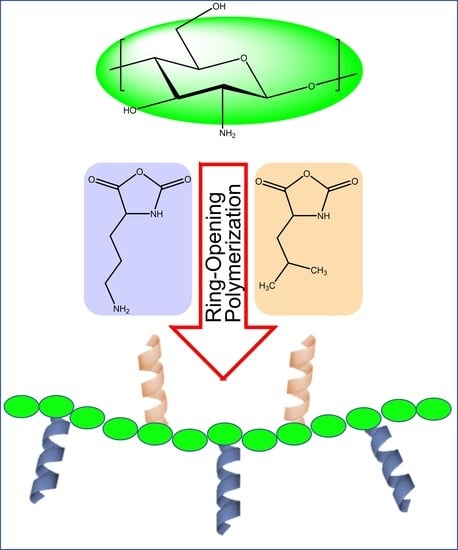
Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides
Abstract
:1. Introduction
2. Results and Discussion
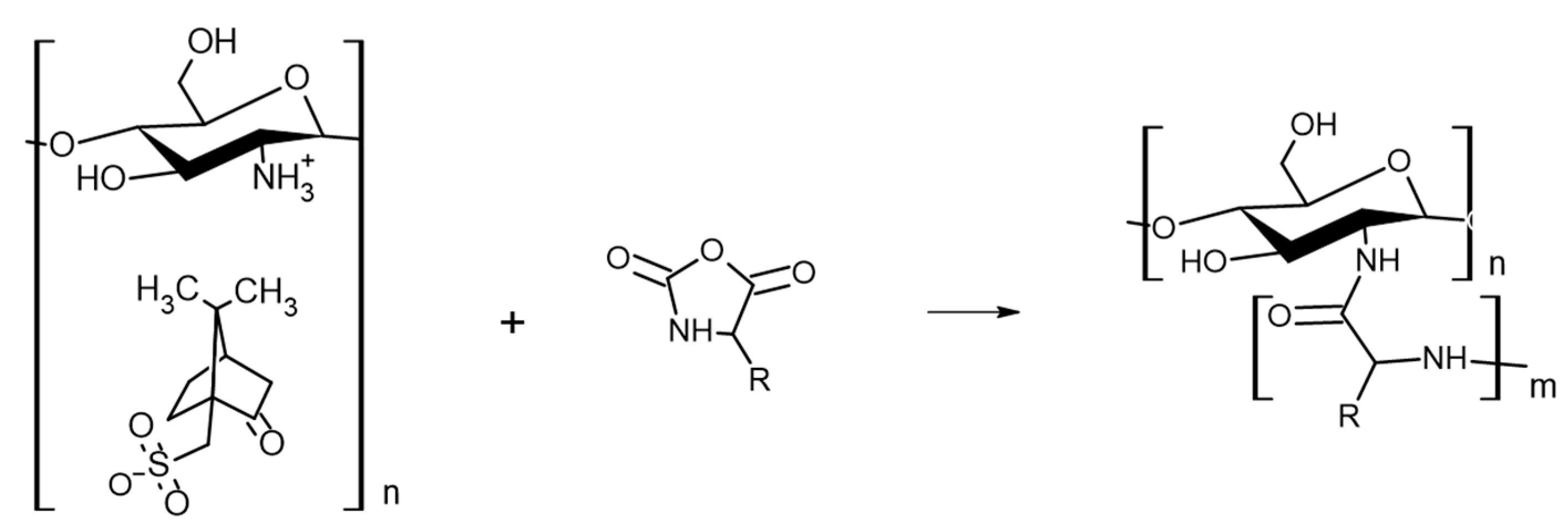
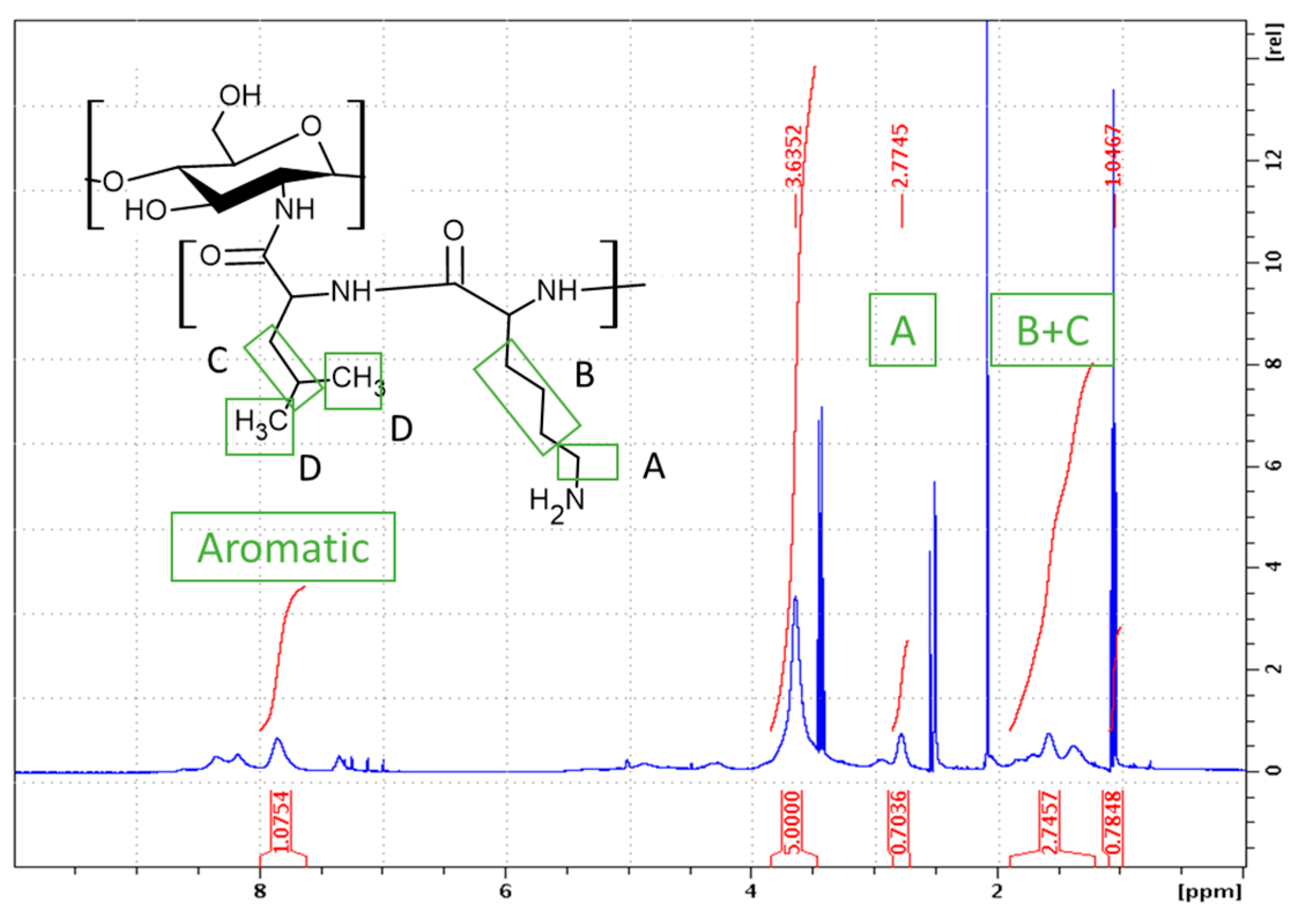
2.1. Synthesis of GCPs
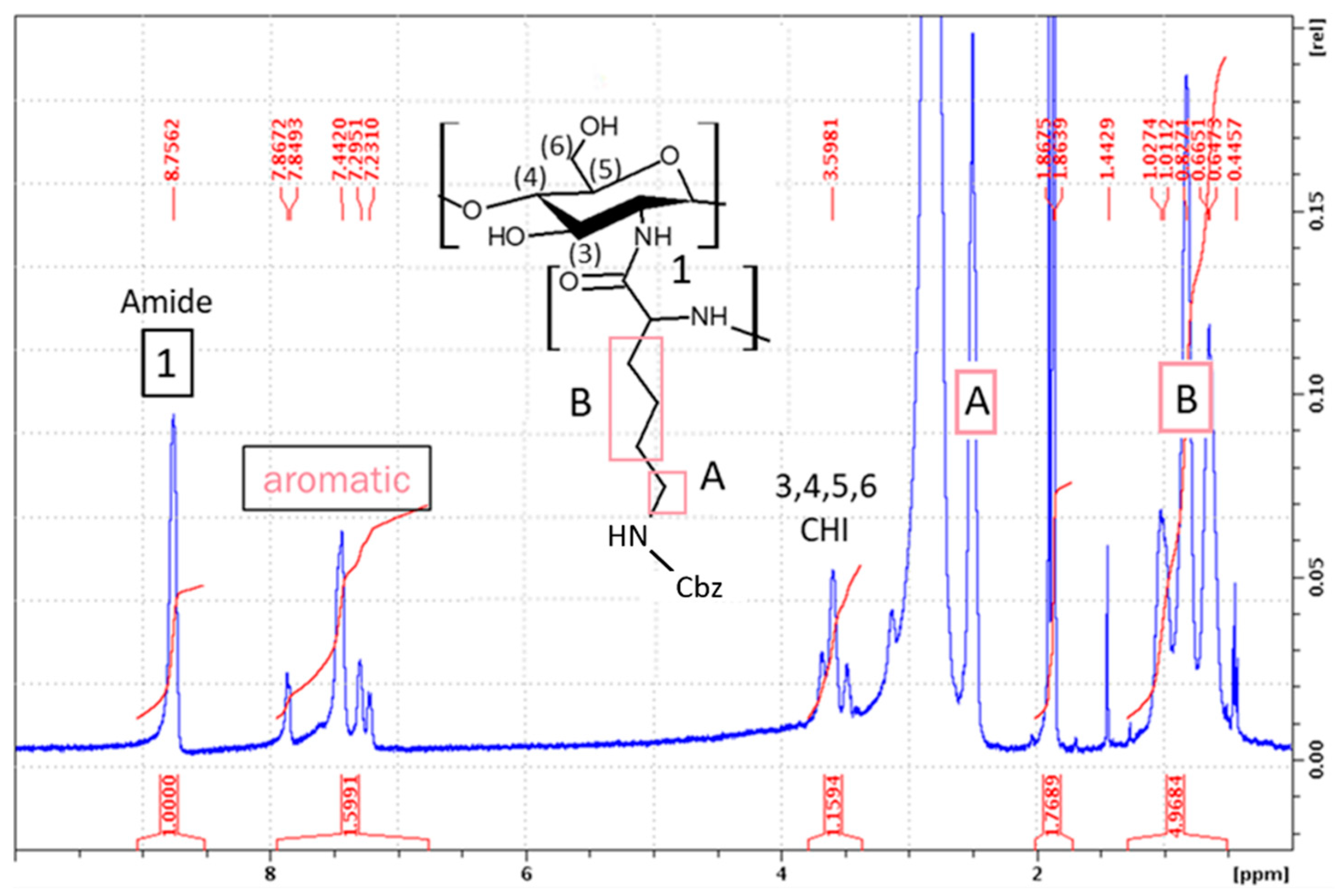
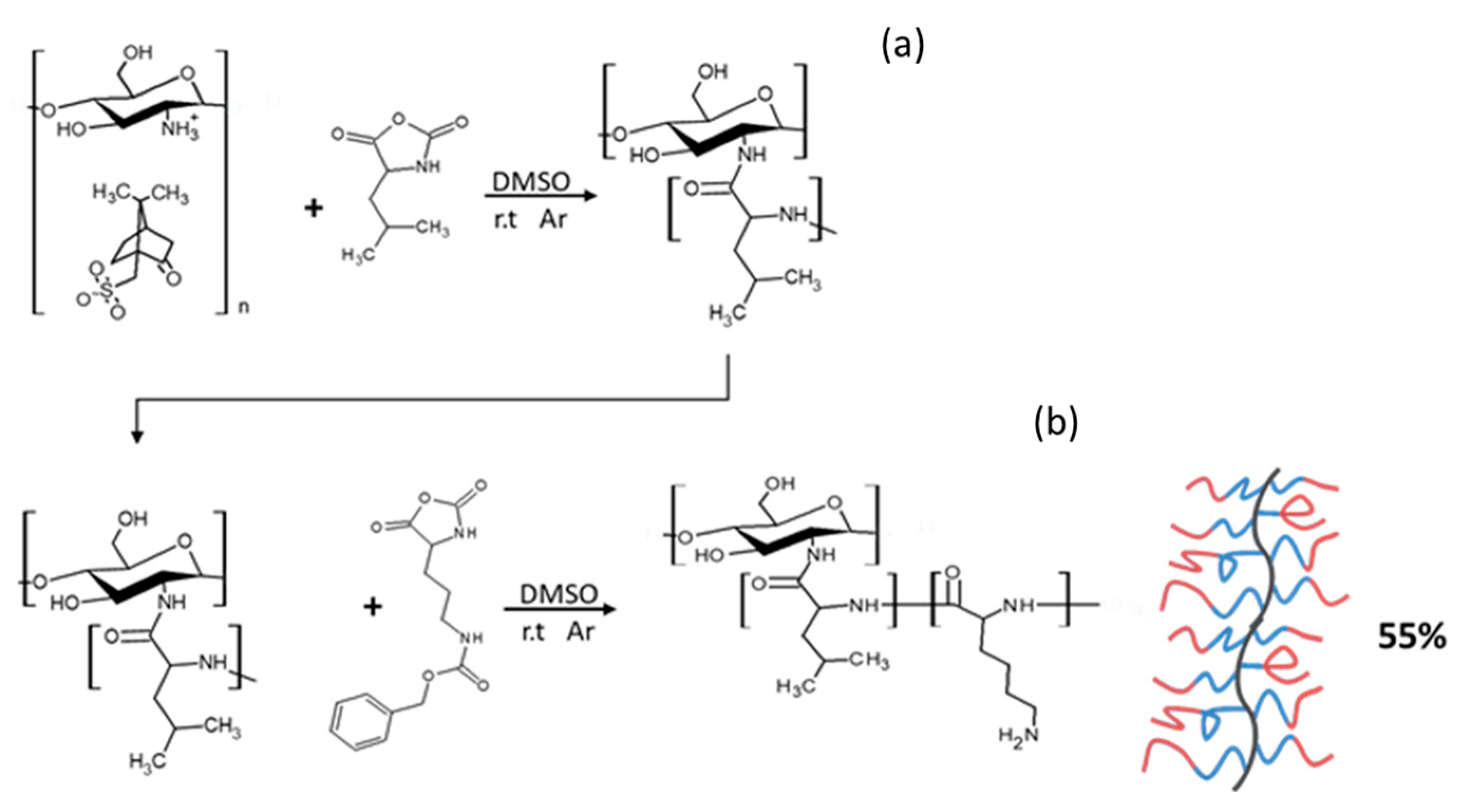
2.1.1. Synthesis of CHI-graft-poly(l-lysine(Z))
2.1.2. Synthesis of CHI-graft-poly(l-leucine-co-l-lysine)
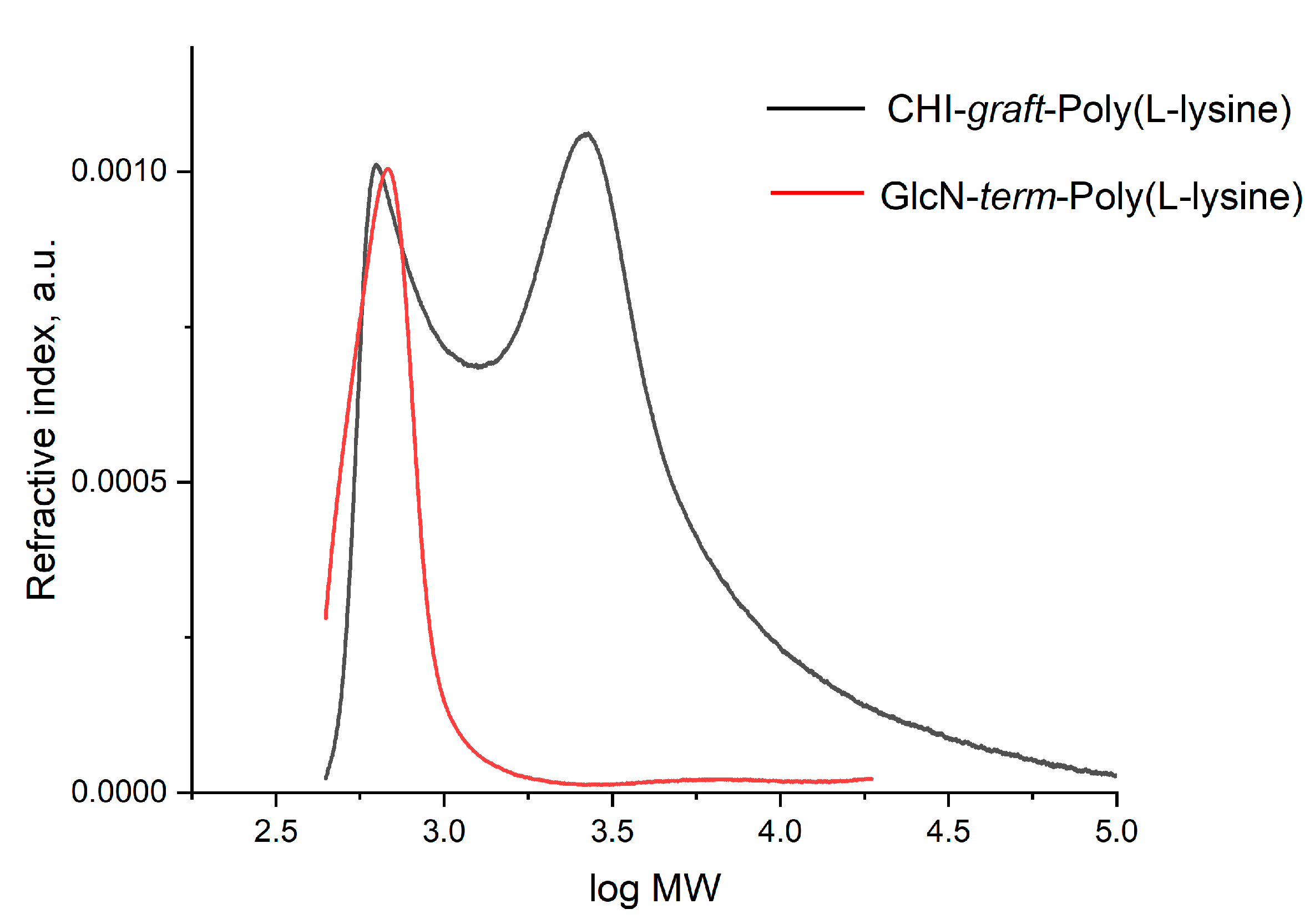
2.1.3. Synthesis of Block GCPs
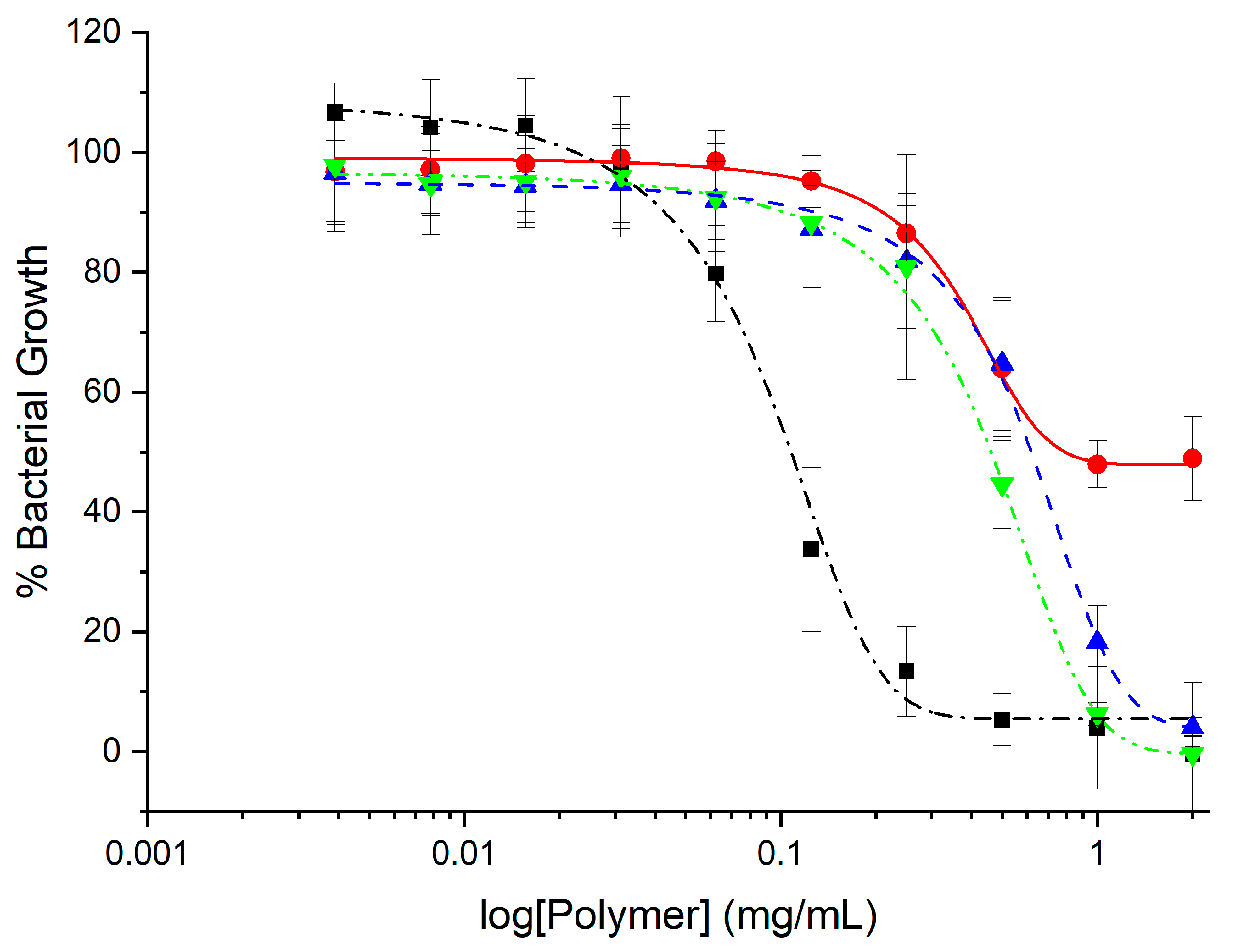
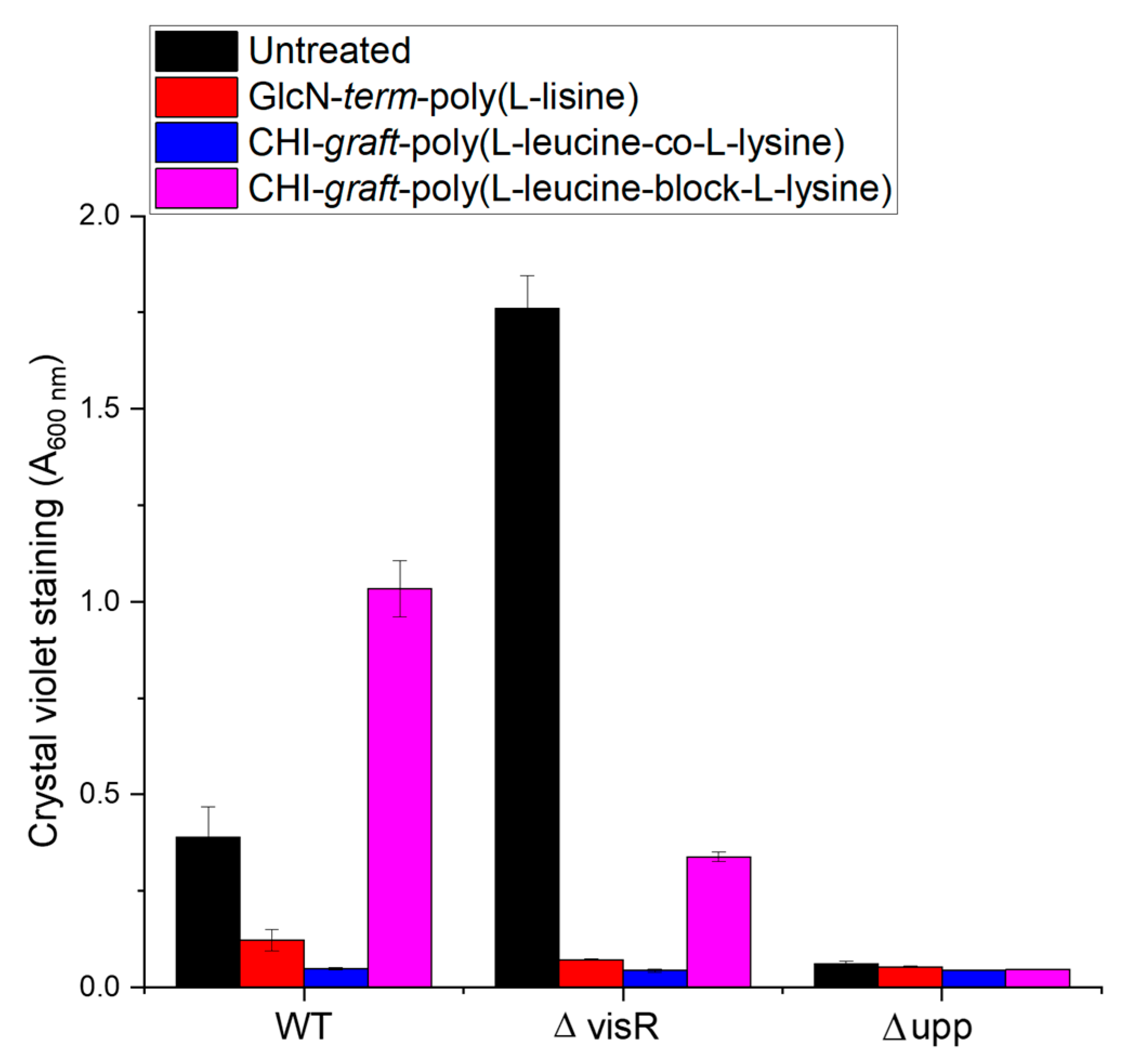
2.2. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
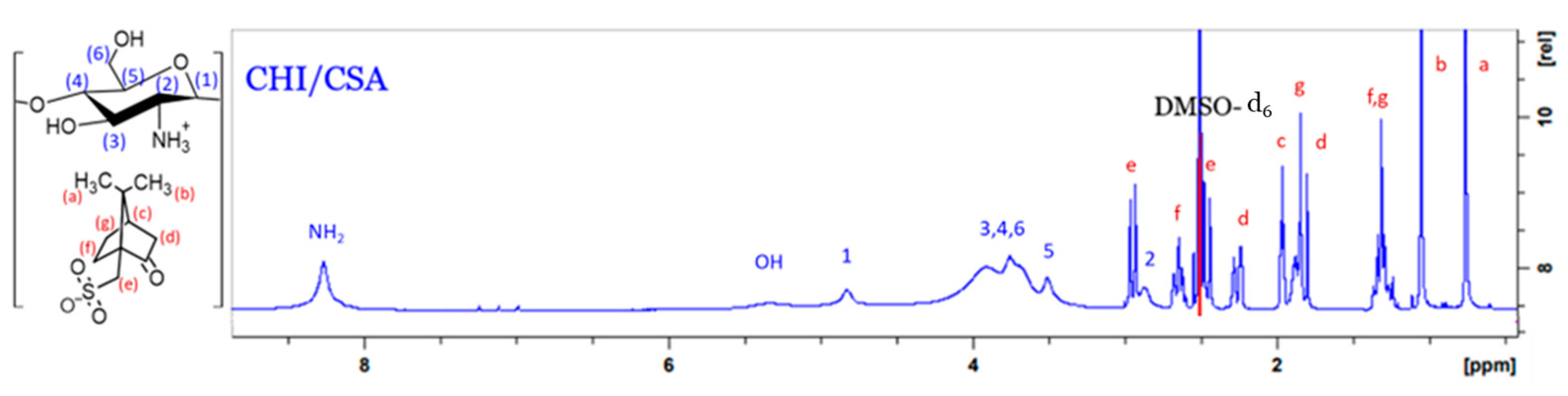
3.2.1. Synthesis of CHI-CSA Salt
3.2.2. Synthesis of l-lysine(Z)-NCA
3.2.3. Synthesis of CHI-graft-poly(l-lysine(Z))
3.2.4. Synthesis of CHI-graft-poly(l-leucine-co-l-lysine(Z))
3.2.5. Synthesis of CHI-graft-poly(l-leucine-block-lysine(Z))
3.2.6. Synthesis of Linear GlcN-term-poly(l-lysine(Z))
3.2.7. Deprotection of N-ε-Carbobenzyloxy-l-lysine (l-lysine(Z)) Moieties
3.3. Characterization
3.3.1. Minimum Inhibitory Concentrations (MICs)
3.3.2. LIVE/DEAD Assay to Examine Bacterial Viability
3.3.3. Crystal Violet Biofilm Assay
3.3.4. Test of Activity against Biofilms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- D’Souza, A.R.; Necelis, M.R.; Kulesha, A.; Caputo, G.A.; Makhlynets, O.V. Beneficial Impacts of Incorporating the Non-Natural Amino Acid Azulenyl-Alanine into the Trp-Rich Antimicrobial Peptide buCATHL4B. Biomolecules 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Yang, M.-S.; Liang, M. The synthesis, characterization and antibacterial activity of quaternized poly(2,6-dimethyl-1,4-phenylene oxide)s modified with ammonium and phosphonium salts. React. Funct. Polym. 2010, 70, 944–950. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [Green Version]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef]
- Popa, A.; Davidescu, C.M.; Trif, R.; Ilia, G.; Iliescu, S.; Dehelean, G. Study of quaternary ‘onium’ salts grafted on polymers: Antibacterial activity of quaternary phosphonium salts grafted on ‘gel-type’ styrene–divinylbenzene copolymers. React. Funct. Polym. 2003, 55, 151–158. [Google Scholar] [CrossRef]
- Costanza, F.; Padhee, S.; Wu, H.; Wang, Y.; Revenis, J.; Cao, C.; Li, Q.; Cai, J. Investigation of antimicrobial PEG-poly(amino acid)s. RSC Adv. 2014, 4, 2089–2095. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Wang, H.; Jeremy Tan, P.K.; Fan, W.; Venkatraman, S.S.; Li, L.; Yang, Y.-Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nano 2009, 4, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Arnusch, C.J.; Branderhorst, H.; de Kruijff, B.; Liskamp, R.M.J.; Breukink, E.; Pieters, R.J. Enhanced Membrane Pore Formation by Multimeric/Oligomeric Antimicrobial Peptides. Biochemistry 2007, 46, 13437–13442. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Hou, Z.; Shankar, Y.V.; Liu, Y.; Ding, F.; Subramanion, J.L.; Ravikumar, V.; Zamudio-Vázquez, R.; Keogh, D.; Lim, H.; Tay, M.Y.F.; et al. Nanoparticles of Short Cationic Peptidopolysaccharide Self-Assembled by Hydrogen Bonding with Antibacterial Effect against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 38288–38303. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Hitoshi, S.; Yoshihiro, S.; René, R. Dissolution of Chitosan in Dimethyl Sulfoxide by Salt Formation. Chem. Lett. 2000, 29, 596–597. [Google Scholar]
- Weinhold, M.X.; Sauvageau, J.C.M.; Keddig, N.; Matzke, M.; Tartsch, B.; Grunwald, I.; Kübel, C.; Jastorff, B.; Thöming, J. Strategy to improve the characterization of chitosan for sustainable biomedical applications: SAR guided multi-dimensional analysis. Green Chem. 2009, 11, 498–509. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of Chitosan by Acids. Sci. World J. 2013, 2013, 508540. [Google Scholar] [CrossRef] [Green Version]
- Chi, P.; Wang, J.; Liu, C. Synthesis and characterization of polycationic chitosan-graft-poly (l-lysine). Mater. Lett. 2008, 62, 147–150. [Google Scholar] [CrossRef]
- Kurita, K.; Yoshida, A.; Koyama, Y. Studies on chitin. 13. New polysaccharide/polypeptide hybrid materials based on chitin and poly(.gamma.-methyl L-glutamate). Macromolecules 1988, 21, 1579–1583. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X.; Lu, T.; Sun, J.; Tian, H.; Hu, J.; Wang, Y.; Zhang, P.; Jing, X. Poly(l-lysine)-Graft-Chitosan Copolymers: Synthesis, Characterization, and Gene Transfection Effect. Biomacromolecules 2007, 8, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Perdih, P.; Pahovnik, D.; Cegnar, M.; Miklavžin, A.; Kerč, J.; Žagar, E. Synthesis of chitosan-graft-poly(sodium-l-glutamate) for preparation of protein nanoparticles. Cellulose 2014, 21, 3469–3485. [Google Scholar] [CrossRef]
- Dimitrov, I.; Schlaad, H. Synthesis of nearly monodisperse polystyrene–polypeptide block copolymers via polymerisation of N-carboxyanhydrides. Chem. Commun. 2003, 23, 2944–2945. [Google Scholar] [CrossRef] [PubMed]
- Knobler, Y.; Bittner, S.; Frankel, M. 749. Reaction of N-carboxy-α-amino-acid anhydrides with hydrochlorides of hydroxylamine, O-alkylhydroxylamines, and amines; syntheses of amino-hydroxamic acids, amido-oxy-peptides, and α-amino-acid amides. J. Chem. Soc. (Resumed) 1964, 3941–3951. [Google Scholar] [CrossRef]
- Breedveld, V.; Nowak, A.P.; Sato, J.; Deming, T.J.; Pine, D.J. Rheology of Block Copolypeptide Solutions: Hydrogels with Tunable Properties. Macromolecules 2004, 37, 3943–3953. [Google Scholar] [CrossRef] [Green Version]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Haidacher, D.; Horváth, C. Physicochemical Basis of Hydrophobic Interaction Chromatography. In Theoretical Advancement in Chromatography and Related Separation Techniques; Dondi, F., Guiochon, G., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 443–480. [Google Scholar]
- Holbrook, R.D.; Galyean, A.A.; Gorham, J.M.; Herzing, A.; Pettibone, J. Chapter 2—Overview of Nanomaterial Characterization and Metrology. In Frontiers of Nanoscience; Baalousha, M., Lead, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 47–87. [Google Scholar]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of α-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, R.J.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Kaur, M.; Kumari, A.; Kumari, L.; Pawar, S.V.; Singh, R.; Salunke, D.B. Facially Amphiphilic Cholic Acid–Lysine Conjugates as Promising Antimicrobials. ACS Omega 2020, 5, 3952–3963. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Hou, Y.; Wu, C.; Fan, Y.; Liu, L.; et al. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef]
- Gomes Von Borowski, R.; Gnoatto, S.C.B.; Macedo, A.J.; Gillet, R. Promising Antibiofilm Activity of Peptidomimetics. Frontiers in microbiology 2018, 9, 2157. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. JCMA 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Høiby, N.; Ulrich, M.; Molin, S.; Riethmüller, J.; Döring, G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Han, F.; Ma, S.; Yu, W. Carboxymethyl chitosan prevents formation of broad-spectrum biofilm. Carbohydr. Polym. 2011, 84, 1365–1370. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [Green Version]
- Arslan, S.Y.; Leung, K.P.; Wu, C.D. The effect of lactoferrin on oral bacterial attachment. Oral Microbiol. Immunol. 2009, 24, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhou, C.; Liu, Z.; Young, A.W.; Shi, Z.; Ren, D.; Kallenbach, N.R. Antimicrobial dendrimer active against Escherichia coli biofilms. Bioorganic Med. Chem. Lett. 2009, 19, 5478–5481. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Lee, M.-Y.; Kim, J.-Y.; Kim, H.; Jung, M.; Shin, M.-K.; Lee, W.-K.; Cheong, G.-W.; Lee, J.R.; Jang, M.-K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. [Google Scholar] [CrossRef] [Green Version]
- Hall-Edgefield, D.L.; Shi, T.; Nguyen, K.; Sidorenko, A. Hybrid molecular brushes with chitosan backbone: Facile synthesis and surface grafting. ACS Appl. Mater. Interfaces 2014, 6, 22026–22033. [Google Scholar] [CrossRef]
- Vernaez, O.; Neubert, K.J.; Kopitzky, R.; Kabasci, S. Compatibility of Chitosan in Polymer Blends by Chemical Modification of Bio-based Polyesters. Polymers 2019, 11, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 1564–1569. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.; Currier, T.C.; Gordon, M.P.; Chilton, M.D.; Nester, E.W. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 1975, 123, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kim, J.; Koestler, B.J.; Choi, J.-H.; Waters, C.M.; Fuqua, C. Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile-to-sessile switch. Mol. Microbiol. 2013, 89, 929–948. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.; Niles, A.; Duellman, S.; Benink, H.; Worzella, T.; Minor, L. Cell viability assays assay guidance manual. Assay Guid. Man. 2004, 1–23. [Google Scholar]





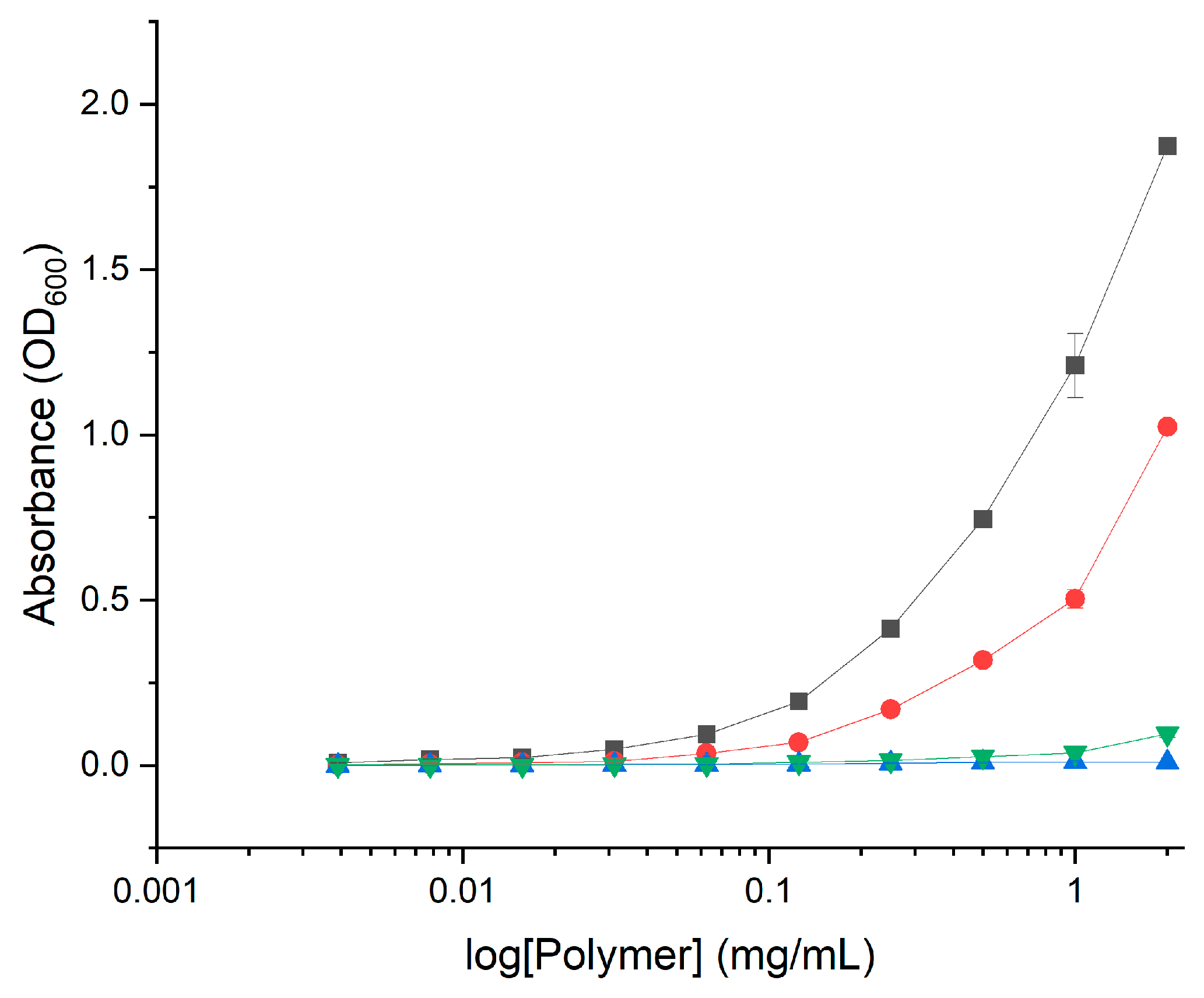


| Polymer | E. coli | S. aureus | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| GlcN-term-poly(l-lysine) | 0.16 ± 0.09 | 0.12 | 0.26 ± 0.12 | 0.25 |
| CHI-graft-poly(l-lysine) | 0.65 ± 0.11 | >2.0 | >2.0 | >2.0 |
| CHI-graft-poly(l-leucine-co-l-lysine) | 1.2 ± 0.02 | 2.0 | >2.0 | >2.0 |
| CHI-graft-poly(l-leucine-block-l-lysine) | 0.76 ± 0.04 | 1.0 | >2.0 | >2.0 |
| Magainin II | 0.04 ± 0.03 | 0.06 | Not tested | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enright, T.P.; Garcia, D.L.; Storti, G.; Heindl, J.E.; Sidorenko, A. Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides. Mar. Drugs 2023, 21, 243. https://doi.org/10.3390/md21040243
Enright TP, Garcia DL, Storti G, Heindl JE, Sidorenko A. Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides. Marine Drugs. 2023; 21(4):243. https://doi.org/10.3390/md21040243
Chicago/Turabian StyleEnright, Timothy P., Dominic L. Garcia, Gia Storti, Jason E. Heindl, and Alexander Sidorenko. 2023. "Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides" Marine Drugs 21, no. 4: 243. https://doi.org/10.3390/md21040243
APA StyleEnright, T. P., Garcia, D. L., Storti, G., Heindl, J. E., & Sidorenko, A. (2023). Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides. Marine Drugs, 21(4), 243. https://doi.org/10.3390/md21040243