Chrysomycin A Regulates Proliferation and Apoptosis of Neuroglioma Cells via the Akt/GSK-3β Signaling Pathway In Vivo and In Vitro
Abstract
:1. Introduction
2. Results
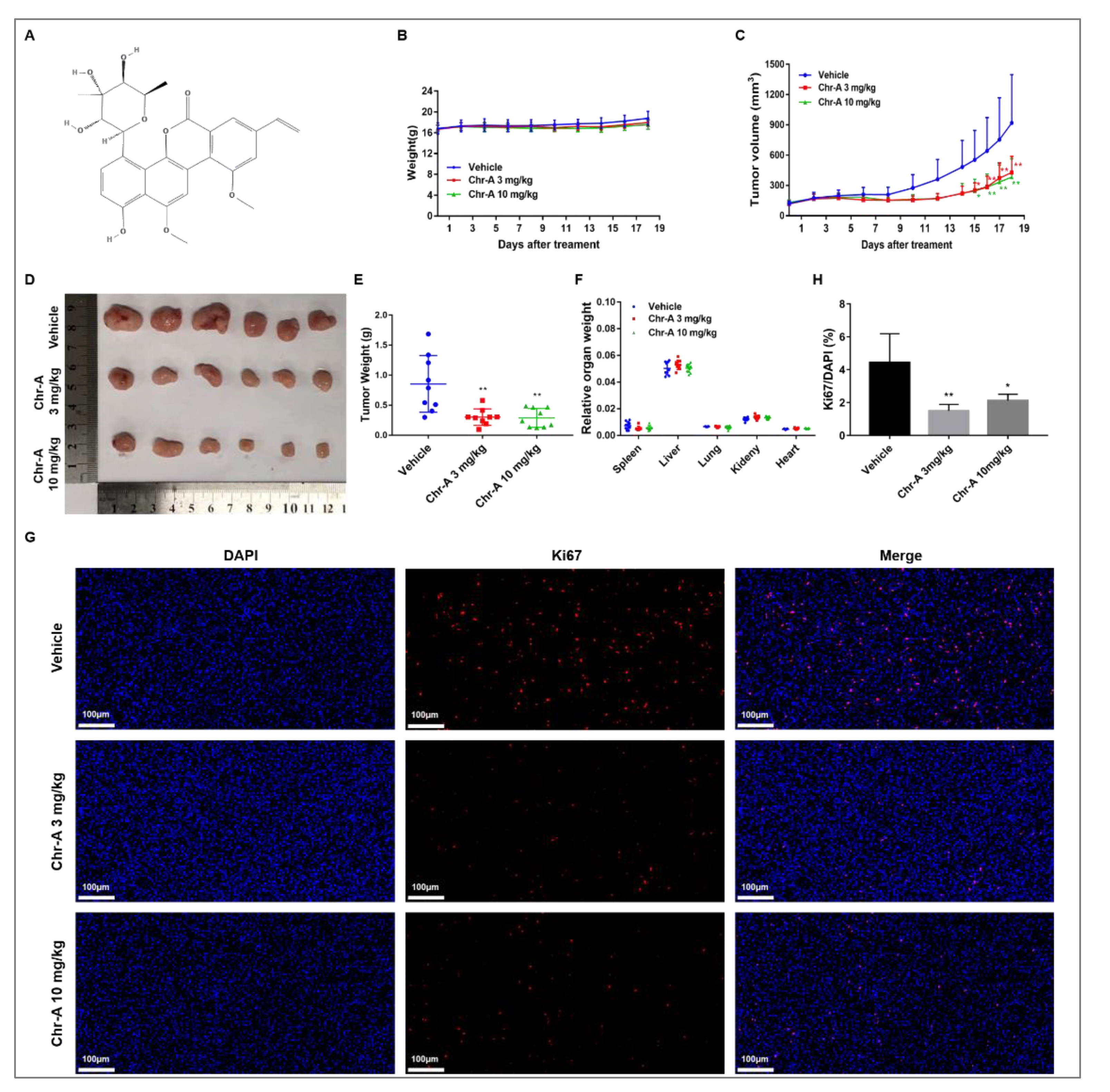
2.1. Chr-A Suppressed the Tumorigenicity in Human Glioma U87 Xenografted Hairless Mice
2.2. Chr-A Regulates Akt/GSK-3β Signaling Pathway of Glioblastoma Cells in Human Glioma U87 Xenografted Hairless Mice
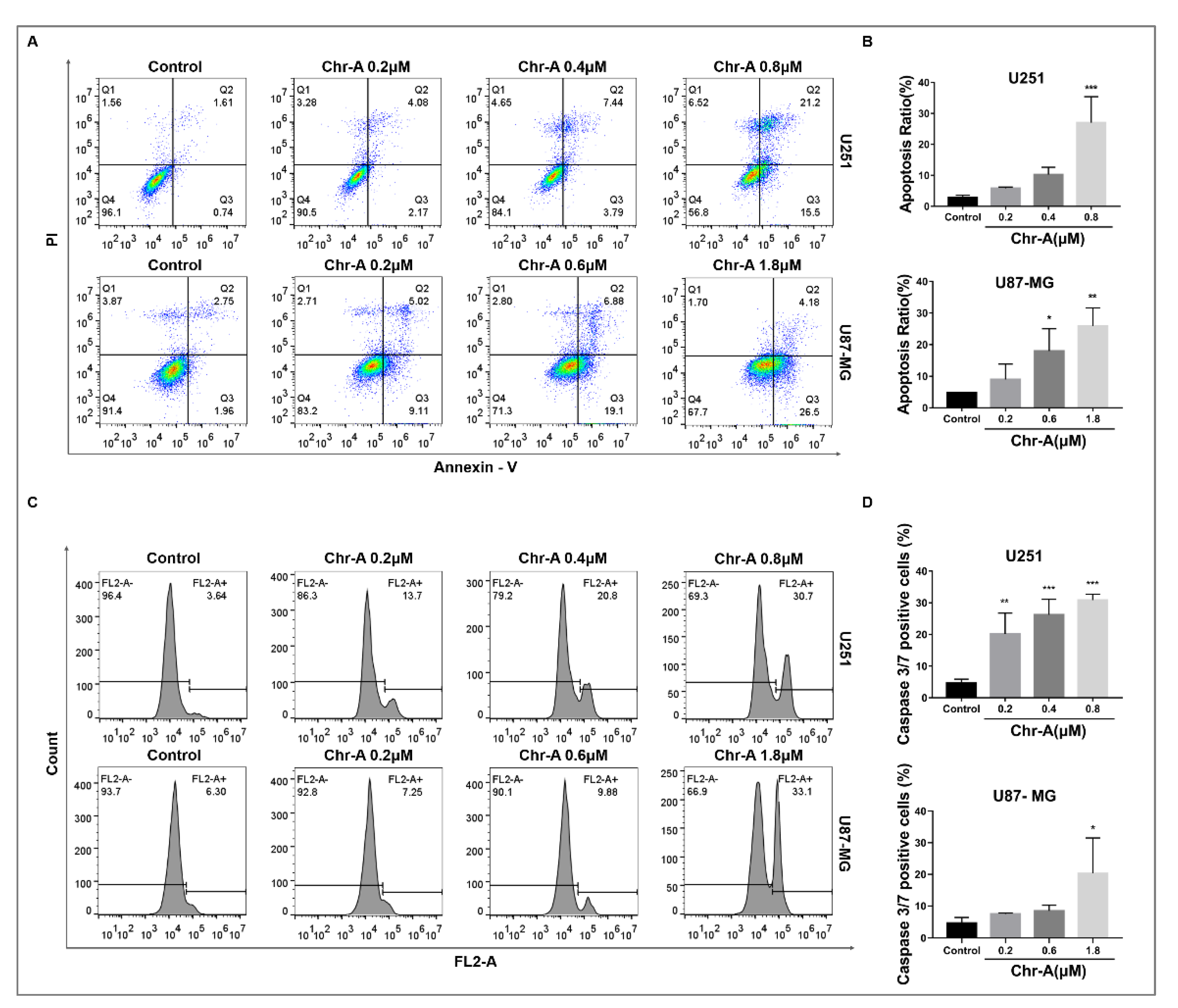
2.3. Chr-A Induces Apoptosis of U251 and U87-MG Cells
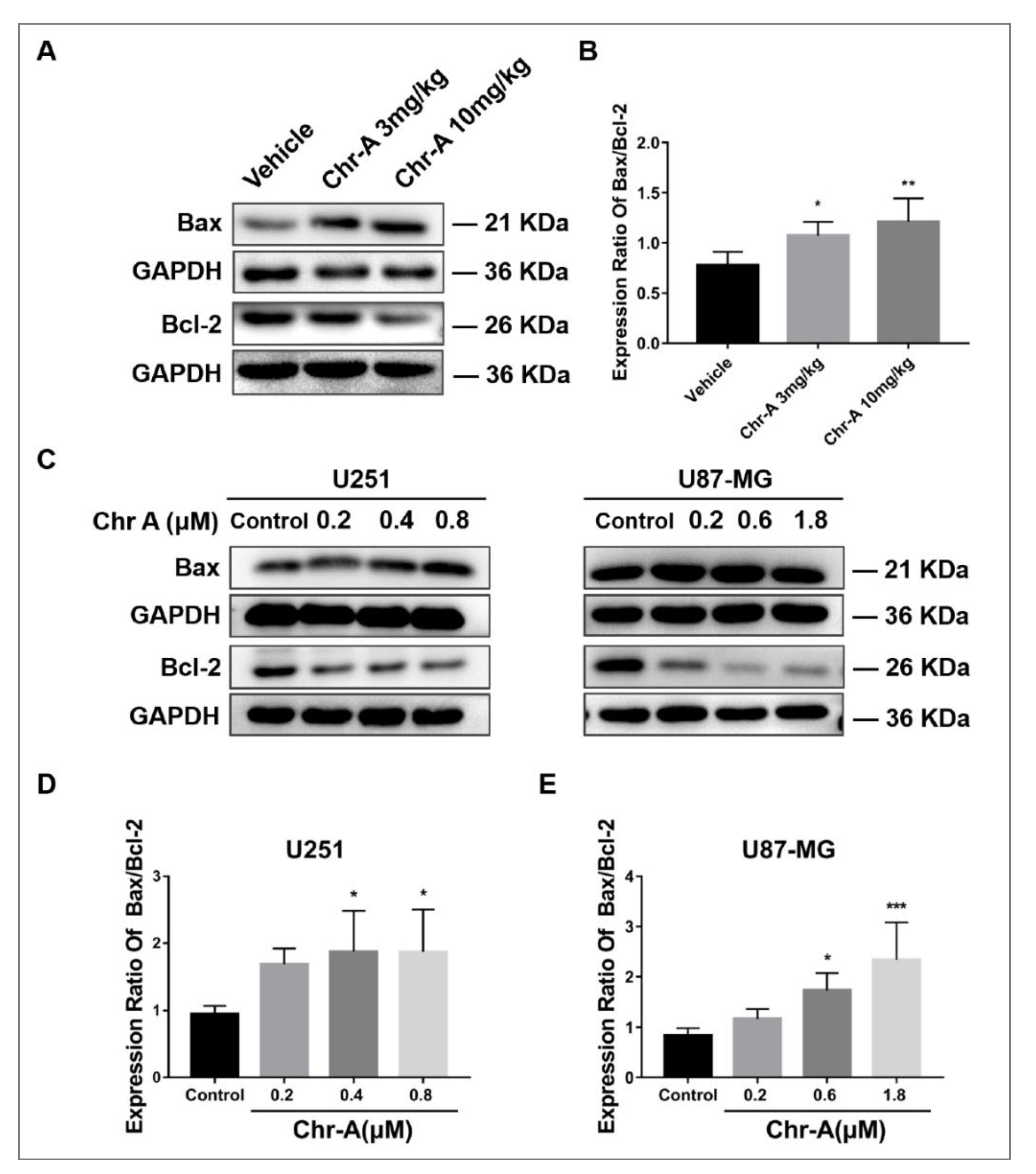
2.4. Chr-A Increases Ratio of Bax to Bcl-2 of Glioblastoma Cells In Vivo and In Vitro
2.5. Chr-A Activates a Caspase Cascade Reaction of Glioblastoma Cells In Vivo and In Vitro
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Antitumor Activity in Xenografted Tumor Mouse Models
4.3. Ki67 Staining via Immunofluorescence
4.4. Chr-A-Related Target Identification via RNA-Sequencing
4.5. Glioblastoma-Related Target Collection and Analysis
4.6. Glioblastoma Cell Culture and Treatment
4.7. Apoptosis Assay
4.8. Caspase 3/7 Activity Detection
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar]
- Kyriakou, I.; Yarandi, N.; Polycarpou, E. Efficacy of cannabinoids against glioblastoma multiforme: A systematic review. Phytomedicine 2021, 88, 153533. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; He, X.; Yu, W.; Chen, Y.; Long, Y.; Wang, K.; Zhu, S.; Liu, Q. p53-targeted lncRNA ST7-AS1 acts as a tumour suppressor by interacting with PTBP1 to suppress the Wnt/beta-catenin signalling pathway in glioma. Cancer Lett. 2021, 503, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Balca-Silva, J.; Matias, D.; do Carmo, A.; Girao, H.; Moura-Neto, V.; Sarmento-Ribeiro, A.B.; Lopes, M.C. Tamoxifen in combination with temozolomide induce a synergistic inhibition of PKC-pan in GBM cell lines. Biochim. Biophys. Acta 2015, 1850, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24 (Suppl. 5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Huang, X.S.; Ishida, K.; Maier, A.; Kelter, G.; Jiang, Y.; Peschel, G.; Menzel, K.D.; Li, M.G.; Wen, M.L.; et al. Plasticity in gilvocarcin-type C-glycoside pathways: Discovery and antitumoral evaluation of polycarcin V from Streptomyces polyformus. Org. Biomol. Chem. 2008, 6, 3601–3605. [Google Scholar] [CrossRef]
- Wada, S.I.; Sawa, R.; Iwanami, F.; Nagayoshi, M.; Kubota, Y.; Iijima, K.; Hayashi, C.; Shibuya, Y.; Hatano, M.; Igarashi, M.; et al. Structures and biological activities of novel 4’-acetylated analogs of chrysomycins A and B. J. Antibiot. 2017, 70, 1078–1082. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S.; Gao, X.; Hong, Y.; Cai, Y.; Zhang, Q.; Xiang, J.; Xie, D.; Song, F.; Zhang, H.; et al. Mechanochemical preparation of chrysomycin A self-micelle solid dispersion with improved solubility and enhanced oral bioavailability. J. Nanobiotech. 2021, 19, 164. [Google Scholar] [CrossRef]
- Ni, H.J.; Lv, S.Y.; Sheng, Y.T.; Wang, H.; Chu, X.H.; Zhang, H.W. Optimization of fermentation conditions and medium compositions for the production of chrysomycin a by a marine-derived strain Streptomyces sp. 891. Prep. Biochem. Biotechnol. 2021, 51, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.T.; Byrne, K.M.; Warnick-Pickle, D.; Greenstein, M. Studies on the mechanism of actin of gilvocarcin V and chrysomycin A. J. Antibiot. 1982, 35, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Yoshihira, K.; Highet, R.J.; White, R.J.; Wei, T.T. The chemistry of the antibiotics chrysomycin A and B. Antitumor activity of chrysomycin A. J. Antibiot. 1982, 35, 1194–1201. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.S.; Liu, D.N.; Yang, Y.L.; Wang, Y.H.; Du, G.H. Chrysomycin A Attenuates Neuroinflammation by Down-Regulating NLRP3/Cleaved Caspase-1 Signaling Pathway in LPS-Stimulated Mice and BV2 Cells. Int. J. Mol. Sci. 2021, 22, 6799. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.N.; Liu, M.; Zhang, S.S.; Shang, Y.F.; Song, F.H.; Zhang, H.W.; Du, G.H.; Wang, Y.H. Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects. Molecules 2022, 27, 6148. [Google Scholar] [CrossRef]
- Jeyamohan, S.; Moorthy, R.K.; Kannan, M.K.; Arockiam, A.J. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol. Lett. 2016, 38, 1251–1260. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Brunetti, A.; Marinelli, O.; Morelli, M.B.; Iannarelli, R.; Amantini, C.; Russotti, D.; Santoni, G.; Maggi, F.; Nabissi, M. Isofuranodiene synergizes with temozolomide in inducing glioma cells death. Phytomedicine 2019, 52, 51–59. [Google Scholar] [CrossRef]
- Karachi, A.; Dastmalchi, F.; Mitchell, D.A.; Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018, 20, 1566–1572. [Google Scholar] [CrossRef]
- Muralikrishnan, B.; Dan, V.M.; Vinodh, J.S.; Jamsheena, V.; Ramachandran, R.; Thomas, S.; Dastager, S.G.; Kumar, K.S.; Lankalapalli, R.S.; Kumar, R.A. Anti-microbial activity of chrysomycin A produced by Streptomyces sp. against Mycobacterium tuberculosis. RSC Adv. 2017, 7, 36335–36339. [Google Scholar]
- Pawlowska, E.; Szczepanska, J.; Szatkowska, M.; Blasiak, J. An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective. Int. J. Mol. Sci. 2018, 19, 889. [Google Scholar] [CrossRef]
- Trejo-Solis, C.; Serrano-Garcia, N.; Escamilla-Ramirez, A.; Castillo-Rodriguez, R.A.; Jimenez-Farfan, D.; Palencia, G.; Calvillo, M.; Alvarez-Lemus, M.A.; Flores-Najera, A.; Cruz-Salgado, A.; et al. Autophagic and Apoptotic Pathways as Targets for Chemotherapy in Glioblastoma. Int. J. Mol. Sci. 2018, 19, 3773. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Park, J.H.; Kim, M.J.; Kim, W.J.; Ha, K.T.; Choi, B.T.; Lee, S.Y.; Shin, H.K. Isolinderalactone regulates the BCL-2/caspase-3/PARP pathway and suppresses tumor growth in a human glioblastoma multiforme xenograft mouse model. Cancer Lett. 2019, 443, 25–33. [Google Scholar] [CrossRef]
- Chu, G.; Zhou, X.; Hu, Y.; Shi, S.; Yang, G. Rev-erbalpha Inhibits Proliferation and Promotes Apoptosis of Preadipocytes through the Agonist GSK4112. Int. J. Mol. Sci. 2019, 20, 4524. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Deng, H.; Liu, Y.; Lei, X.; He, P.; Dong, W. Thymoquinone inhibits the proliferation and invasion of esophageal cancer cells by disrupting the AKT/GSK-3beta/Wnt signaling pathway via PTEN upregulation. Phytother. Res. 2020, 34, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Zhao, P.; Zhao, H.; Gao, W.; Wang, L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and Angiogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Xu, H.; Liu, J.; Ren, L.; Yang, Y.; Li, S.; Wang, J.; Ji, T.; Du, G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 2021, 11, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Tchokonte-Nana, V.; Lotfi, M.; Lotfi, M.; Ghorbani, A.; Sadeghnia, H.R. Wnt/beta-catenin and PI3K/Akt/mTOR Signaling Pathways in Glioblastoma: Two Main Targets for Drug Design: A Review. Curr. Pharm. Des. 2020, 26, 1729–1741. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Jin, R.; Xu, H.; Li, Y.; Chen, Y.; Zhao, G. Pyrvinium pamoate regulates MGMT expression through suppressing the Wnt/beta-catenin signaling pathway to enhance the glioblastoma sensitivity to temozolomide. Cell. Death Discov. 2021, 7, 288. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Lei, S.; Wang, W. SKA3 promotes glioblastoma proliferation and invasion by enhancing the activation of Wnt/beta-catenin signaling via modulation of the Akt/GSK-3beta axis. Brain Res. 2021, 1765, 147500. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/beta-catenin signaling via transactivation of GSK-3beta and Axin2 expression. Cell. Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, J.; Xu, P.; Deng, G.; Liu, B.; Yuan, F.; Tan, Y.; Sun, Q.; Xu, Y.; Zhang, H.; et al. AKT Inhibitor SC66 Inhibits Proliferation and Induces Apoptosis in Human Glioblastoma Through Down-Regulating AKT/beta-Catenin Pathway. Front. Pharmacol. 2020, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-N.; Liu, M.; Zhang, S.-S.; Shang, Y.-F.; Zhang, W.-F.; Song, F.-H.; Zhang, H.-W.; Du, G.-H.; Wang, Y.-H. Chrysomycin A Regulates Proliferation and Apoptosis of Neuroglioma Cells via the Akt/GSK-3β Signaling Pathway In Vivo and In Vitro. Mar. Drugs 2023, 21, 329. https://doi.org/10.3390/md21060329
Liu D-N, Liu M, Zhang S-S, Shang Y-F, Zhang W-F, Song F-H, Zhang H-W, Du G-H, Wang Y-H. Chrysomycin A Regulates Proliferation and Apoptosis of Neuroglioma Cells via the Akt/GSK-3β Signaling Pathway In Vivo and In Vitro. Marine Drugs. 2023; 21(6):329. https://doi.org/10.3390/md21060329
Chicago/Turabian StyleLiu, Dong-Ni, Man Liu, Shan-Shan Zhang, Yu-Fu Shang, Wen-Fang Zhang, Fu-Hang Song, Hua-Wei Zhang, Guan-Hua Du, and Yue-Hua Wang. 2023. "Chrysomycin A Regulates Proliferation and Apoptosis of Neuroglioma Cells via the Akt/GSK-3β Signaling Pathway In Vivo and In Vitro" Marine Drugs 21, no. 6: 329. https://doi.org/10.3390/md21060329
APA StyleLiu, D.-N., Liu, M., Zhang, S.-S., Shang, Y.-F., Zhang, W.-F., Song, F.-H., Zhang, H.-W., Du, G.-H., & Wang, Y.-H. (2023). Chrysomycin A Regulates Proliferation and Apoptosis of Neuroglioma Cells via the Akt/GSK-3β Signaling Pathway In Vivo and In Vitro. Marine Drugs, 21(6), 329. https://doi.org/10.3390/md21060329







