Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage
Abstract
:1. Introduction
2. Results
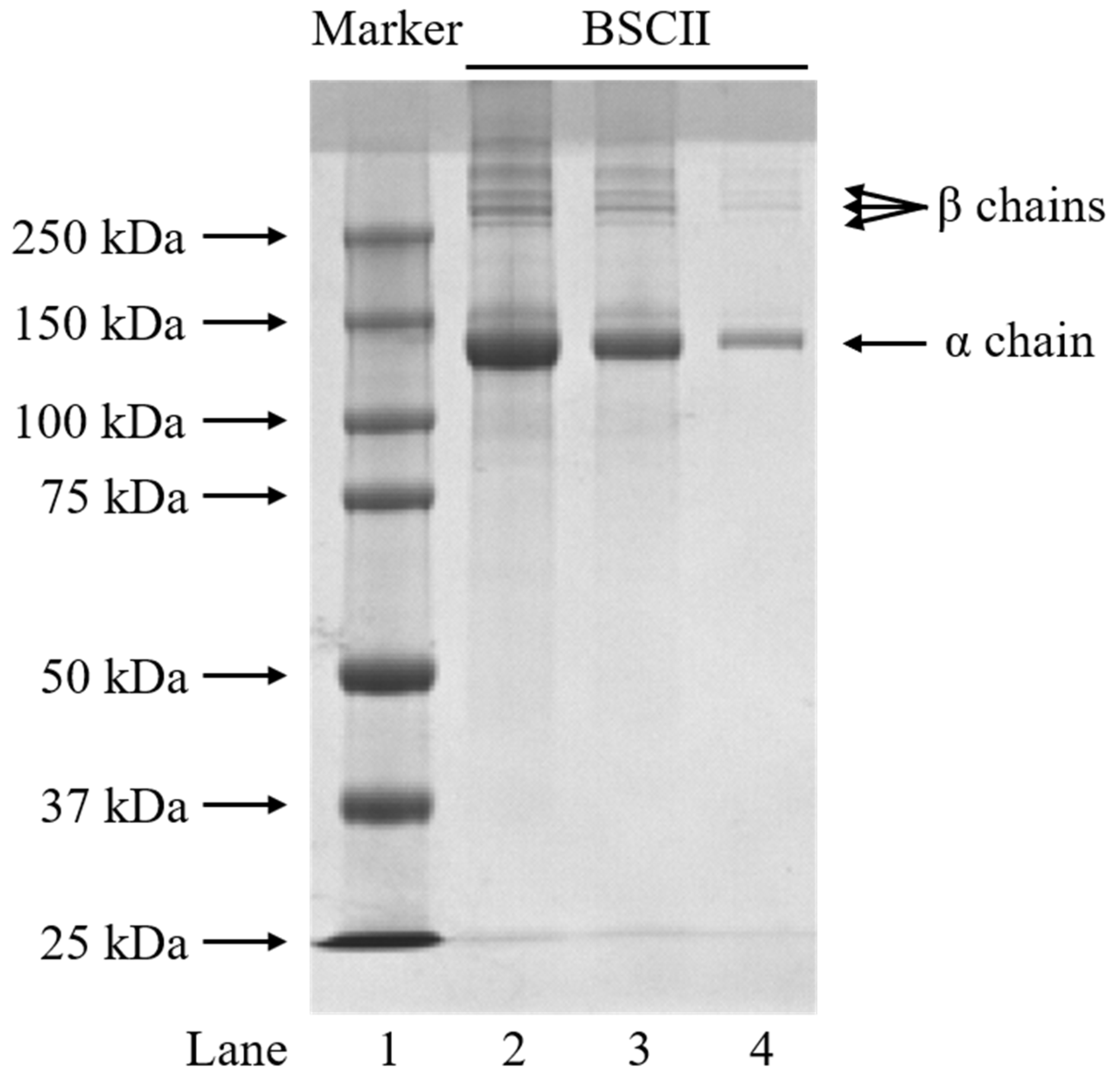
2.1. Protein Pattern of BSCII
2.2. Total Sugar Content of BSCII
2.3. Microstructural Analysis
2.4. Amino Acid Composition of BSCII
2.5. Ultraviolet (UV) Absorption Spectrum
2.6. Secondary Structure Analysis
2.7. Thermal Stability Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Raw Materials and Pretreatment
4.3. Isolation of BSCII
4.4. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Determination of Total Sugar Content
4.6. Scanning Electron Microscopy
4.7. Atomic Force Microscope
4.8. Amino Acid Composition
4.9. UV Absorption Spectrum
4.10. Circular Dichroism (CD) Spectrum
4.11. Fourier Transform Infrared (FTIR) Spectra
4.12. Temperature Sweep Test
4.13. Differential Scanning Calorimetry (DSC)
4.14. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, J.; Chen, X.; Li, J.; Luo, B.; Fan, T.; Li, R.; Liu, X.; Song, B.; Jia, X.; Zhong, S. Preparation and Characterization of Nano-Selenium Decorated by Chondroitin Sulfate Derived from Shark Cartilage and Investigation on Its Antioxidant Activity. Mar. Drugs 2022, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kwon, D.H.; Choi, Y.H.; Noh, J.S. Inhibitory effects of skate cartilage chondroitin sulfate-rich extract on the production of inflammatory mediators and ROS in lipopolysaccharide-treated murine macrophages: A comparison with shark cartilage chondroitin sulfate. In Vitr. Cell. Dev. Biol. Anim. 2020, 56, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenserbaerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef]
- Li, Z.; Bai, X.; Fan, Y.; Jia, Q.; Zhang, H.; Hou, H. Structure of type II collagen from sturgeon cartilage and its effect on adjuvant-induced rheumatoid arthritis in rats. Food Funct. 2022, 13, 6152–6165. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Wang, Y.; Sun, X.; Li, B.; Poungchawanwong, S.; Hou, H. Structural feature and self-assembly properties of type II collagens from the cartilages of skate and sturgeon. Food Chem. 2020, 331, 127340. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.M.; Qiu, Y.T.; Chi, C.F.; Luo, H.Y.; Wang, B. Gelatin from cartilage of Siberian sturgeon (Acipenser baerii): Characterization and protective function on ultraviolet-A injured human skin fibroblasts. Front. Mar. Sci. 2022, 9, 925407. [Google Scholar] [CrossRef]
- Jeevithan, E.; Bao, B.; Zhang, J.; Hong, S.; Wu, W. Purification, characterization and antioxidant properties of low molecular weight collagenous polypeptide (37 kDa) prepared from whale shark cartilage (Rhincodon typus). J. Food Sci. Technol. 2015, 52, 6312–6322. [Google Scholar] [CrossRef]
- Rabbani-Chadegani, A.; Abdossamadi, S.; Bargahi, A.; Yousef-Masboogh, M. Identification of low-molecular-weight protein (SCP1) from shark cartilage with anti-angiogenesis activity and sequence similarity to parvalbumin. J. Pharm. Biomed. Anal. 2008, 46, 563–567. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Jeevithan, E.; Wu, W.; Wang, N.; Lan, H.; Bao, B. Isolation, purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014, 49, 1767–1777. [Google Scholar] [CrossRef]
- Chen, M.; Ding, X.; Wu, S.; Chen, J. Efficacy of agkistrodon type II collagen in treatment of rats with collagen-induced arthritis and related mechanism. Zhejiang Med. 2021, 43, 467–470+580. [Google Scholar]
- Jiang, X.; Chen, S.; Ding, J.; Wang, H.; Ma, J.; Wu, L.; Xu, H. Study on isolation, purification and identification of collagen type II from porcine articular cartilage. J. Jiangsu Univ. Med. Sci. 2006, 16, 389–391. [Google Scholar]
- Li, Z.; Sha, L. Isolation, purification and identification of type II collagen from sheep cartilage. Food Sci. 2013, 38, 233–236. [Google Scholar]
- Lu, X.; Xu, S.; Cao, H. Extraction of type II collagen from chicken sternal cartilage powder and its structural analysis. Food Sci. 2009, 30, 76–80. [Google Scholar]
- Tao, P.; Zhu, W.; Han, Y.; Ning, Q.; Zhang, F.; Meng, L.; Lyu, D. Isolation of rat collagen type II to induce collagen-induced arthritis in rats. J. Xi’an Jiaotong Univ. Med. Sci. 2020, 41, 603–605+616. [Google Scholar]
- Wang, H.; Feng, Z.; Zhu, J.; Wu, X.; Li, J. Identification of zaocys type II collagen and its effect on arthritis in mice with collagen-induced arthritis. J. Chin. Med. Mater. 2014, 37, 1020–1024. [Google Scholar]
- Jeevithan, E.; Zhang, J.; Wang, N.; He, L.; Bao, B.; Wu, W. Physico-chemical, antioxidant and intestinal absorption properties of whale shark type-II collagen based on its solubility with acid and pepsin. Process Biochem. 2015, 50, 463–472. [Google Scholar] [CrossRef]
- Che, S.; Du, F.; Liu, C.; Li, B. The structure analysis of type II collagen from sturgeon (Acipenser sinensis) cartilage. Sci. Technol. Food Ind. 2018, 39, 60–63. [Google Scholar]
- Guo, X.; He, L.; Wei, X.; Wang, N. Extracting technology and structure characterization of type II collagen from squid cartilage. Prog. Biomed. Eng. 2016, 37, 1–5. [Google Scholar]
- Xiao, X.; Shi, W.; Yao, Q.; Chen, H.; Wang, J.; Li, X.; Gao, L. Isolation, purification and identification of collagen type II from sheep cartilage. J. Ningxia Med. Univ. 2009, 31, 730–731+735. [Google Scholar]
- Pang, J.; Li, J.; Wu, X.; Wu, H. The effect of the type II collagen protein from zaocys on cytokines production by synoviocytes in rats with adjuvant arthritis. J. Chin. Med. Mater. 2009, 32, 556–560. [Google Scholar]
- Wang, H.; Feng, Z.; Zhu, J.; Li, J. Zaocys type II collagen regulates mesenteric lymph node Treg/Th17 cell balance in mice with collagen-induced arthritis. J. South. Med. Univ. 2014, 34, 622–626. [Google Scholar]
- Zhang, Z.; Liu, A.; Liu, Y.; Jian, J. Suppression of sheep type II collagen by oral administration on KM mice with adjuvant-induced arthritis. J. Hebei. Agric. Univ. 2006, 29, 76–80+90. [Google Scholar]
- Cao, H.; Xu, S.; Ge, H.; Xu, F. Molecular characterisation of type II collagen from chick sternal cartilage and its anti-rheumatoid arthritis activity. Food Agric. Immunol. 2014, 25, 119–136. [Google Scholar] [CrossRef]
- Li, W.; Tian, S.; Dai, X.; Chen, X. Research progress of blue shark Prionace glauca fishery biology. Mar. Fish 2016, 38, 540–550. [Google Scholar]
- Campana, S.E.; Joyce, W.; Manning, M.J. Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar. Ecol. Prog. Ser. 2009, 387, 241–253. [Google Scholar] [CrossRef]
- Campana, S.E.; Marks, L.; Joyce, W.; Kohler, N.E. Effects of recreational and commercial fishing on blue sharks (Prionace glauca) in Atlantic Canada, with inferences on the North Atlantic population. Can. J. Fish Aquat. Sci. 2006, 63, 670–682. [Google Scholar] [CrossRef]
- Francis, M.P.; Griggs, L.H.; Baird, S.J. Pelagic shark bycatch in the New Zealand tuna longline fishery. Mar. Freshwater Res. 2001, 52, 165–178. [Google Scholar] [CrossRef]
- Kerstetter, D.W.; Graves, J.E. Effects of circle versus J-style hooks on target and non-target species in a pelagic longline fishery. Fish. Res. 2006, 80, 239–250. [Google Scholar] [CrossRef]
- Megalofonou, P.; Damalas, D.; Yannopoulos, C. Composition and abundance of pelagic shark by-catch in the eastern Mediterranean Sea. Cybium 2005, 29, 135–140. [Google Scholar]
- Ward, P.; Myers, R.A.; Blanchard, W. Fish lost at sea: The effect of soak time on pelagic longline catches. Fish. Bull. 2004, 102, 179–195. [Google Scholar]
- Cardeñosa, D.; Shea, K.; Zhang, H.; Feldheim, K.; Fischer, G.; Chapman, D. Small fins, large trade: A snapshot of the species composition of low-value shark fins in the Hong Kong markets. Anim. Conserv. 2020, 23, 203–211. [Google Scholar] [CrossRef]
- Phanat, K.; Soottawat, B.; Wonnop, V.; Takashi, N.; Munehiko, T. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2004, 89, 363–372. [Google Scholar]
- Phanat, K.; Soottawat, B.; Wonnop, V.; Fereidoon, S. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT-Food Sci. Technol. 2010, 43, 792–800. [Google Scholar]
- Song, Z.; Liu, H.; Chen, L.; Chen, L.; Zhou, C.; Hong, P.; Deng, C. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chem. 2021, 340, 128–139. [Google Scholar] [CrossRef]
- Schuetz, T.; Richmond, N.; Harmon, M.E.; Schuetz, J.; Castaneda, L.; Slowinska, K. The microstructure of collagen type I gel cross-linked with gold nanoparticles. Colloids Surf. 2013, 101, 118–125. [Google Scholar] [CrossRef]
- Rigby, B. Amino-acid composition and thermal stability of the skin collagen of the Antarctic ice-fish. Nature 1968, 219, 166–167. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Song, W.; Wang, W.; Qian, G. Purification, optimization and physicochemical properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2016, 89, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zheng, L.; Zhao, M. Effect of temperature and pH on the structure, viscosity and thermal stability of type II collagen from chicken cartilage. Food Sci. 2020, 41, 66–71. [Google Scholar]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Jackson, M.; Choo, L.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim. Biophys. Acta Mol. Basis Dis. 1995, 1270, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2006, 103, 906–912. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, P. Extraction and structural identification of collagen type II from sturgeon cartilage. J. Food Saf. Qual. 2021, 12, 2433–2438. [Google Scholar]
- Gao, L.; Hou, C.; Gao, Y.; Wang, Z.; Zhang, D. Research advances of thermal stability of collagen. J. Chin. Inst. Food Sci. Technol. 2018, 18, 195–207. [Google Scholar]
- Ge, B.; Hou, C.; Bao, B.; Pan, Z.; de Val, J.E.M.S.; Jeevithan, E.; Wu, W. Comparison of physicochemical and structural properties of acid-soluble and pepsin-soluble collagens from blacktip reef shark skin. Mar. Drugs 2022, 20, 376. [Google Scholar] [CrossRef]
- Cuesta, G.; Suarez, N.; Bessio, M.I. Quantitative determination of pneumococcal capsular polysaccharide serotype 14 using a modification of phenol–sulfuric acid method. J. Microbiol. Methods 2003, 1, 69–73. [Google Scholar] [CrossRef]
- Shi, C.; Bi, C.; Ding, M.; Xie, J.; Xu, C.; Qiao, R.; Wang, X.; Zhong, J. Polymorphism and stability of nanostructures of three types of collagens from bovine flexor tendon, rat tail, and tilapia skin. Food Hydrocoll. 2019, 93, 253–260. [Google Scholar] [CrossRef]
- Cao, H.; Shi, F.; Xu, F.; Yu, J. Molecular structure and physicochemical properties of pepsin-solubilized type II collagen from the chick sternal cartilage. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1427–1437. [Google Scholar] [PubMed]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderias, A.J.; Herranz, B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]





| Amino Acid | Sources of CII | ||||||
|---|---|---|---|---|---|---|---|
| Blue Shark (Prionace glauca) Cartilage | Chicken Sternal Cartilage [16] | Chinese Sturgeon (Acipenser sinensis) Cartilage [20] | Squid CARTILAGE [13] | Whale Shark (Rhincodon typus) Cartilage [19] | Silvertip Shark (Carcharhinus albimarginatus) Head Bone [12] | Silvertip Shark (Carcharhinus albimarginatus) Skeletal Bone [12] | |
| Tryptophan (Trp) | N.D. 1 | N.D. 1 | − 2 | N.D. 1 | − 2 | − 2 | − 2 |
| Aspartic acid (Asp)/asparagine (Asn) | 49.04 ± 0.02 | 47 | 62 | 57 | 49.07 | 43.23 ± 1.75 | 45.76 ± 2.13 |
| Threonine (Thr) | 25.16 ± 0.02 | 30 | 23 | 24 | 26.28 | 23.82 ± 2.37 | 25.57 ± 3.55 |
| Serine (Ser) | 25.38 ± 0.05 | 25 | 51 | 31 | 32.83 | 37.68 ± 2.82 | 38.27 ± 1.81 |
| Glutamic acid (Glu)/glutamine (Gln) | 94.80 ± 0.24 | 94 | 70 | 90 | 87.43 | 71.88 ± 3.71 | 76.20 ± 2.07 |
| Glycine (Gly) | 353.83 ± 0.19 | 313 | 311 | 341 | 289.84 | 322.57 ± 21.06 | 319.69 ± 9.99 |
| Alanine (Ala) | 117.12 ± 0.62 | 103 | 104 | 75 | 96.51 | 135.02 ± 12.69 | 132.63 ± 8.46 |
| Cysteine (Cys) | N.D. 1 | 17 | 3 | 1 | 2.36 | 7.93 ± 0.87 | 4.26 ± 0.94 |
| Valine (Val) | 21.26 ± 0.04 | 22 | 28 | 24 | 31.39 | 16.78 ± 1.35 | 25.43 ± 1.05 |
| Methionine (Met) | 0.42 ± 0.03 | 2 | 15 | 12 | 18.35 | 13.41 ± 0.74 | 13.54 ± 0.93 |
| Isoleucine (Ile) | 12.57 ± 0.12 | 13 | 17 | 18 | 25.70 | 19.59 ± 1.04 | 21.81 ± 2.35 |
| Leucine (Leu) | 33.38 ± 0.02 | 31 | 57 | 30 | 81.67 | 40.33 ± 3.93 | 29.95 ± 2.04 |
| Tyrosine (Tyr) | 1.86 ± 0.07 | 5 | 5 | 3 | 8.04 | 7.37 ± 1.44 | 7.19 ± 0.90 |
| Phenylalanine (Phe) | 17.66 ± 0.18 | 15 | 24 | 10 | 28.06 | 16.20 ± 0.55 | 14.97 ± 0.51 |
| Lysine (Lys) | 25.59 ± 0.05 | 15 | 17 | 13 | 25.40 | 22.33 ± 0.33 | 29.37 ± 0.77 |
| Histidine (His) | 5.57 ± 0.09 | 4 | 12 | 6 | − 2 | 5.52 ± 0.81 | 9.38 ± 2.28 |
| Arginine (Arg) | 55.22 ± 0.08 | 53 | 77 | 56 | 43.12 | 40.94 ± 0.65 | 49.87 ± 1.06 |
| Proline (Pro) | 144.16 ± 0.11 | 94 | 125 | 96 | 98.83 | 124.29 ± 1.23 | 49.25 ± 1.28 |
| Hydroxyproline (Hyp) | 74.92 ± 0.09 | 118 | − 2 | 113 | 58.03 | 51.11 ± 3.99 | 106.78 ± 0.79 |
| Total | 1000 | ||||||
| Imino acid | 219.08 | 212 | − 2 | 209 | 156.86 | 175.40 | 156.03 |
| Region | Wavenumber (cm−1) | Cause | References |
|---|---|---|---|
| Amide A | 3304 | N−H stretch, hydrogen bond | Doyle et al. [43] |
| Amide B | 2938 | CH2 asymmetrical stretch | Muyonga et al. [44] |
| Amide I | 1633 | C=O stretch | Jackson et al. [45] |
| Amide II | 1547 | N−H bend coupled with C−N stretch | Jackson et al. [45] |
| Amide III | 1238 | C−N stretch, N−H in-plane bend, CH2 wag | Jackson et al. [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Ge, B.; Wei, M.; Elango, J.; Wu, W. Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage. Mar. Drugs 2023, 21, 260. https://doi.org/10.3390/md21050260
Pan Z, Ge B, Wei M, Elango J, Wu W. Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage. Marine Drugs. 2023; 21(5):260. https://doi.org/10.3390/md21050260
Chicago/Turabian StylePan, Zhilin, Baolin Ge, Mingjun Wei, Jeevithan Elango, and Wenhui Wu. 2023. "Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage" Marine Drugs 21, no. 5: 260. https://doi.org/10.3390/md21050260
APA StylePan, Z., Ge, B., Wei, M., Elango, J., & Wu, W. (2023). Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage. Marine Drugs, 21(5), 260. https://doi.org/10.3390/md21050260









