Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences
Abstract
:1. Introduction
2. Results
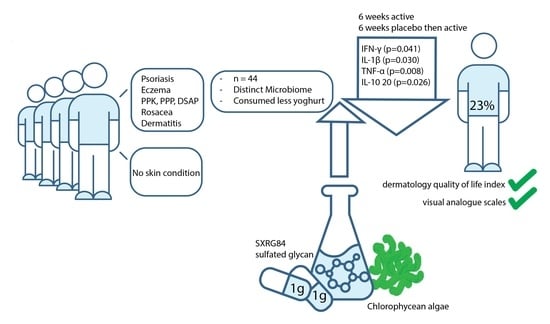
2.1. Participants
2.2. Differences in Gut Microbiome between the Subsets of Skin Cohort and Non-Skin Cohort from the Original Clinical Trial
2.3. Baseline Characteristics of Participants with Inflammatory Skin Conditions
2.4. Changes in Outcome Variables
2.5. Changes in Gut Microbiota
2.6. Skin Responders and Non-Responders to the Intervention
2.7. Dietary Intake
3. Discussion
4. Materials and Methods
4.1. Ethics and Clinical Trial Registration
4.2. Participants and Study Design
4.3. Clinic Visits
4.4. Blood and Faecal Sample Analysis
4.5. Dietary Intake
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.; Maguire, G. The role of microbiota, and probiotics and prebiotics in skin health. Arch. Dermatol. Res. 2017, 309, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef] [PubMed]
- Yeşilova, Y.; Çalka, Ö.; Akdeniz, N.; Berktaş, M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann. Dermatol. 2012, 24, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Meneghin, F.; Fabiano, V.; Mameli, C.; Zuccotti, G.V. Probiotics and atopic dermatitis in children. Pharmaceuticals 2012, 5, 727–744. [Google Scholar] [CrossRef] [Green Version]
- Fanfaret, I.S.; Boda, D.; Ion, L.M.; Hosseyni, D.; Leru, P.; Ali, S.; Corcea, S.; Bumbacea, R. Probiotics and prebiotics in atopic dermatitis: Pros and cons (Review). Exp. Ther. Med. 2021, 22, 1376. [Google Scholar] [CrossRef]
- Oomizu, S.; Onishi, N.; Suzuki, H.; Ueda, K.; Mochizuki, M.; Morimoto, K.; Kawamoto, S.; Ono, K.; Kameyoshi, Y.; Hide, M. Oral administration of pulverized Konjac glucomannan prevents the increase of plasma immunoglobulin E and immunoglobulin G levels induced by the injection of syngeneic keratinocyte extracts in BALB/c mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2006, 36, 102–110. [Google Scholar] [CrossRef]
- Roach, L.A.; Meyer, B.J.; Fitton, J.H.; Winberg, P. Improved Plasma Lipids, Anti-Inflammatory Activity, and Microbiome Shifts in Overweight Participants: Two Clinical Studies on Oral Supplementation with Algal Sulfated Polysaccharide. Mar. Drugs 2022, 20, 500. [Google Scholar] [CrossRef]
- Guerra, L.; Castori, M.; Didona, B.; Castiglia, D.; Zambruno, G. Hereditary palmoplantar keratodermas. Part I. Non-syndromic palmoplantar keratodermas: Classification, clinical and genetic features. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 704–719. [Google Scholar] [CrossRef]
- Patel, S.; Zirwas, M.; English, J.C., 3rd. Acquired palmoplantar keratoderma. Am. J. Clin. Dermatol. 2007, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schiller, S.; Seebode, C.; Hennies, H.C.; Giehl, K.; Emmert, S. Palmoplantar keratoderma (PPK): Acquired and genetic causes of a not so rare disease. J. Der Dtsch. Dermatol. Ges. = J. Ger. Soc. Dermatol. 2014, 12, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Reich, A.; Szepietowski, J.C. Frontiers in Neuroscience Clinical Aspects of Itch: Psoriasis. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hamilton, M.P.; Ntais, D.; Griffiths, C.E.; Davies, L.M. Psoriasis treatment and management—A systematic review of full economic evaluations. Br. J. Dermatol. 2015, 172, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic bacteria induce a ‘glow of health’. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef] [Green Version]
- Saarialho-Kere, U. The gut-skin axis. J. Pediatr. Gastroenterol. Nutr. 2004, 39 (Suppl. 3), S734–S735. [Google Scholar] [CrossRef]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methé, B.A.; Blaser, M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: Development of a new Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 12754. [Google Scholar] [CrossRef]
- Watanabe, S.; Narisawa, Y.; Arase, S.; Okamatsu, H.; Ikenaga, T.; Tajiri, Y.; Kumemura, M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003, 111, 587–591. [Google Scholar] [CrossRef]
- Mah, K.W.; Björkstén, B.; Lee, B.W.; van Bever, H.P.; Shek, L.P.; Tan, T.N.; Lee, Y.K.; Chua, K.Y. Distinct pattern of commensal gut microbiota in toddlers with eczema. Int. Arch. Allergy Immunol. 2006, 140, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kang, M.J.; Lee, S.Y.; Lee, E.; Kim, K.; Won, S.; Suh, D.I.; Kim, K.W.; Sheen, Y.H.; Ahn, K.; et al. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J. Allergy Clin. Immunol. 2018, 141, 1310–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607.e8. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; Chen, X.; Peng, C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front. Microbiol. 2020, 11, 589726. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar]
- Yeom, G.; Yun, D.M.; Kang, Y.W.; Kwon, J.S.; Kang, I.O.; Kim, S.Y. Clinical efficacy of facial masks containing yoghurt and Opuntia humifusa Raf. (F-YOP). J. Cosmet. Sci. 2011, 62, 505–514. [Google Scholar]
- Puch, F.; Samson-Villeger, S.; Guyonnet, D.; Blachon, J.L.; Rawlings, A.V.; Lassel, T. Consumption of functional fermented milk containing borage oil, green tea and vitamin E enhances skin barrier function. Exp. Derm. 2008, 17, 668–674. [Google Scholar] [CrossRef]
- Matsumoto, M.; Aranami, A.; Ishige, A.; Watanabe, K.; Benno, Y. LKM512 yogurt consumption improves the intestinal environment and induces the T-helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clin. Exp. Allergy 2007, 37, 358–370. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, X.; Huang, L.; Shen, R.; Qin, H. Gut Microbiota Alteration After Long-Term Consumption of Probiotics in the Elderly. Probiotics Antimicrob. Proteins 2019, 11, 655–666. [Google Scholar] [CrossRef]
- Sprague, R. Fusicatenibacter Is Associated with Kefir Drinking. BioRxiv 2017, 218313. [Google Scholar] [CrossRef] [Green Version]
- Mori, G.; Rampelli, S.; Orena, B.S.; Rengucci, C.; De Maio, G.; Barbieri, G.; Passardi, A.; Casadei Gardini, A.; Frassineti, G.L.; Gaiarsa, S.; et al. Shifts of Faecal Microbiota during Sporadic Colorectal Carcinogenesis. Sci. Rep. 2018, 8, 10329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-R.; Lin, C.-S.; Chang, C.-J.; Lin, T.-L.; Martel, J.; Ko, Y.-F.; Ojcius, D.; Lu, C.-C.; Young, J.; Lai, H.-C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2018, 68, 248–262. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2010, 163, 250–259. [Google Scholar] [CrossRef]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M.; Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: Present state and perspectives. Microbiology 2010, 156 Pt 11, 3205–3215. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Guida, B.; Napoleone, A.; Trio, R.; Nastasi, A.; Balato, N.; Laccetti, R.; Cataldi, M. Energy-restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: A randomized control clinical trial. Clin. Nutr. 2014, 33, 399–405. [Google Scholar] [CrossRef]
- Wolters, M. Diet and psoriasis: Experimental data and clinical evidence. Br. J. Dermatol. 2005, 153, 706–714. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Langley, R.G.; Ellis, C.N. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J. Am. Acad. Dermatol. 2004, 51, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]




| Genus | Average Abundance in Those with Skin Condition n = 35 | Average Abundance in Those without Skin Condition n = 18 | Contribution to Dissimilarity % | Cumulative % |
|---|---|---|---|---|
| Thalassospira | 6.00 | 5.72 | 5.61 | 5.61 |
| Bifidobacterium | 6.50 a | 3.77 b | 5.05 | 10.66 |
| Kluyvera | 4.72 | 2.50 | 4.96 | 15.62 |
| Akkermansia | 6.43 | 6.97 | 4.90 | 20.52 |
| Sutterella | 6.83 | 7.61 | 4.48 | 25.00 |
| Catenibacterium | 3.23 | 3.87 | 4.47 | 29.47 |
| Parasutterella | 4.97 | 6.41 | 4.44 | 33.91 |
| Prevotella | 5.04 | 2.55 | 4.33 | 38.25 |
| Phascolarctobacterium | 7.83 | 6.98 | 4.20 | 42.44 |
| Finegoldia | 4.16 | 2.70 | 4.04 | 46.48 |
| Peptoniphilus | 4.03 | 2.40 | 4.00 | 50.48 |
| Asteroleplasma | 1.60 a | 3.54 b | 3.93 | 54.41 |
| Corynebacterium | 3.70 | 2.82 | 3.86 | 58.27 |
| Megasphaera | 3.12 | 1.66 | 3.86 | 62.13 |
| Butyrivibrio | 5.44 a | 5.74 b | 3.22 | 65.35 |
| Megamonas | 1.47 | 1.64 | 3.01 | 68.37 |
| Klebsiella | 1.62 | 1.53 | 2.99 | 71.35 |
| Alloprevotella | 1.00 | 1.75 | 2.63 | 73.98 |
| Peptoclostridium | 6.63 | 5.94 | 2.57 | 76.55 |
| Intestinibacter | 7.91 | 8.33 | 1.78 | 78.33 |
| Sarcina | 9.40 a | 9.88 b | 1.72 | 80.05 |
| Lachnospira | 8.72 | 8.10 | 1.69 | 81.74 |
| Erysipelatoclostridium | 8.08 | 8.20 | 1.50 | 83.24 |
| Subdoligranulum | 9.31 | 9.95 | 1.48 | 84.72 |
| Alistipes | 9.65 | 10.37 | 1.47 | 86.19 |
| Streptococcus | 8.14 | 7.58 | 1.46 | 87.65 |
| Anaeroplasma | 0.72 | 0.57 | 1.29 | 88.93 |
| Fusicatenibacter | 9.59 | 9.71 | 1.15 | 90.08 |
| Group AB: Placebo Then SXRG Treatment (n = 25) | Group BA: SXRG Treatment Then Placebo (n = 19) | p-Value 1 | |
|---|---|---|---|
| Gender, F, (%) | 13 (52%) | 9 (47%) | 0.76 |
| Age (years) | 51.2 ± 14.6 | 53.6 ± 11.4 | 0.55 |
| BMI | 27.5 (24.8, 30.4) | 29.7 (28.1, 33.3) | 0.02 |
| Skin Category | |||
| Psoriasis, n (%) | 17 (68%) | 13 (68%) | |
| Eczema, n (%) | 1 (4%) | 1 (5%) | |
| Rosacea, n (%) | 1 (4%) | 1 (5%) | |
| Dermatitis, n (%) * | 2 (8%) | 3 (16%) | |
| PPK, n (%) | 0 (0%) | 1 (5%) | |
| DSAP, n (%) | 1 (4%) | 0 (0%) | |
| PPP, n (%) | 3 (12%) | 0 (0%) | |
| Skin Measures | |||
| PASI § | 2.4 (0.6, 4.4) | 3.0 (1.6, 4.9) | 0.18 |
| VAS § | 5.0 (4.1, 6.2) | 5.0 (5.0, 5.0) | 0.90 |
| DQLI § | 3.0 (2.0, 5.5) | 5.0 (3.0, 11.0) | 0.11 |
| Inflammation | |||
| C-reactive protein (mg/L) § | 0.0 (0.0, 3.2) | 0.0 (0.0, 1.7) | 0.78 |
| IFN-gamma (pg/mL) § | 3.2 (1.8, 5.1) | 3.5 (1.8, 5.3) | 0.95 |
| IL-1 beta (pg/mL) § | 18.0 (9.2, 26.9) | 16.1 (10.1, 26.3) | 0.88 |
| IL-6 (pg/mL) § | 11.9 (8.0, 20.2) | 12.3 (8.4, 18.1) | 0.84 |
| TNF-alpha (pg/mL) § | 7.7 (3.9, 12.0) | 5.4 (3.1, 12.9) | 0.84 |
| IL-10 (pg/mL) § | 1.3 (0.7, 2.3) | 1.0 (0.8, 2.5) | 0.91 |
| IL-8 (pg/mL) § | 5.6 (3.7, 10.4) | 5.4 (3.5, 7.5) | 0.88 |
| AA 6 Weeks from Baseline (Placebo) n = 25 | AB 12 Weeks from Baseline (Placebo Then Active) n = 25 | BB 6 Weeks from Baseline (Active) n = 19 | BA 12 Weeks from Baseline (Active Then Placebo) n = 19 | p-Value * | |
|---|---|---|---|---|---|
| BMI | 0.0 (−0.3, 0.6) | 0.0 (−0.4, 0.4) | 0.3 (−0.3, 0.4) | 0.0 (−0.9, 0.4) | 0.520 |
| Skin Scores | |||||
| PASI | −0.4 (−1.7, 0.0) | −0.8 (−2.1, 0.0) | −1.2 (−1.8, 0.0) | −0.6 (−2.5, 0.4) | 0.664 |
| VAS | 0.0 (−2.0, 2.2) | 0.2 (−2.0, 2.5) | 1.2 (−0.4, 3.1) | 0.0 (−1.5, 2.5) | 0.828 |
| DQLI | −1.0 (−2.0, 0.5) | 0.0 (−2.0, 1.0) | −2.0 (−5.0, 0.0) | −2.0 (−4.0, 1.0) | 0.694 |
| Inflammation | |||||
| C-reactive protein (mg/L) | 0.0 (0.0, 2.2) | 0.0 (0.0, 0.8) | 0.0 (0.0, 6.1) | 0.0 (0.0, 0.1) | 0.559 |
| IFN-gamma (pg/mL) | 0.2 (−0.7, 1.6) a | −0.4 (−1.8, 0.7) b | 0.4 (−0.9, 1.5) ab | −0.8 (−2.7, 0.1) b | 0.041 |
| IL-1 beta (pg/mL) | −0.3 (−4.5, 8.3) a | −0.5 (−8.5, 4.9) b | 0.8 (−7.2, 5.7) ab | −5.4 (−11.3, 0.2) b | 0.030 |
| IL-6 (pg/mL) | 1.1 (−4.9, 7.4) | −0.7 (−4.9, 3.6) | 1.3 (−2.9, 3.9) | −1.3 (−6.0, 3.0) | 0.284 |
| TNF-alpha (pg/mL) | 1.7 (−1.1, 7.8) a | 0.7 (−1.8, 3.4) a,b | 1.0 (−2.4, 3.4) a,b | −2.0 (−6.3, 1.8) b | 0.008 |
| IL-10 (pg/mL) | 0.2 (−0.3, 0.9) a | 0.1 (−0.3, 0.5) a,b | 0.0 (−0.6, 0.7) a,b | −0.3 (−0.9, 0.2) b | 0.026 |
| IL-8 (pg/mL) | 0.0 (−1.1, 3.7) | −1.2 (−2.8, 0.0) | 0.2 (−2.5, 2.4) | −0.5 (−2.2, 0.6) | 0.378 |
| Skin Condition | Number That Participated in Trial | Responders, n (%) |
|---|---|---|
| Psoriasis | 30 | 8 (27%) |
| Eczema | 2 | 2 (100%) |
| Rosacea | 2 | 0 |
| Dermatitis | 5 | 0 |
| PPK | 1 | 0 |
| DSAP | 1 | 0 |
| PPP | 3 | 0 |
| Total | 44 | 10 (23%) |
| Outcome Measure | Responder vs. Non-Responder Mean Difference (Standard Error) | p-Value |
|---|---|---|
| PASI | −1.56 (0.87) | 0.08 |
| VAS | 3.08 (0.76) | <0.001 |
| DQLI | −2.04 (1.01) | 0.049 |
| C-reactive protein (mg/L) | −1.97 (2.26) | 0.39 |
| IFN-gamma (pg/mL) | −0.32 (0.76) | 0.68 |
| IL-1 beta (pg/mL) | −3.56 (2.68) | 0.19 |
| IL-6 (pg/mL) | −2.37 (2.36) | 0.32 |
| TNF-alpha (pg/mL) | −2.55 (1.63) | 0.13 |
| IL-10 (pg/mL) | −0.38 (0.27) | 0.17 |
| IL-8 (pg/mL) | −0.79 (1.66) | 0.64 |
| Skin Measure | Genus | Spearman’s Correlation Co-Efficient | p-Value |
|---|---|---|---|
| Baseline PASI score | Peptoclostridium | 0.335 | 0.04 |
| Baseline VAS | Clostridium | 0.367 | 0.02 |
| Coprobacter | −0.480 | 0.03 | |
| Faecalibacterium | 0.322 | 0.04 | |
| Flavobacterium | −0.434 | 0.04 | |
| Mogibacterium | −0.604 | 0.00 | |
| Peptococcus | −0.460 | 0.0 | |
| Baseline DQLI | Dialister | 0.453 | 0.03 |
| Lactobacillus | 0.426 | 0.03 | |
| Peptoclostridium | 0.372 | 0.02 | |
| Streptococcus | −0.418 | 0.01 |
| AA: 6-week measure after placebo consumption |
| AB: 12-week measure after placebo (for 6 weeks) then SXRG84 treatment consumption (for 6 weeks) |
| BB: 6-week measure after SXRG84 treatment consumption |
| BA: 12-week measure after SXRG84 treatment (for 6 weeks) then placebo consumption (for 6 weeks) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roach, L.A.; Meyer, B.J.; Fitton, J.H.; Winberg, P. Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences. Mar. Drugs 2023, 21, 379. https://doi.org/10.3390/md21070379
Roach LA, Meyer BJ, Fitton JH, Winberg P. Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences. Marine Drugs. 2023; 21(7):379. https://doi.org/10.3390/md21070379
Chicago/Turabian StyleRoach, Lauren A., Barbara J. Meyer, J. Helen Fitton, and Pia Winberg. 2023. "Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences" Marine Drugs 21, no. 7: 379. https://doi.org/10.3390/md21070379
APA StyleRoach, L. A., Meyer, B. J., Fitton, J. H., & Winberg, P. (2023). Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences. Marine Drugs, 21(7), 379. https://doi.org/10.3390/md21070379