Microbial Interactions between Marine Microalgae and Fungi: From Chemical Ecology to Biotechnological Possible Applications
Abstract
1. Introduction
2. Microalgae and Fungi Interactions
2.1. Microalgae–Fungi Saprophytic Relationship
2.2. Microalgae–Fungi Parasitic Relationship
2.3. Microalgae–Fungi Microbial Competition
3. Possible Biotechnological Applications
3.1. Compounds for Human Health Applications
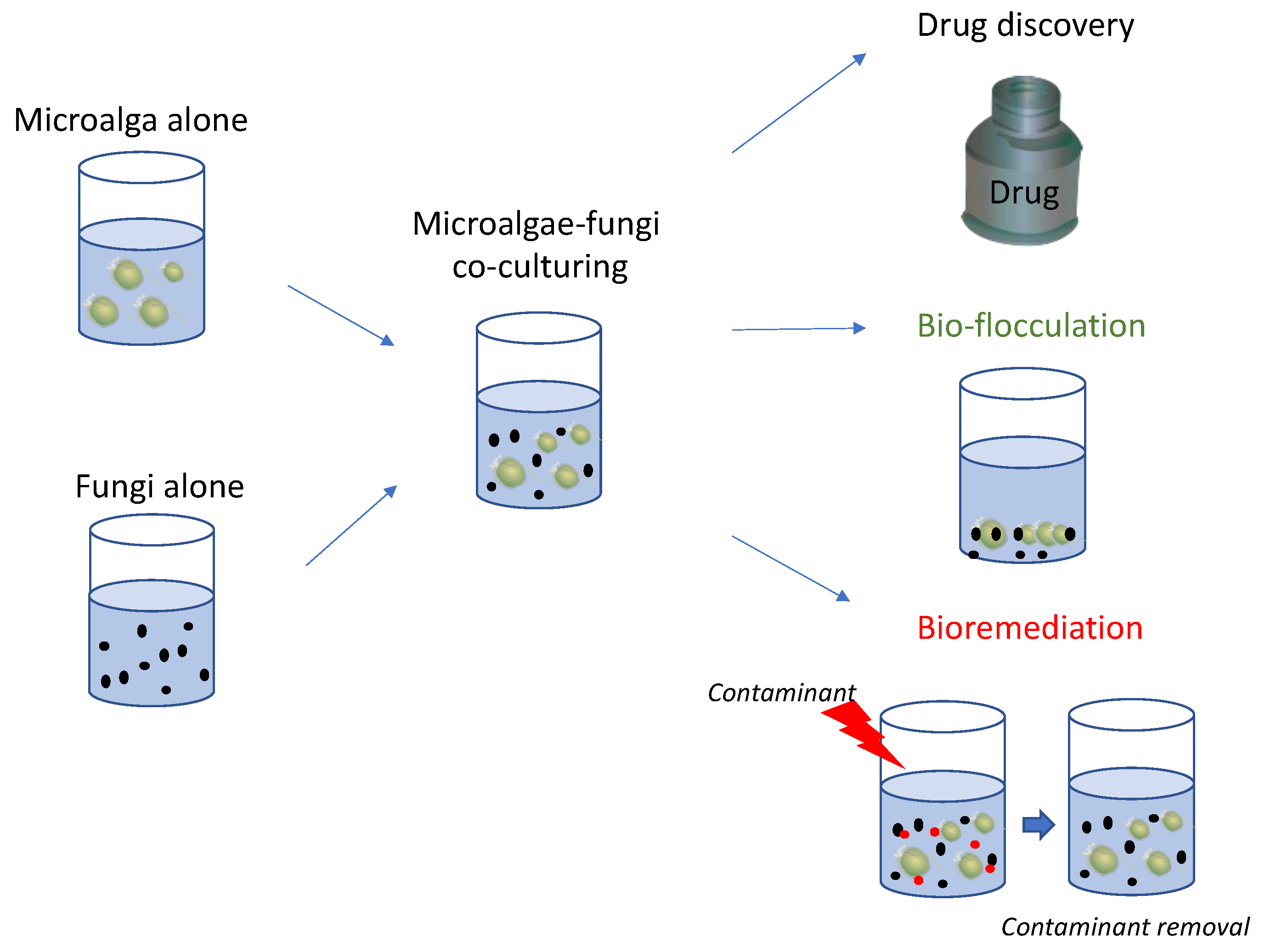
3.2. Bio-Flocculation
3.3. Fungi–Microalgae Consortia for Wastewater Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pohnert, G. Chemical Defense Strategies of Marine Organisms. Top. Curr. Chem. 2004, 239, 179–219. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G.; Steinke, M.; Tollrian, R. Chemical Cues, Defence Metabolites and the Shaping of Pelagic Interspecific Interactions. Trends Ecol. Evol. 2007, 22, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.E. Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engström-Öst, J.; Halsband, C.; Sonnenschein, E.; Legrand, C.; Llewellyn, C.A.; et al. The Relevance of Marine Chemical Ecology to Plankton and Ecosystem Function: An Emerging Field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Verdes, A.; Anand, P.; Gorson, J.; Jannetti, S.; Kelly, P.; Leffler, A.; Simpson, D.; Ramrattan, G.; Holford, M. From Mollusks to Medicine: A Venomics Approach for the Discovery and Characterization of Therapeutics from Terebridae Peptide Toxins. Toxins 2016, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.; Berdalet, E.; Burford, M.; Pitcher, G.; Zhou, M. Harmful Algal Blooms and the Importance of Understanding Their Ecology and Oceanography. In Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Berlin/Heidelberg, Germany, 2018; pp. 9–25. ISBN 978-3-319-70068-7. [Google Scholar]
- Lauritano, C.; Ianora, A. Chemical Defense in Marine Organisms. Mar. Drugs 2020, 18, 518. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde Suppression of Copepod Recruitment in Blooms of a Ubiquitous Planktonic Diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Zupo, V.; Lauritano, C.; Caramiello, D.; Ianora, A.; Budillon, A.; Romano, G.; Nuzzo, G.; D’Ippolito, G.; et al. Toxigenic Effects of Two Benthic Diatoms upon Grazing Activity of the Sea Urchin: Morphological, Metabolomic and de Novo Transcriptomic Analysis. Sci. Rep. 2018, 8, 5622. [Google Scholar] [CrossRef]
- Ribalet, F.; Berges, J.A.; Ianora, A.; Casotti, R. Growth Inhibition of Cultured Marine Phytoplankton by Toxic Algal-Derived Polyunsaturated Aldehydes. Aquat. Toxicol. Amst. Neth. 2007, 85, 219–227. [Google Scholar] [CrossRef]
- Ribalet, F.; Intertaglia, L.; Lebaron, P.; Casotti, R. Differential Effect of Three Polyunsaturated Aldehydes on Marine Bacterial Isolates. Aquat. Toxicol. Amst. Neth. 2008, 86, 249–255. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The Insidious Effect of Diatoms on Copepod Reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Orefice, I.; Di Dato, V.; Sardo, A.; Lauritano, C.; Romano, G. Lipid Mediators in Marine Diatoms. Aquat. Ecol. 2022, 56, 377–397. [Google Scholar] [CrossRef]
- Simon, C.A.; Bentley, M.G.; Caldwell, G.S. 2,4-Decadienal: Exploring a Novel Approach for the Control of Polychaete Pests on Cultured Abalone. Aquaculture 2010, 310, 52–60. [Google Scholar] [CrossRef]
- Long, J.D.; Smalley, G.W.; Barsby, T.; Anderson, J.T.; Hay, M.E. Chemical Cues Induce Consumer-Specific Defenses in a Bloom-Forming Marine Phytoplankton. Proc. Natl. Acad. Sci. USA 2007, 104, 10512–10517. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.B.; Smith, A.G. Exploring Mutualistic Interactions between Microalgae and Bacteria in the Omics Age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae–Bacteria Symbiosis in Microalgal Growth and Biofuel Production: A Review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Falkowski, P.G. The Role of Phytoplankton Photosynthesis in Global Biogeochemical Cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the Recent Structural Advances in Open and Closed Systems for Carbon Capture through Algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Wang, Y.; Barth, D.; Tamminen, A.; Wiebe, M.G. Growth of Marine Fungi on Polymeric Substrates. BMC Biotechnol. 2016, 16, 3. [Google Scholar] [CrossRef]
- Cunliffe, M.; Hollingsworth, A.; Bain, C.; Sharma, V.; Taylor, J.D. Algal Polysaccharide Utilisation by Saprotrophic Planktonic Marine Fungi. Fungal Ecol. 2017, 30, 135–138. [Google Scholar] [CrossRef]
- Berry, O.; Briand, E.; Bagot, A.; Chaigné, M.; Meslet-Cladière, L.; Wang, J.; Grovel, O.; Jansen, J.J.; Ruiz, N.; du Pont, T.R.; et al. Deciphering Interactions between the Marine Dinoflagellate Prorocentrum Lima and the Fungus Aspergillus Pseudoglaucus. Environ. Microbiol. 2023, 25, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Küpper, F.C.; Vyverman, W.; Ólafsson, H.G.; Karsten, U. Chytridiomycosis of Marine Diatoms—The Role of Stress Physiology and Resistance in Parasite-Host Recognition and Accumulation of Defense Molecules. Mar. Drugs 2017, 15, 26. [Google Scholar] [CrossRef]
- Hayashi, A.; Crombie, A.; Lacey, E.; Richardson, A.J.; Vuong, D.; Piggott, A.M.; Hallegraeff, G. Aspergillus Sydowii Marine Fungal Bloom in Australian Coastal Waters, Its Metabolites and Potential Impact on Symbiodinium Dinoflagellates. Mar. Drugs 2016, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Vallet, M.; Baumeister, T.U.H.; Kaftan, F.; Grabe, V.; Buaya, A.; Thines, M.; Svatoš, A.; Pohnert, G. The Oomycete Lagenisma Coscinodisci Hijacks Host Alkaloid Synthesis during Infection of a Marine Diatom. Nat. Commun. 2019, 10, 4938. [Google Scholar] [CrossRef] [PubMed]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Ocean Plankton. Eukaryotic Plankton Diversity in the Sunlit Ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Satake, M.; Yasumoto, T. Antimicrobial Activities of Polyether Compounds of Dinoflagellate Origins. J. Appl. Phycol. 1990, 2, 305–308. [Google Scholar] [CrossRef]
- Kagami, M.; Miki, T.; Takimoto, G. Mycoloop: Chytrids in Aquatic Food Webs. Front. Microbiol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Gleason, F.; Kagami, M.; Lefevre, E.; Sime-Ngando, T. The Ecology of Chytrids in Aquatic Ecosystems: Roles in Food Web Dynamics. Fungal Biol. Rev. 2008, 22, 17–25. [Google Scholar] [CrossRef]
- Laundon, D.; Chrismas, N.; Bird, K.; Thomas, S.; Mock, T.; Cunliffe, M. A Cellular and Molecular Atlas Reveals the Basis of Chytrid Development. eLife 2022, 11, e73933. [Google Scholar] [CrossRef]
- Kamoun, S. Molecular Genetics of Pathogenic Oomycetes. Eukaryot. Cell 2003, 2, 191–199. [Google Scholar] [CrossRef]
- Wetsteyn, L.P.M.J.; Peperzak, L. Field Observations in the Oosterschelde (The Netherlands) OnCoscinodiscus Concinnus AndCoscinodiscus Granii (Bacillariophyceae) Infected by the Marine FungusLagenisma Coscinodisci (Oomycetes). Hydrobiol. Bull. 1991, 25, 15–21. [Google Scholar] [CrossRef]
- Buaya, A.; Kraberg, A.; Thines, M. Dual Culture of the Oomycete Lagenisma Coscinodisci Drebes and Coscinodiscus Diatoms as a Model for Plankton/Parasite Interactions. Helgol. Mar. Res. 2019, 73, 2. [Google Scholar] [CrossRef]
- Robinson, S.M.; Bostock, R.M. β-Glucans and Eicosapolyenoic Acids as MAMPs in Plant–Oomycete Interactions: Past and Present. Front. Plant Sci. 2015, 5, 797. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.H.; Jara, A.M.; Pantoja, S. Fungal Parasites Infect Marine Diatoms in the Upwelling Ecosystem of the Humboldt Current System off Central Chile. Environ. Microbiol. 2016, 18, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Anabalón, V.; Morales, C.E.; Escribano, R.; Angélica Varas, M. The Contribution of Nano- and Micro-Planktonic Assemblages in the Surface Layer (0–30 m) under Different Hydrographic Conditions in the Upwelling Area off Concepción, Central Chile. Prog. Oceanogr. 2007, 75, 396–414. [Google Scholar] [CrossRef]
- Burge, C.A.; Kim, C.J.S.; Lyles, J.M.; Harvell, C.D. Special Issue Oceans and Humans Health: The Ecology of Marine Opportunists. Microb. Ecol. 2013, 65, 869–879. [Google Scholar] [CrossRef]
- Krespach, M.K.C.; García-Altares, M.; Flak, M.; Schoeler, H.; Scherlach, K.; Netzker, T.; Schmalzl, A.; Mattern, D.J.; Schroeckh, V.; Komor, A.; et al. Lichen-like Association of Chlamydomonas Reinhardtii and Aspergillus Nidulans Protects Algal Cells from Bacteria. ISME J. 2020, 14, 2794–2805. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Xie, J. Diatoms as Cell Factories for High-Value Products: Chrysolaminarin, Eicosapentaenoic Acid, and Fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical Structure and Biological Activity of a Highly Branched (1→3,1→6)-β-D-Glucan from Isochrysis Galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Ma, M.; Li, Y.; Chen, J.; Wang, F.; Yuan, L.; Li, Y.; Zhang, B.; Ye, D.; Han, D.; Jin, H.; et al. High-Cell-Density Cultivation of the Flagellate Alga Poterioochromonas Malhamensis for Biomanufacturing the Water-Soluble β-1,3-Glucan with Multiple Biological Activities. Bioresour. Technol. 2021, 337, 125447. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Shen, Y.; Jiang, D.; Wang, C.; Li, X.; Zhang, H.; Pan, Y.; Xie, C.; Xie, T.; Zhang, Y.; et al. Nutrient Deprivation Coupled with High Light Exposure for Bioactive Chrysolaminarin Production in the Marine Microalga Isochrysis Zhangjiangensis. Mar. Drugs 2022, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Vogler, B.W.; Brannum, J.; Chung, J.W.; Seger, M.; Posewitz, M.C. Characterization of the Nannochloropsis Gaditana Storage Carbohydrate: A 1,3-Beta Glucan with Limited 1,6-Branching. Algal Res. 2018, 36, 152–158. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary Characterization, Antioxidant Properties and Production of Chrysolaminarin from Marine Diatom Odontella Aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef]
- Carballo, C.; Chronopoulou, E.G.; Letsiou, S.; Maya, C.; Labrou, N.E.; Infante, C.; Power, D.M.; Manchado, M. Antioxidant Capacity and Immunomodulatory Effects of a Chrysolaminarin-Enriched Extract in Senegalese Sole. Fish Shellfish Immunol. 2018, 82, 1–8. [Google Scholar] [CrossRef]
- Yu, F.; Lu, S.; Yu, F.; Shi, J.; McGuire, P.M.; Wang, R. Cytotoxic Activity of an Octadecenoic Acid Extract from Euphorbia Kansui (Euphorbiaceae) on Human Tumour Cell Strains. J. Pharm. Pharmacol. 2008, 60, 253–259. [Google Scholar] [CrossRef]
- Seo, M.-J.; Shin, K.-C.; Oh, D.-K. Production of 5,8-Dihydroxy-9,12(Z,Z)-Octadecadienoic Acid from Linoleic Acid by Whole Recombinant Escherichia Coli Cells Expressing Diol Synthase from Aspergillus Nidulans. Appl. Microbiol. Biotechnol. 2014, 98, 7447–7456. [Google Scholar] [CrossRef]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological Significance of Toxic Marine Dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Liu, J.; Sidell, N. Anti-Estrogenic Effects of Conjugated Linoleic Acid through Modulation of Estrogen Receptor Phosphorylation. Breast Cancer Res. Treat. 2005, 94, 161–169. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Xu, H.; Zhang, X.; Li, X.-M. Olanzapine Attenuates the Okadaic Acid-Induced Spatial Memory Impairment and Hippocampal Cell Death in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2005, 30, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Identifier: 405238731, Chrysolaminarin. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/405238731#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 446512, Okadoic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/446512#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 6437058, Dinophysistoxin 1. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6437058#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 23872092, Sydowinin A. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/23872092#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 45359153, Sydowinin B. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/45359153#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 275388622, Sydowinol. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/275388622#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 14197386, Sydowic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14197386#section=2D-Structure (accessed on 29 March 2023).
- National Center for Biotechnology Information. PubChem Identifier: 64961, 9H-Pyrido[3,4-B]indole. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/64961#section=2D-Structure (accessed on 29 March 2023).
- Galasso, C.; Celentano, S.; Costantini, M.; D’Aniello, S.; Ianora, A.; Sansone, C.; Romano, G. Diatom-Derived Polyunsaturated Aldehydes Activate Similar Cell Death Genes in Two Different Systems: Sea Urchin Embryos and Human Cells. Int. J. Mol. Sci. 2020, 21, 5201. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-Derived Polyunsaturated Aldehydes Activate Cell Death in Human Cancer Cell Lines but Not Normal Cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.T.; Desbois, A.P. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus Cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef]
- Hayashi, A.; José Dorantes-Aranda, J.; Bowman, J.P.; Hallegraeff, G. Combined Cytotoxicity of the Phycotoxin Okadaic Acid and Mycotoxins on Intestinal and Neuroblastoma Human Cell Models. Toxins 2018, 10, 526. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic Polyketides from the Deep-Sea-Derived Fungus Engyodontium Album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Luo, Z.; Liao, Y.; Zhang, B.; Ke, W.; Shao, Z.; Li, F.; Chen, J. Secondary Metabolites Isolated from the Deep Sea-Derived Fungus Aspergillus Sydowii C1-S01-A7. Nat. Prod. Res. 2019, 33, 3077–3082. [Google Scholar] [CrossRef]
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive Marine Xanthones: A Review. Mar. Drugs 2022, 20, 58. [Google Scholar] [CrossRef]
- Song, Y.; Kesuma, D.; Wang, J.; Deng, Y.; Duan, J.; Wang, J.H.; Qi, R.Z. Specific Inhibition of Cyclin-Dependent Kinases and Cell Proliferation by Harmine. Biochem. Biophys. Res. Commun. 2004, 317, 128–132. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Chen, H.; Ma, Y.; Liu, X.; Hou, X.; Guan, H.; Xu, A. DNA Binding Properties of 9-Substituted Harmine Derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 1557–1563. [Google Scholar] [CrossRef]
- Kamal, A.; Sathish, M.; Nayak, V.L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N.; Nagesh, N. Design and Synthesis of Dithiocarbamate Linked β-Carboline Derivatives: DNA Topoisomerase II Inhibition with DNA Binding and Apoptosis Inducing Ability. Bioorg. Med. Chem. 2015, 23, 5511–5526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Park, E.-J.; Kondratyuk, T.P.; Pezzuto, J.M.; Sun, D. Synthesis and Structure-Activity Relationships of Tetrahydro-β-Carboline Derivatives as Anticancer and Cancer-Chemopreventive Agents. Anticancer Res. 2018, 38, 4425–4433. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Saha, S.T.; Perumal, S.; Gu, L.; Ebenezer, O.; Singh, P.; Kaur, M.; Kumar, V. Design, Synthesis, Antiproliferative Evaluation, and Molecular Docking Studies of N-(3-Hydroxyindole)-Appended β-Carbolines/Tetrahydro-β-Carbolines Targeting Triple-Negative and Non-Triple-Negative Breast Cancer. ACS Omega 2020, 5, 28907–28917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, F.; Liang, T.; Xie, Z.; Yang, Y.; Cao, C.; Gao, J.; Lu, T.; Chen, X. A Review of Synthetic Bioactive Tetrahydro-β-Carbolines: A Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2021, 225, 113815. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef]
- Moreiras-Figueruelo, A.; Nuzzo, G.; Galasso, C.; Sansone, C.; Crocetta, F.; Mazzella, V.; Gallo, C.; Barra, G.; Sardo, A.; Iuliano, A.; et al. Probing the Therapeutic Potential of Marine Phyla by SPE Extraction. Mar. Drugs 2021, 19, 640. [Google Scholar] [CrossRef]
- Brillante, S.; Galasso, C.; Lauritano, C.; Carrella, S. From the Sea for the Sight: Marine Derived Products for Human Vision. Front. Aging Neurosci. 2022, 14, 892764. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Riccio, G.; Ruocco, N.; Galasso, C.; Zupo, V.; Greco, S. Editorial: Cyanobacterial and Microalgal Compounds: Chemical Ecology and Biotechnological Potentials. Front. Mar. Sci. 2022, 9, 984160. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, S.; Das, D. Microalgal Bio-Flocculation: Present Scenario and Prospects for Commercialization. Environ. Sci. Pollut. Res. Int. 2021, 28, 26294–26312. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing Oil Production and Harvest by Combining the Marine Alga Nannochloropsis Oceanica and the Oleaginous Fungus Mortierella Elongata. Biotechnol. Biofuels 2018, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Hu, B. Mycoalgae Biofilm: Development of a Novel Platform Technology Using Algae and Fungal Cultures. Biotechnol. Biofuels 2016, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [PubMed]
- Gultom, S.O.; Zamalloa, C.; Hu, B. Microalgae Harvest through Fungal Pelletization—Co-Culture of Chlorella Vulgaris and Aspergillus Niger. Energies 2014, 7, 4417–4429. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-Culture of Fungi-Microalgae Consortium for Wastewater Treatment: A Review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef]
- Grimm, L.H.; Kelly, S.; Völkerding, I.I.; Krull, R.; Hempel, D.C. Influence of Mechanical Stress and Surface Interaction on the Aggregation of Aspergillus Niger Conidia. Biotechnol. Bioeng. 2005, 92, 879–888. [Google Scholar] [CrossRef]
- Arikan, E.; Işık, Z.; Bouras, H.D.; Dizge, N. Investigation of Immobilized Filamentous Fungi for Treatment of Real Textile Industry Wastewater Using up Flow Packed Bed Bioreactor. Bioresour. Technol. Rep. 2019, 7, 100197. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Wang, Q. A Novel One-Step Method for Oil-Rich Biomass Production and Harvesting by Co-Cultivating Microalgae with Filamentous Fungi in Molasses Wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Lauritano, C.; Martínez, K.A.; Battaglia, P.; Granata, A.; de la Cruz, M.; Cautain, B.; Martín, J.; Reyes, F.; Ianora, A.; Guglielmo, L. First Evidence of Anticancer and Antimicrobial Activity in Mediterranean Mesopelagic Species. Sci. Rep. 2020, 10, 4929. [Google Scholar] [CrossRef] [PubMed]
- Di Dato, V.; Orefice, I.; Amato, A.; Fontanarosa, C.; Amoresano, A.; Cutignano, A.; Ianora, A.; Romano, G. Animal-like Prostaglandins in Marine Microalgae. ISME J. 2017, 11, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First Identification of Marine Diatoms with Anti-Tuberculosis Activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Thor, P.; Toth, G.; Pavia, H. Copepods Induce Paralytic Shellfish Toxin Production in Marine Dinoflagellates. Proc. R. Soc. B Biol. Sci. 2006, 273, 1673–1680. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Gunasegavan, R.D.-N.; Mustar, S.; Lee, J.C.; Mohd Noh, M.F. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.-S. Microalgae-Based Wastewater Treatment—Microalgae-Bacteria Consortia, Multi-Omics Approaches and Algal Stress Response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-Bacteria Consortium for Wastewater Treatment and Biomass Production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
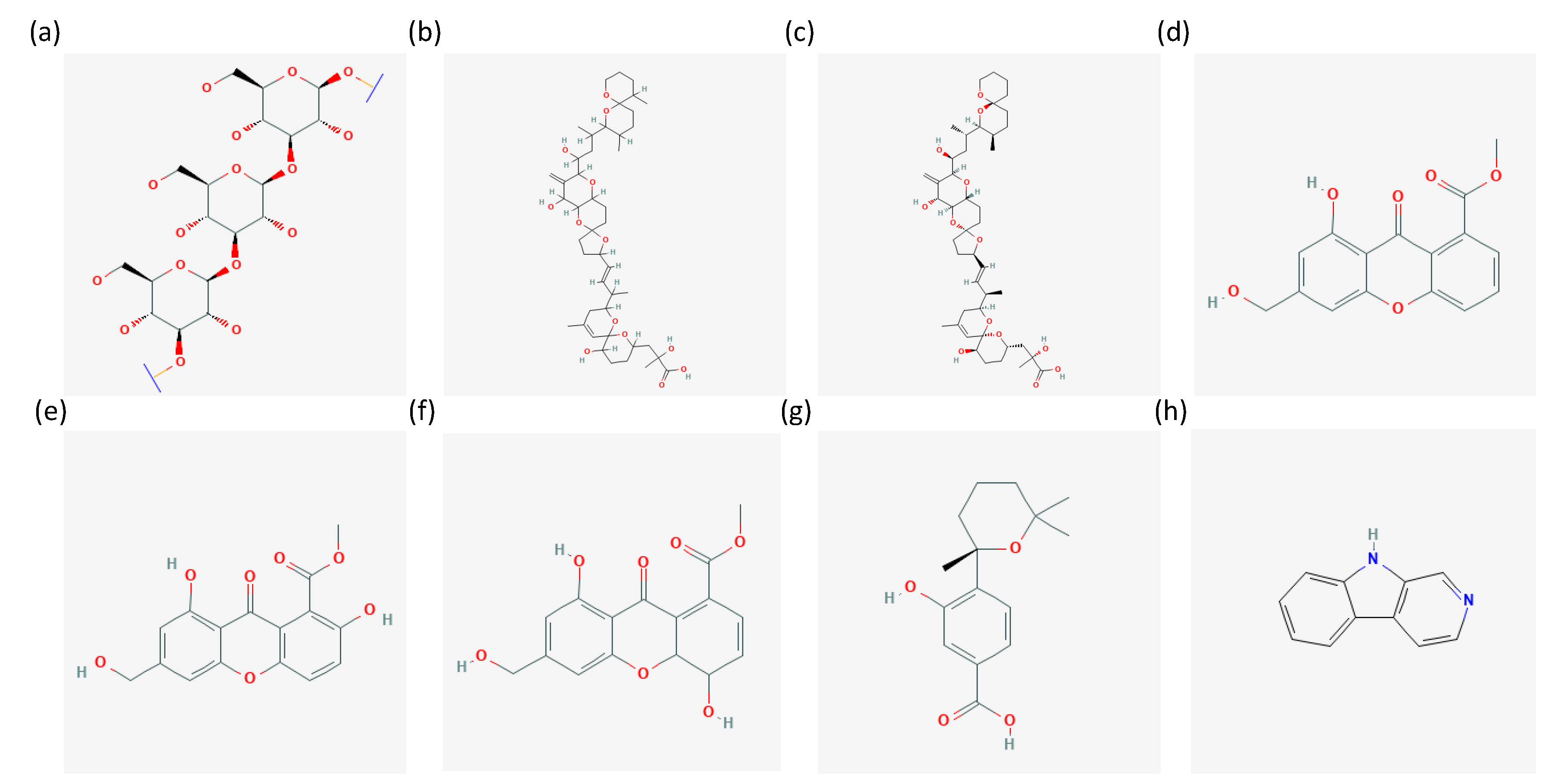
| Compound | Producer | Interaction with | Reference |
|---|---|---|---|
| Chrysolaminarin | Diatom Phaeodactylum tricornutum | Fungus Cladosporium spp. | [22] |
| Okadaic acid and Dinophysistoxin 1 | Dinoflagellate Prorocentrum lima PL4V | Fungus Aspergillus pseudoglaucus MMS1589 | [23] |
| 5S,8R-Dihydroxy-9Z,12Z-octadecadienoic acid | Fungus Aspergillus pseudoglaucus MMS1589 | Dinoflagellate Prorocentrum lima PL4V | [23] |
| Aldehydes and PUFAs | Diatoms | Chytrids | [24] |
| Sydowinin A and B, sydowinol and sydowic acid | Fungus Aspergillus sydowii | Dinoflagellates Symbiodinium spp. | [25] |
| 4-carboxy-2,3,4,9-tetrahydro-1H-β-carboline (4-CTC) and β-carboline | Diatom Coscinodiscus granii | Fungus Langenisma coscinosdisci | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauritano, C.; Galasso, C. Microbial Interactions between Marine Microalgae and Fungi: From Chemical Ecology to Biotechnological Possible Applications. Mar. Drugs 2023, 21, 310. https://doi.org/10.3390/md21050310
Lauritano C, Galasso C. Microbial Interactions between Marine Microalgae and Fungi: From Chemical Ecology to Biotechnological Possible Applications. Marine Drugs. 2023; 21(5):310. https://doi.org/10.3390/md21050310
Chicago/Turabian StyleLauritano, Chiara, and Christian Galasso. 2023. "Microbial Interactions between Marine Microalgae and Fungi: From Chemical Ecology to Biotechnological Possible Applications" Marine Drugs 21, no. 5: 310. https://doi.org/10.3390/md21050310
APA StyleLauritano, C., & Galasso, C. (2023). Microbial Interactions between Marine Microalgae and Fungi: From Chemical Ecology to Biotechnological Possible Applications. Marine Drugs, 21(5), 310. https://doi.org/10.3390/md21050310







