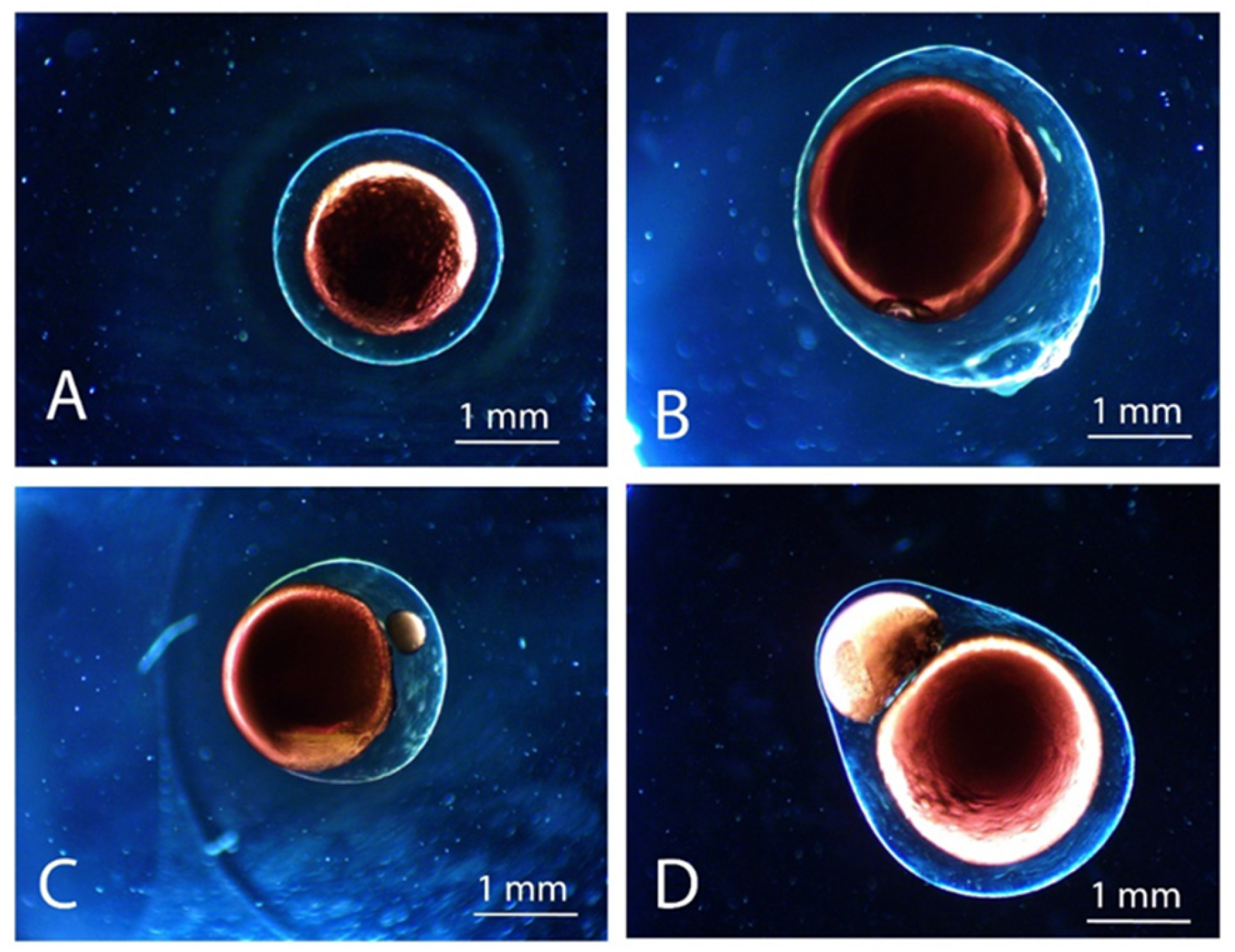
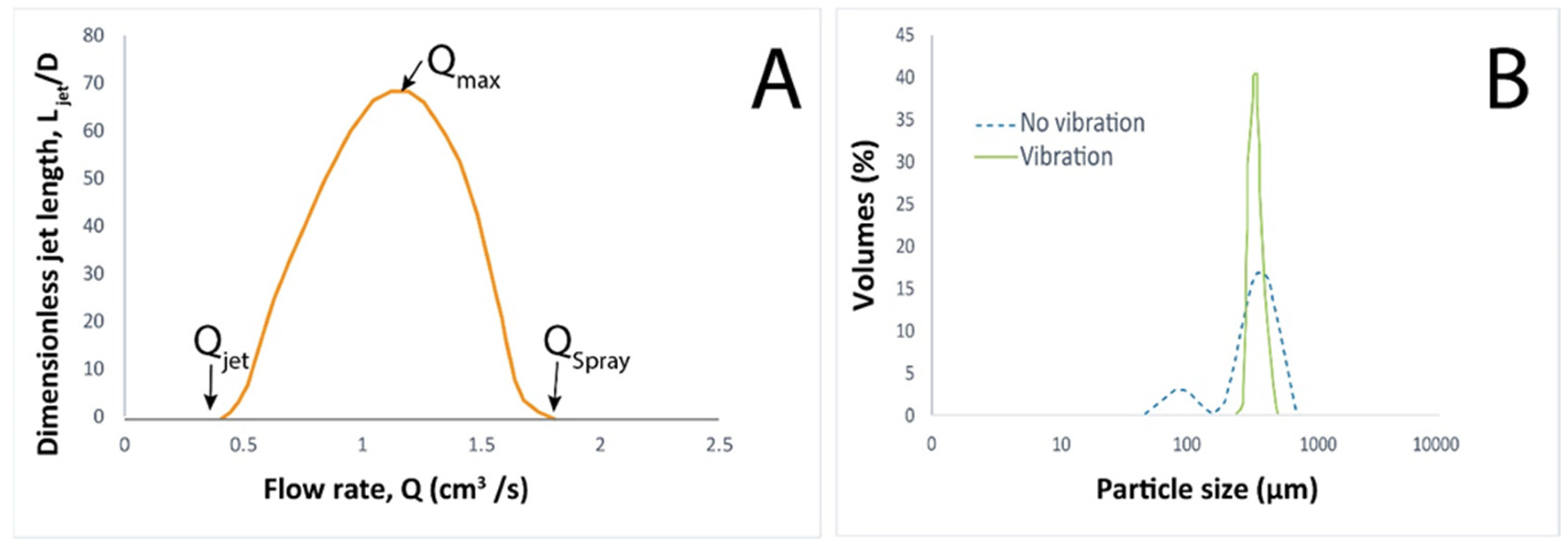
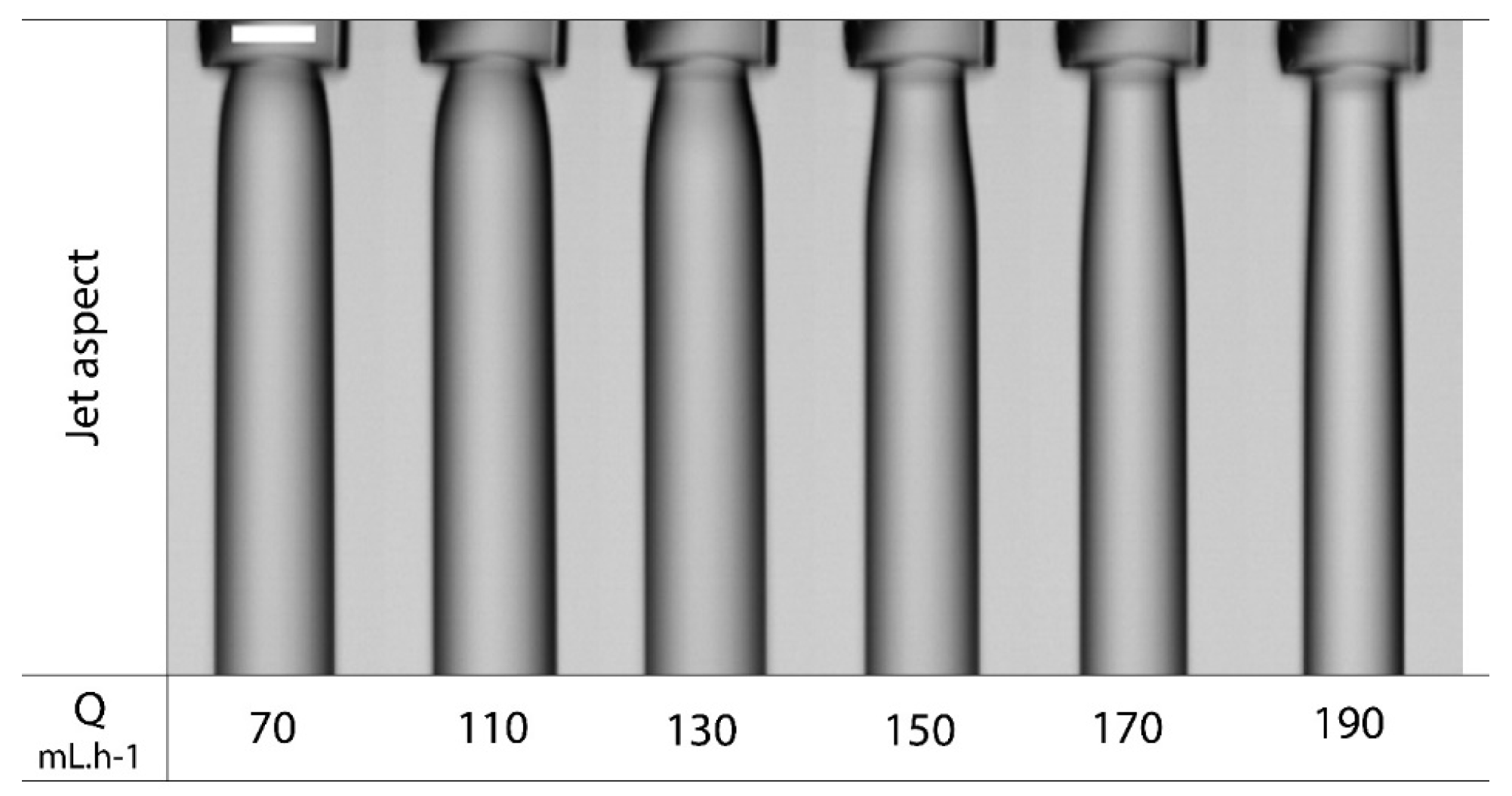
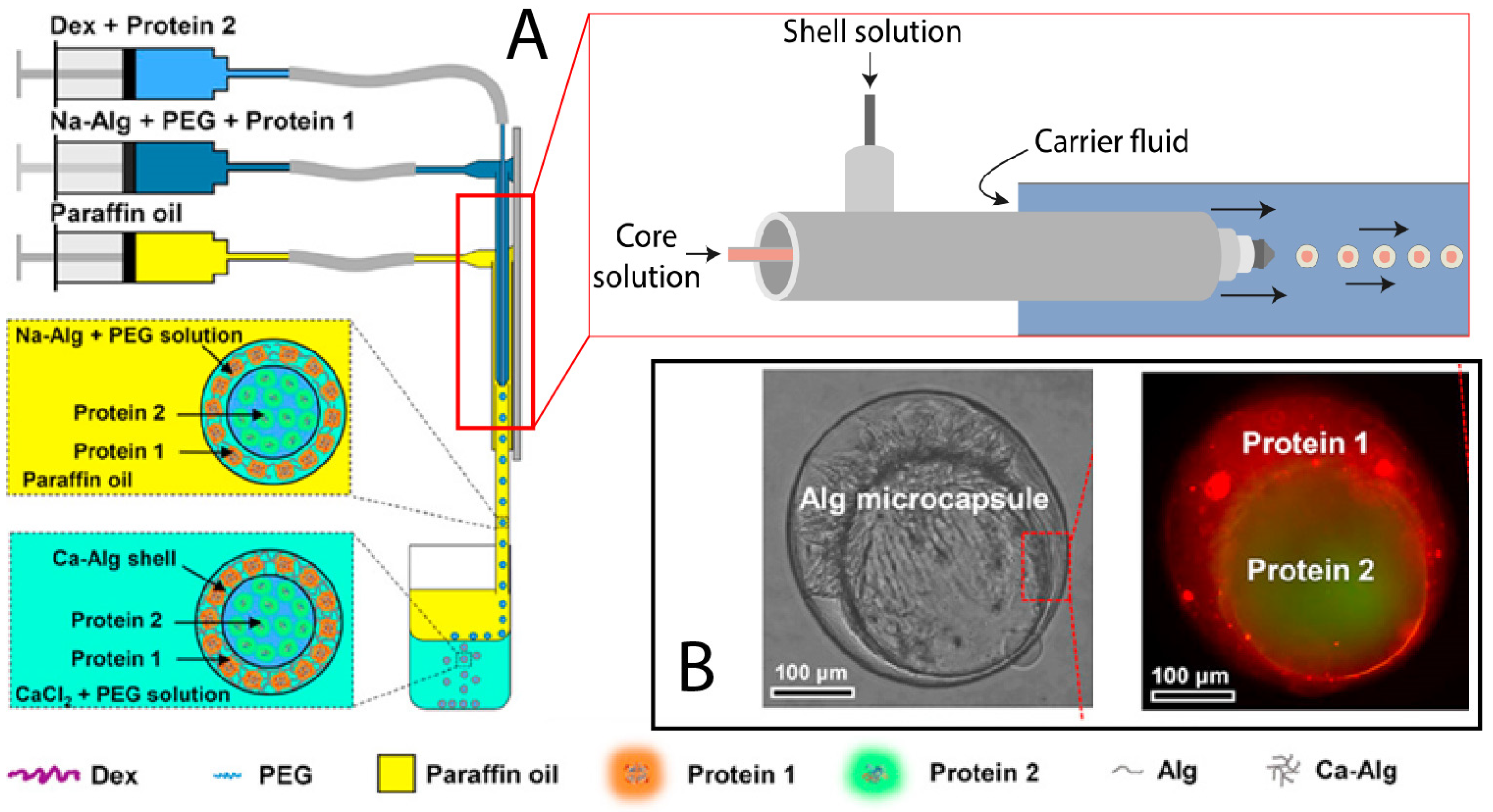
2.1. Dripping Coextrusion System
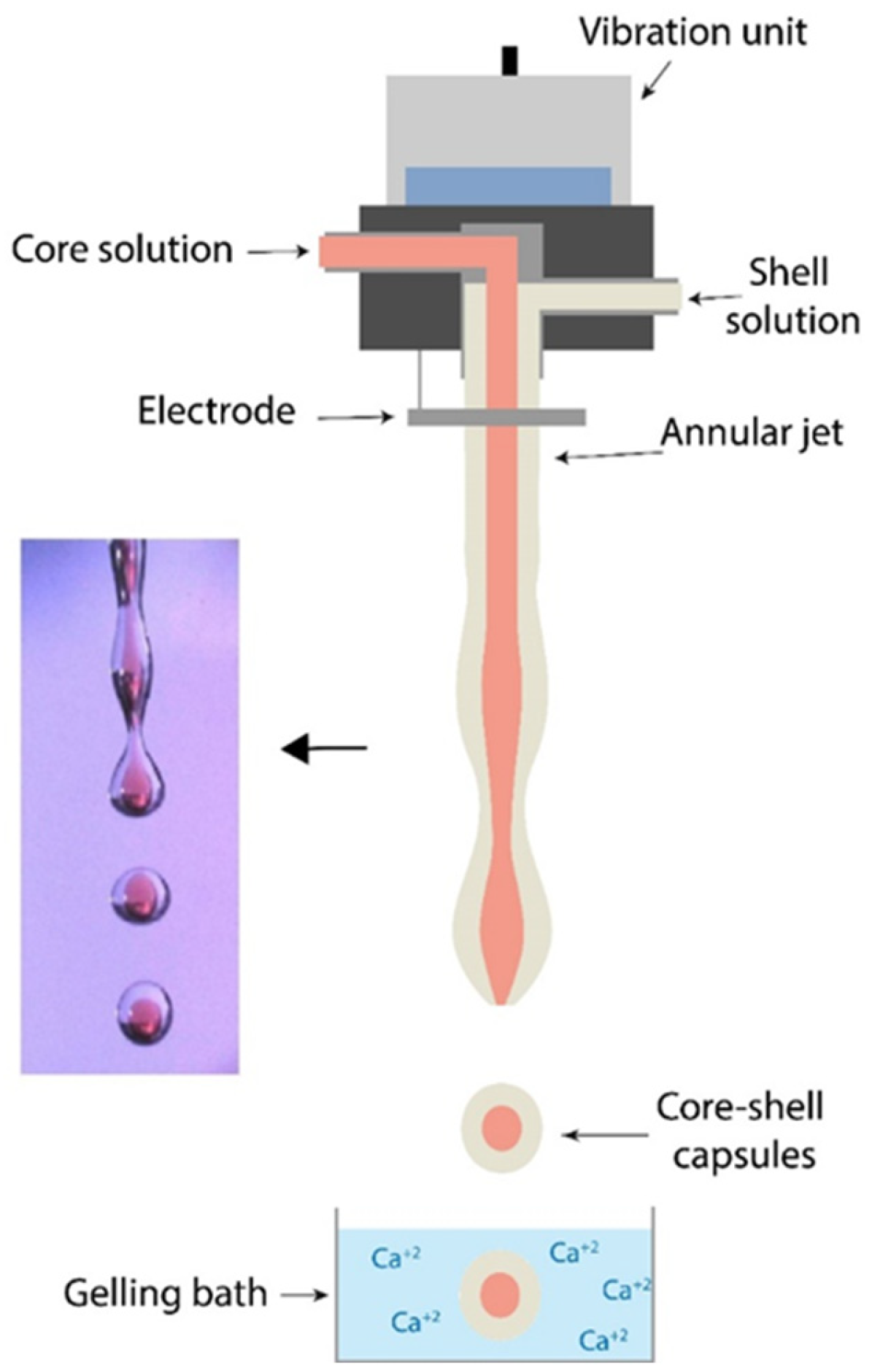
2.2. Vibrating Coextrusion System
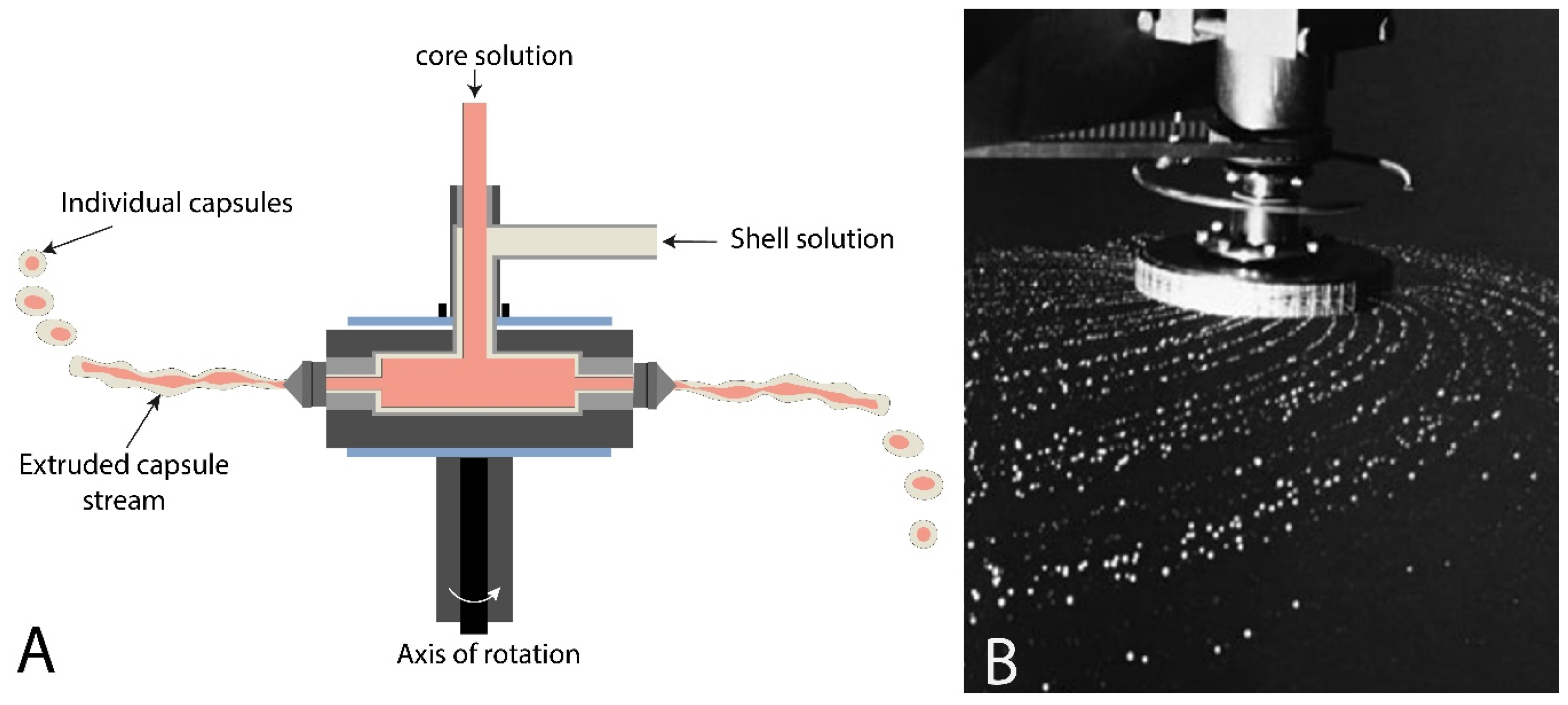
2.3. Centrifugal Coextrusion System
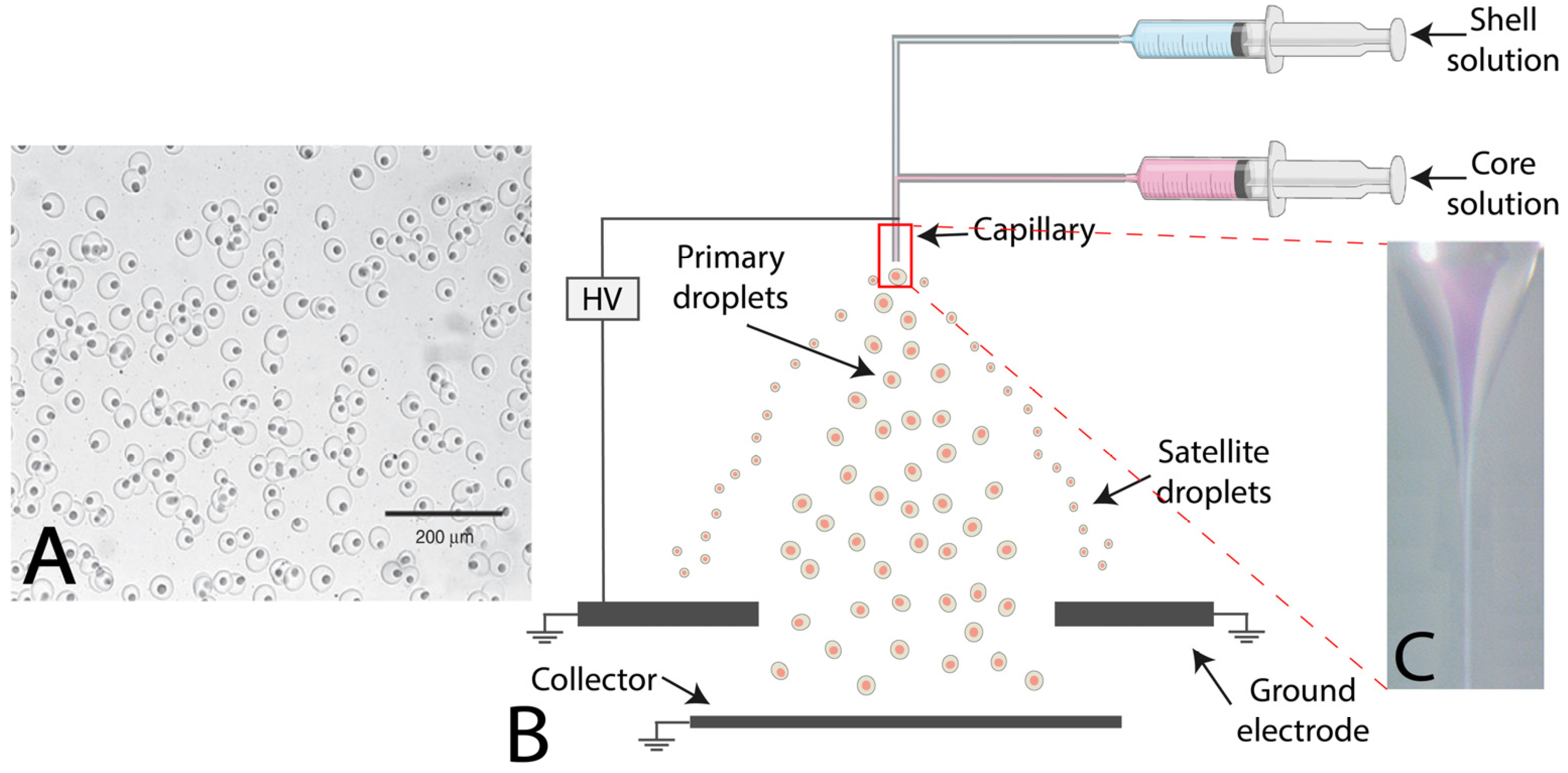
2.4. Electrohydrodynamic Coextrusion or Electrospraying
2.5. Other Coextrusion Systems
3. Advantages and Limitations of Coextrusion Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bangham, A.D.; Horne, R.W. Action of Saponin on Biological Cell Membranes. Nat. Int. J. Sci. 1962, 196, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Green, B.K.; Schleicher, L. Them Oil-Containing Microscopic Capsules and Methods of Making. U.S Patent No. 2,800,457, 23 July 1957. [Google Scholar]
- Cairns, W. Appartus for Sugar Coating Confectionery, Pilsn Etc. U.S Patent No.159,899, 16 February 1985. [Google Scholar]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to Microencapsulation and Controlled Delivery in Foods; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124045682. [Google Scholar]
- Lim, F.; Richard, D. Process for Producing Controlled Porosity Microcapsules. U.S Patent No. 4,322,311, 30 March 1982. [Google Scholar]
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M.; Potdar, S.B. Current Overview of Encapsulation. In Encapsulation of Active Molecules and Their Delivery System; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128193631. [Google Scholar]
- Acharya, S.; Jakeer, S.; Shilpa, P.; Andhale, S. Review: Flavor Encapsulation by Spray Drying Technique. Int. J. Chem. Stud. 2021, 9, 1836–1840. [Google Scholar] [CrossRef]
- Potdar, S.B.; Landge, V.K.; Barkade, S.S.; Potoroko, I.; Sonawane, S.H. Flavor Encapsulation and Release Studies in Food. In Encapsulation of Active Molecules and Their Delivery System; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128193631. [Google Scholar]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin. Mol. Impr. Sens. 2020, 25, 1–18. [Google Scholar]
- Laha, B.; Maiti, S. Design of Core-Shell Stearyl Pullulan Nanostructures for Drug Delivery. Mater. Today Proc. 2019, 11, 620–627. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, Y.; Ashaolu, T.J.; Ye, F.; Zhao, G. Physicochemical and Rheological Characterization of Pectin-Rich Fraction from Blueberry (Vaccinium Ashei) Wine Pomace. Int. J. Biol. Macromol. 2019, 128, 629–637. [Google Scholar] [CrossRef]
- Bae, S.B.; Nam, H.C.; Park, W.H. Electrospraying of Environmentally Sustainable Alginate Microbeads for Cosmetic Additives. Int. J. Biol. Macromol. 2019, 133, 278–283. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Veiga, M.; Baptista, P.; Tavaria, F.K.; Pintado, M.E. Textile Dyes Loaded Chitosan Nanoparticles: Characterization, Biocompatibility and Staining Capacity. Carbohydr. Polym. 2021, 251, 117120. [Google Scholar] [CrossRef]
- Matos, J.C.; Pereira, L.C.J.; Waerenborgh, J.C.; Gonçalves, M.C. Encapsulation of Active Molecules in Pharmaceutical Sector: The Role of Ceramic Nanocarriers. In Encapsulation of Active Molecules and Their Delivery System; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128193631. [Google Scholar]
- Mejías, F.J.R.; Trasobares, S.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Macías, F.A. One-Step Encapsulation of Ortho-Disulfides in Functionalized Zinc MOF. Enabling Metal–organic Frameworks in Agriculture. ACS Appl. Mater. Interfaces 2021, 13, 7997–8005. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on Alginate Use for Spherification to Encapsulate Biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Whelehan, M.; Marison, I.W. Microencapsulation Using Vibrating Technology. J. Microencapsul. 2011, 28, 669–688. [Google Scholar] [CrossRef]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.M.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and Characterization of Alginate Extracted from Brown Seaweed Collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Banks, S.R.; Enck, K.; Wright, M.; Opara, E.C.; Welker, M.E. Chemical Modification of Alginate for Controlled Oral Drug Delivery. J. Agric. Food Chem. 2019, 67, 10481–10488. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D Printing Process and Application of Sodium Alginate Based Hydrogels in Soft Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of Probiotics in Multi-Polysaccharide Microcapsules by Electro-Hydrodynamic Atomization and Incorporation into Ice-Cream Formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Shahabivand, S.; Mortazavi, S.S.; Mahdavinia, G.R.; Darvishi, F. Phenol Biodegradation by Immobilized Rhodococcus Qingshengii Isolated from Coking Effluent on Na-Alginate and Magnetic Chitosan-Alginate Nanocomposite. J. Environ. Manag. 2022, 307, 114586. [Google Scholar] [CrossRef]
- How, Y.H.; Hubert, C.; Pui, L.P. Encapsulation of Probiotic Strain Lactobacillus Rhamnosus GG with Black Bean Extract in Alginate-Pectin Microcapsules. Malays. J. Microbiol. 2021, 17, 190–199. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial Appraisal of Existing Technologies and Trends. In Trends in Food Science and Technology; Elsevier: Amsterdam, The Netherland, 2004; Volume 15, pp. 330–347. [Google Scholar]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of Bioactive Compounds by “Extrusion” Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition Design and Medical Application of Liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Fangmeier, M.; Lehn, D.N.; Maciel, M.J.; Volken de Souza, C.F. Encapsulation of Bioactive Ingredients by Extrusion with Vibrating Technology: Advantages and Challenges. Food Bioproc. Tech. 2019, 12, 1472–1486. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles—A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Oxley, J.D. Coextrusion for Food Ingredients and Nutraceutical Encapsulation: Principles and Technology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Doméjean, H. Formation de Capsules d’hydrogel à Coeur Aqueux Par Fragmentation d’un Jet Composé de Fluides Complexes; Université Pierre et Marie Curie: Paris, France, 2014. [Google Scholar]
- Yildirim, O.E.; Xu, Q.; Basaran, O.A. Analysis of the Drop Weight Method. Phys. Fluids 2005, 17, 1–13. [Google Scholar] [CrossRef]
- Rolland, L. Propriétés Physico-Chimiques de Capsules d’hydrogel à Cœur Liquide; Université Pierre et Marie Curie: Paris, France, 2013. [Google Scholar]
- Berthier, J. The Physics of Droplets; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9781455725502. [Google Scholar]
- Bremond, N.; Santanach-Carreras, E.; Chu, L.Y.; Bibette, J. Formation of Liquid-Core Capsules Having a Thin Hydrogel Membrane: Liquid Pearls. Soft Matter 2010, 6, 2484–2488. [Google Scholar] [CrossRef]
- Pereda, M.; Poncelet, D.; Renard, D. Characterization of Core-Shell Alginate Capsules. Food Biophys. 2019, 14, 467–478. [Google Scholar] [CrossRef]
- Saqib, M.N.; Ahammed, S.; Liu, F.; Zhong, F. Customization of Liquid-Core Sodium Alginate Beads by Molecular Engineering. Carbohydr. Polym. 2022, 284, 119047. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, D.; Huang, Y. Fabrication of Double-Layered Alginate Capsules Using Coaxial Nozzle. J. Micro Nanomanuf. 2017, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Farahmand, A.; Emadzadeh, B.; Ghorani, B.; Poncelet, D. Droplet-Based Millifluidic Technique for Encapsulation of Cinnamon Essential Oil: Optimization of the Process and Physicochemical Characterization. Food Hydrocoll. 2022, 129, 107609. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Malekpour-Galogahi, F.; Sreejith, K.R.; Takei, T.; Nguyen, N.T. Surfactant-Free, UV-Curable Core-Shell Microcapsules in a Hydrophilic PDMS Microfluidic Device. AIP Adv. 2020, 10, 065101. [Google Scholar] [CrossRef]
- Dormer, N.H.; Berkland, C.J.; Singh, M. Monodispersed Microencapsulation Technology; Elsevier: Amsterdam, The Netherland, 2014; ISBN 9780124045682. [Google Scholar]
- Mitchell, B.R.; Klewicki, J.C.; Korkolis, Y.P.; Kinsey, B.L. Normal Impact Force of Rayleigh Jets. Phys. Rev. Fluids 2019, 4, 1–23. [Google Scholar] [CrossRef]
- Heinzen, C.; Berger, A.; Marison, I. Use of Vibration Technology for Jet Break-Up for Encapsulation of Cells and Liquids. Fundam. Cell Immobil. Biotechnol. 2004, 257–275. [Google Scholar] [CrossRef]
- Doméjean, H.; De La Motte Saint Pierre, M.; Funfak, A.; Atrux-Tallau, N.; Alessandri, K.; Nassoy, P.; Bibette, J.; Bremond, N. Controlled Production of Sub-Millimeter Liquid Core Hydrogel Capsules for Parallelized 3D Cell Culture. Lab Chip 2017, 17, 110–119. [Google Scholar] [CrossRef]
- Clanet, C.; Lasheras, J.C. Transition from Dripping to Jetting. J. Fluid Mech. 1999, 383, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Fang, Y.; She, L.; Su, R.; Fu, X. Dripping–Jetting Transition of Liquid Stream from Plate-Type Micro-Orifice Affected by Wetting and Dewetting. Exp. Fluid Sci. 2021, 122, 110302. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. On the Instability of Jets. Proc. Lond. Math. Soc. 1878, s1–s10, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.C.; Nyam, K.L. Microencapsulation of Kenaf Seed Oil by Co-Extrusion Technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Brandau, T. Preparation of Monodisperse Controlled Release Microcapsules. Int. J. Pharm. 2002, 242, 179–184. [Google Scholar] [CrossRef]
- Jaworek, A.Ã.; Sobczyk, A.T. Electrospraying Route to Nanotechnology: An Overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients; Taylor & Francis: Oxfordshire, UK, 2005; Volume 23, ISBN 8229535892. [Google Scholar]
- Jeyakumari, A.; Zynudheen, A.; Parvathy, U. Microencapsulation of Bioactive Food Ingredients and Controlled Release—A Review. MOJ Food Process. Technol. 2016, 2, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of Micro-Nanocapsules by a New Electrospraying Method Using Coaxial Jets and Examination of Effective Parameters on Their Production. J. Electrostat. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Jaworek, A. Electrohydrodynamic Microencapsulation Technology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128043073. [Google Scholar]
- Bocanegra, R.; Gaonkar, A.G.; Barrero, A.; Loscertales, I.G.; Pechack, D.; Marquez, M. Production of Cocoa Butter Microcapsules Using an Electrospray Process. J. Food Sci. 2005, 70, e492–e497. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Clasen, C.; Van den Mooter, G. Pharmaceutical Applications of Electrospraying. J. Pharm. Sci. 2016, 105, 2601–2620. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Jiang, B.; Yang, R.; Hou, X.; Zheng, C. One-Step Synthesis of Monodispersed Pt Nanoparticles Anchored on 3D Graphene Foams and Its Application for Electrocatalytic Hydrogen Evolution. Chin. Chem. Lett. 2020, 31, 1540–1544. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- de La Mora, J.F.; Loscertales, I.G. The Current Emitted by Highly Conducting Taylor Cones. J. Fluid Mech. 1994, 260, 155–184. [Google Scholar] [CrossRef]
- Poncelet, D.; Tam, S.K. Microencapsulation Technologies for a Bioartificial Endocrine Pancreas. Pancreas Bioartificial Ther. Other Biohybrid 2009, 661, 37–50. [Google Scholar]
- Uludag, H.; Horvath, V.; Black, J.P.; Sefton, M.V. Viability and Protein Secretion from Human Hepatoma (HepG2) Cells Encapsulated in 400-Pm Polyacrylate Microcapsules by Submerged Nozzle-Liquid Jet Extrusion. Biotechnol. Bioeng. 1994, 44, 119961204. [Google Scholar] [CrossRef]
- Ponrasu, T.; Yang, R.F.; Chou, T.H.; Wu, J.J.; Cheng, Y.S. Core-Shell Encapsulation of Lipophilic Substance in Jelly Fig (Ficus Awkeotsang Makino) Polysaccharides Using an Inexpensive Acrylic-Based Millifluidic Device. Appl. Biochem. Biotechnol. 2020, 191, 360–375. [Google Scholar] [CrossRef]
- Wu, F.; Wang, W.; Liu, L.; Ju, X.J.; Xie, R.; Liu, Z.; Chu, L.Y. Monodisperse Hybrid Microcapsules with an Ultrathin Shell of Submicron Thickness for Rapid Enzyme Reactions. J. Mater. Chem. B 2015, 3, 796–803. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, H.; Tang, Q.; Dong, Y.; Qu, F.; Wang, Y.; Yang, G.; Meng, T. Monodisperse Alginate Microcapsules with Spatially Confined Bioactive Molecules via Microfluid-Generated W/W/O Emulsions. ACS Appl. Mater. Interfaces 2019, 11, 37313–37321. [Google Scholar] [CrossRef]
- Huang, L.; Wu, K.; Zhang, R.; Ji, H. Fabrication of Multicore Milli- And Microcapsules for Controlling Hydrophobic Drugs Release Using a Facile Approach. Ind. Eng. Chem. Res. 2019, 58, 17017–17026. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Kong, B.; Huang, Y.; Yan, D.; Tan, H.; Shang, L. Dual-Core Prebiotic Microcapsule Encapsulating Probiotics for Metabolic Syndrome. ACS Appl. Mater. Interfaces 2020, 12, 42586–42594. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, X.; Ravanbakhsh, H.; Tang, G.; Zhang, J.; Deng, Y.; Braeckmans, K.; De Smedt, S.C.; Xiong, R.; Huang, C. Gas-Shearing Synthesis of Core–Shell Multicompartmental Microparticles as Cell-like System for Enzymatic Cascade Reaction. Chem. Eng. J. 2022, 428, 132607. [Google Scholar] [CrossRef]
- He, F.; Wang, W.; He, X.H.; Yang, X.L.; Li, M.; Xie, R.; Ju, X.J.; Liu, Z.; Chu, L.Y. Controllable Multicompartmental Capsules with Distinct Cores and Shells for Synergistic Release. ACS Appl. Mater. Interfaces 2016, 8, 8743–8754. [Google Scholar] [CrossRef]
- How, Y.H.; Lai, K.W.; Pui, L.P.; In, L.L.A. Co-Extrusion and Extrusion Microencapsulation: Effect on Microencapsulation Efficiency, Survivability through Gastrointestinal Digestion and Storage. J. Food Process. Eng. 2022, 45, 1–16. [Google Scholar] [CrossRef]
- Piazza, L.; Roversi, T. Preliminary Study on Microbeads Production by Co-Extrusion Technology. Procedia Food Sci. 2011, 1, 1374–1380. [Google Scholar] [CrossRef] [Green Version]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-Extrusion Encapsulation of Probiotic Lactobacillus Acidophilus Alone or Together with Apple Skin Polyphenols: An Aqueous and Value-Added Delivery System Using Alginate. Food Bioproc. Tech. 2014, 7, 1581–1596. [Google Scholar] [CrossRef]

| Methods | Advantages | Limitations | Refs |
|---|---|---|---|
| Dripping system |
|
| [33,36,41] |
| Vibrating system |
|
| [18,33,47] |
| Centrifugal system |
|
| [33,44,55] |
| EHD system |
|
| [33,44,57] |
| Common to all methods |
|
| [17,29,73,74,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Mar. Drugs 2023, 21, 235. https://doi.org/10.3390/md21040235
Bennacef C, Desobry-Banon S, Probst L, Desobry S. Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Marine Drugs. 2023; 21(4):235. https://doi.org/10.3390/md21040235
Chicago/Turabian StyleBennacef, Chanez, Sylvie Desobry-Banon, Laurent Probst, and Stéphane Desobry. 2023. "Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies" Marine Drugs 21, no. 4: 235. https://doi.org/10.3390/md21040235
APA StyleBennacef, C., Desobry-Banon, S., Probst, L., & Desobry, S. (2023). Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Marine Drugs, 21(4), 235. https://doi.org/10.3390/md21040235








