New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas
Abstract
:1. Introduction
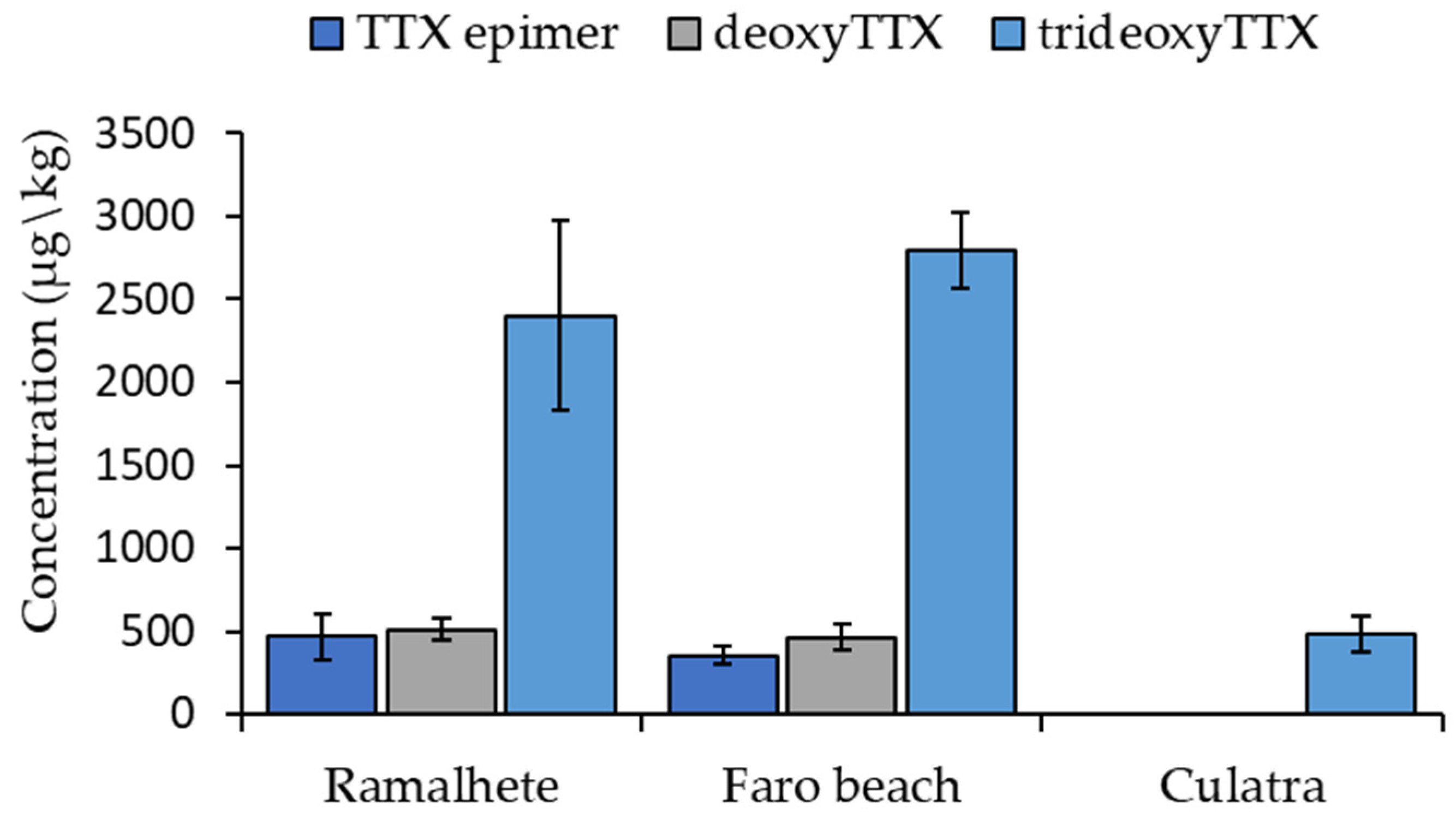
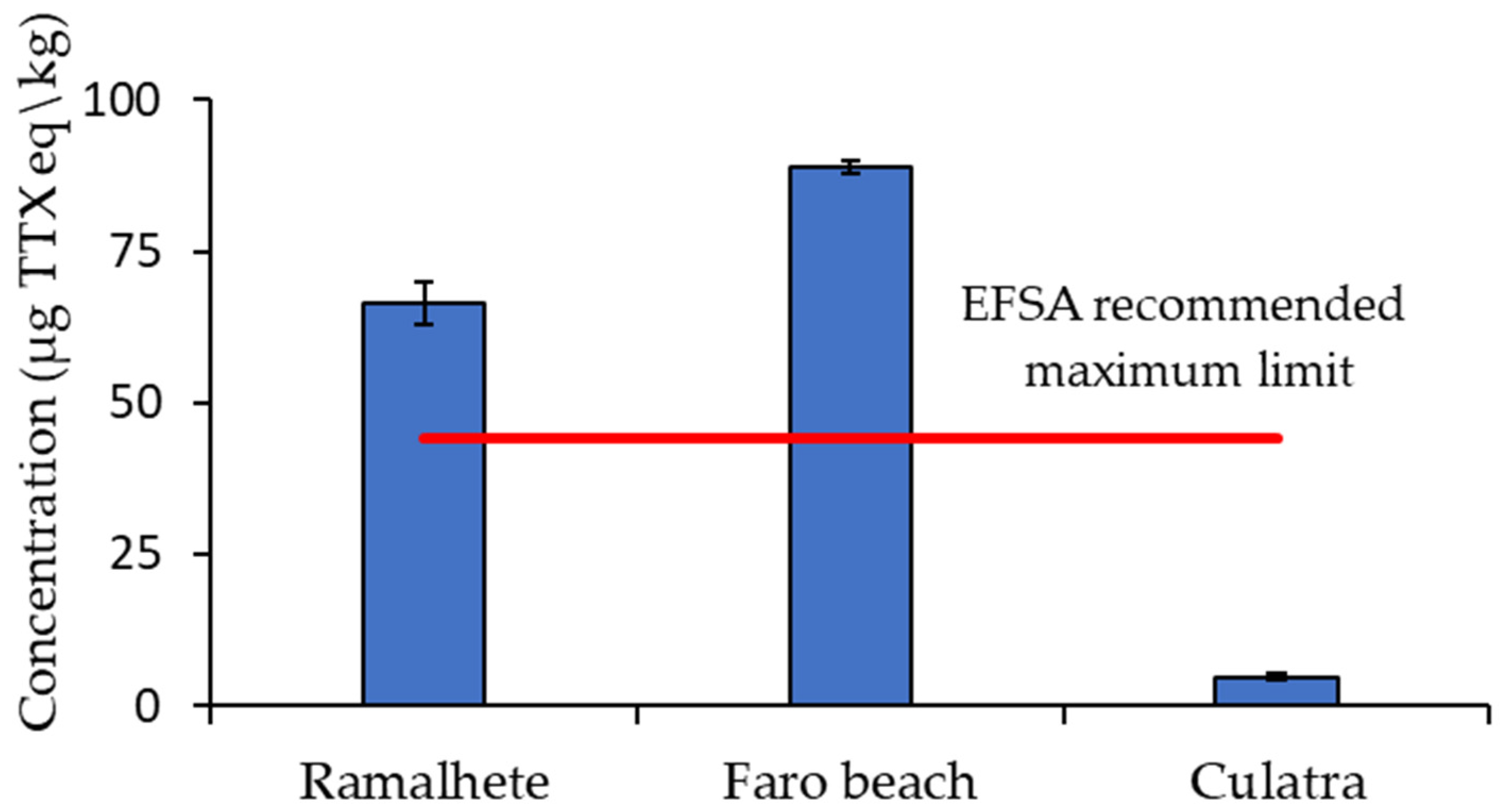
2. Results
3. Discussion
4. Materials and Methods
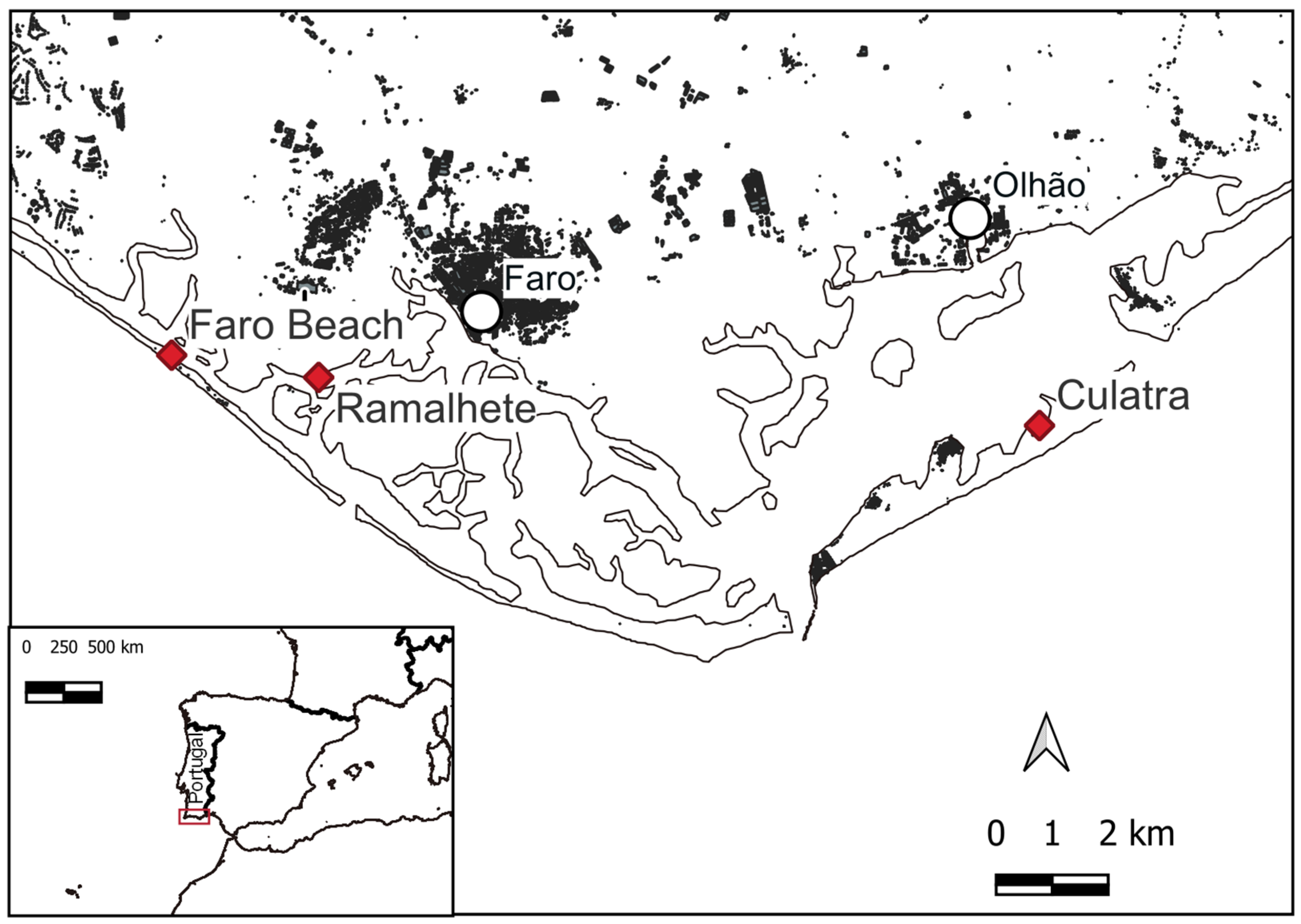
4.1. Sampling
4.2. TTX and Analogues Extraction and Analysis
4.2.1. Materials
4.2.2. Sample Extraction
4.2.3. LC–HRMS Conditions
4.2.4. Quantitation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.J.E.J. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar] [PubMed]
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. Int. Sch. Res. Not. 2011, 2011, 276939. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O. Tetrodotoxin–distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Ho, P.-H.; Hwang, C.-C.; Hwang, P.-A.; Cheng, C.-A.; Hwang, D.-F. Tetrodotoxin in several species of xanthid crabs in southern Taiwan. Food Chem. 2006, 95, 205–212. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5, 6, 11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Arakawa, O.; Noguchi, T.; Shida, Y.; Onoue, Y. Occurrence of 11-Oxotetrodotoxin and 11-Nortetrodotoxin-6 (R)-ol in a Xanthid Crab Atergatis loridus Collected at Kojima, Ishigaki Island. Fish. Sci. 1994, 60, 769–771. [Google Scholar] [CrossRef]
- Kanchanapongkul, J.; Krittayapoositpot, P. An epidemic of tetrodotoxin poisoning following ingestion of the horseshoe crab Carcinoscorpius rotundicauda. Southeast Asian J. Trop. Med. Public Health 1995, 26, 364–367. [Google Scholar]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. 2006, 1, 145–152. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Yotsu-Yamashita, M.; Yamamori, K. Accumulation of tetrodotoxin and 4, 9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon 2008, 51, 1269–1273. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Numano, S.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Temporal variation of the profile and concentrations of paralytic shellfish toxins and tetrodotoxin in the scallop, Patinopecten yessoensis, cultured in a bay of East Japan. Mar. Drugs 2019, 17, 653. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef]
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins occurrence in non-traditional vectors of the north atlantic waters (Portuguese maritime territory, and morocco coast). Toxins 2019, 11, 306. [Google Scholar] [CrossRef]
- Turner, A.; Powell, A.; Schofield, A.; Lees, D.; Baker-Austin, C.J.E. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Euro Surveill. 2015, 20, 21009. [Google Scholar] [CrossRef]
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.H.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- European Commission. Regulation of the European Parliament and of the Council laying down specific hygiene rules for on the hygiene of foodstuffs, 853/2004/EC. Off. J. Eur. Union L 2004, 139, 152. [Google Scholar]
- European Commission. Regulation of the European Parliament and of the Council (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Union L 2019, 131, 51–100. [Google Scholar]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [PubMed]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Accoroni, S.; Romagnoli, T.; Campanelli, A.; Griffoni, F.; Tavoloni, T.; Gorbi, S.; et al. A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Mar. Drugs 2023, 21, 8. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Pinto, E.P.; Oliveira, P.; Pedro, S.; Costa, P.R. Evaluation of the Occurrence of Tetrodotoxin in Bivalve Mollusks from the Portuguese Coast. J. Mar. Sci. Eng. 2019, 7, 232. [Google Scholar] [CrossRef]
- Leão, J.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A.J.M.D. Preliminary results on the evaluation of the occurrence of tetrodotoxin associated to marine Vibrio spp. in bivalves from the Galician rias (northwest of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First detection of tetrodotoxin in bivalves and gastropods from the French mainland coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- Costa, P.R.; Giráldez, J.; Rodrigues, S.M.; Leão, J.M.; Pinto, E.; Soliño, L.; Gago-Martínez, A. High levels of tetrodotoxin (TTX) in trumpet shell Charonia lampas from the Portuguese coast. Toxins 2021, 13, 250. [Google Scholar] [CrossRef]
- Lage, S.; Afonso, I.I.; Reis Costa, P.; Canário, A.V.M.; Da Silva, J.P. Tissue accumulation of tetrodotoxin (TTX) and analogues in trumpet shell Charonia lampas. Food Addit. Contam. Part A 2023, 40, 159–168. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; González-Jartín, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Yamagishi, Y.; Yasumoto, T. 5, 6, 11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett. 1995, 36, 9329–9332. [Google Scholar] [CrossRef]
- Satake, Y.; Adachi, M.; Tokoro, S.; Yotsu-Yamashita, M.; Isobe, M.; Nishikawa, T. Synthesis of 5-and 8-deoxytetrodotoxin. Chem. Asian J. 2014, 9, 1922–1932. [Google Scholar] [CrossRef]
- FAO Fisheries and Aquaculture—Global Capture Production Quantity (1950–2020). Available online: https://www.fao.org/fishery/statistics-query/en/capture/capture_quantity (accessed on 16 February 2023).
- Salvitti, L.R.; Wood, S.A.; McNabb, P.; Cary, S.C. No evidence for a culturable bacterial tetrodotoxin producer in Pleurobranchaea maculata (Gastropoda: Pleurobranchidae) and Stylochoplana sp. (Platyhelminthes: Polycladida). Toxins 2015, 7, 255–273. [Google Scholar] [CrossRef]
- Salvitti, L.; Wood, S.A.; Fairweather, R.; Culliford, D.; McNabb, P.; Cary, S.C. In situ accumulation of tetrodotoxin in non-toxic Pleurobranchaea maculata (Opisthobranchia). Aquat. Sci. 2017, 79, 335–344. [Google Scholar] [CrossRef]
- Wolfrath, B. Field experiments on feeding of European fiddler crab Uca tangeri. Mar. Ecol. Prog. Ser. 1992, 90, 39–43. [Google Scholar] [CrossRef]
- Miller, D.C. The feeding mechanism of fiddler crabs, with ecological considerations of feeding adaptations. Zoologica 1961, 46, 80–101. [Google Scholar] [CrossRef]
- Gaudêncio, M.; Guerra, M.; Almeida, P.; Moreira, F.; Costa, J.; Assis, C. Estrutura e funcionamento das comunidades animais do estuário do Tejo (Bentónicas, Ictiológicas e Ornitológicas), suas relações com alterações antropogénicas. Proj. Povoamentos Macrozoobentónicos 1991, 87294, 1–69. (In Portuguese) [Google Scholar]
- Le Calvez, J.C. Location of the shore crab Carcinus maenas (Linnaeus, 1758), in the food web of a managed estuary ecosystem: The Rance Basin (Brittany, France). Inv. Pesq. Barcelona 1987, 51, 431–442. [Google Scholar]
- Pardal, M.; Baeta, A.; Marques, J.; Cabral, H. Feeding ecology of the green crab, Carcinus maenas (Linnaeus, 1758) in a temperate estuary, Portugal. Crustaceana 2006, 79, 1181–1193. [Google Scholar]
- Hwang, D.F.; Tsai, Y.H. Toxins in toxic Taiwanese crabs. Food Rev. Int. 1999, 15, 145–162. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeon, J.-K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [PubMed]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar]
- Noguchi, T.; Miyazawa, K.; Daigo, K.; Arakawa, O. Paralytic shellfish poisoning (PSP) toxin- and/or tetrodotoxin-contaminated crabs and food poisoning by them. Toxin Rev. 2011, 30, 91–102. [Google Scholar] [CrossRef]
- Do, H.; Kogure, K.; Imada, C.; Noguchi, T.; Ohwada, K.; Simidu, U. Tetrodotoxin production of actinomycetes isolated from marine sediment. J. Appl. Bacteriol. 1991, 70, 464–468. [Google Scholar] [CrossRef]
- Katikou, P. Public health risks associated with tetrodotoxin and its analogues in european waters: Recent advances after the EFSA scientific opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Mariño, C.; Martín, H.; Blanco, J. First Report of Two Gymnodimines and Two Tetrodotoxin Analogues in Invertebrates from the North Atlantic Coast of Spain. Mar. Drugs 2023, 21, 232. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, J.-S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef]
- Nzoughet, J.K.; Campbell, K.; Barnes, P.; Cooper, K.M.; Chevallier, O.P.; Elliott, C.T. Comparison of sample preparation methods, validation of an UPLC–MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013, 136, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T.; et al. First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and Structural Determination of the First 8-epi-type Tetrodotoxin Analogs from the Newt, Cynops ensicauda popei, and Comparison of Tetrodotoxin Analogs Profiles of This Newt and the Puffer Fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef]
- Ueyama, N.; Sugimoto, K.; Kudo, Y.; Onodera, K.I.; Cho, Y.; Konoki, K.; Nishikawa, T.; Yotsu-Yamashita, M. Spiro bicyclic guanidino compounds from pufferfish: Possible biosynthetic intermediates of tetrodotoxin in marine environments. Chemi. Eu. J. 2018, 24, 7250–7258. [Google Scholar] [CrossRef]
- EURLMB TTX Standard Operating Procedure. Determination of Tetrodotoxin by HILIC-MS/MS; European Union Reference Laboratory for Marine Biotoxins: Vigo, Spain, 2017. [Google Scholar]

| Structure | Analogue | R1 | R2 | R3 | R4 | Molecular Formula | [M+H]+ Exact Mass |
|---|---|---|---|---|---|---|---|
 | TTX | H | OH | OH | CH2OH | C11H17N3O8 | 320.10884 |
| 4-epiTTX | OH | H | OH | CH2OH | C11H17N3O8 | 320.10884 | |
| 11-deoxyTTX | H | OH | OH | CH3 | C11H17N3O7 | 304.11393 | |
| 11-norTTX-6(R/S)-ol | H | OH | H | OH | C10H15N3O7 | 290.09828 | |
| 6,11-dideoxyTTX | H | OH | H | CH3 | C11H17N3O6 | 288.11901 | |
| 11-oxoTTX | H | OH | OH | CHO | C11H17N3O9 | 336.10376 | |
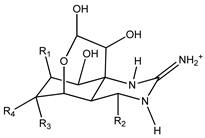 | 5-deoxyTTX | H | OH | OH | CH2OH | C11H17N3O7 | 304.11393 |
| 5,11-dideoxyTTX | H | OH | H | CH3 | C11H17N3O6 | 288.11901 | |
| 5,6,11-trideoxyTTX | H | OH | H | CH3 | C11H17N3O5 | 272.12410 | |
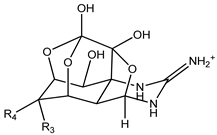 | 4,9-anhydroTTX | ─ | ─ | OH | CH2OH | C11H15N3O7 | 302.09828 |
| 6-epi-4,9-anhydroTTX | ─ | ─ | CH2OH | OH | C11H15N3O7 | 302.09828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage, S.; ten Brink, F.; Canário, A.V.M.; Da Silva, J.P. New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas. Mar. Drugs 2023, 21, 320. https://doi.org/10.3390/md21060320
Lage S, ten Brink F, Canário AVM, Da Silva JP. New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas. Marine Drugs. 2023; 21(6):320. https://doi.org/10.3390/md21060320
Chicago/Turabian StyleLage, Sandra, Felicitas ten Brink, Adelino V. M. Canário, and José P. Da Silva. 2023. "New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas" Marine Drugs 21, no. 6: 320. https://doi.org/10.3390/md21060320
APA StyleLage, S., ten Brink, F., Canário, A. V. M., & Da Silva, J. P. (2023). New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas. Marine Drugs, 21(6), 320. https://doi.org/10.3390/md21060320







