From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability
Abstract
1. Introduction
2. Results and Discussion
2.1. Nutritional Profiling
2.2. Chemical Profiling
2.3. Bioactivity Profiling
3. Materials and Methods
3.1. Chemicals
3.2. Sample Collection, Processing, and Extraction
3.3. Nutritional Profiling
3.3.1. Proximate Composition
3.3.2. Mineral Composition
3.4. Chemical Profiling
3.4.1. Contents of Total Phenolics (TPC), Flavonoids, and Condensed Tannins (CTC)
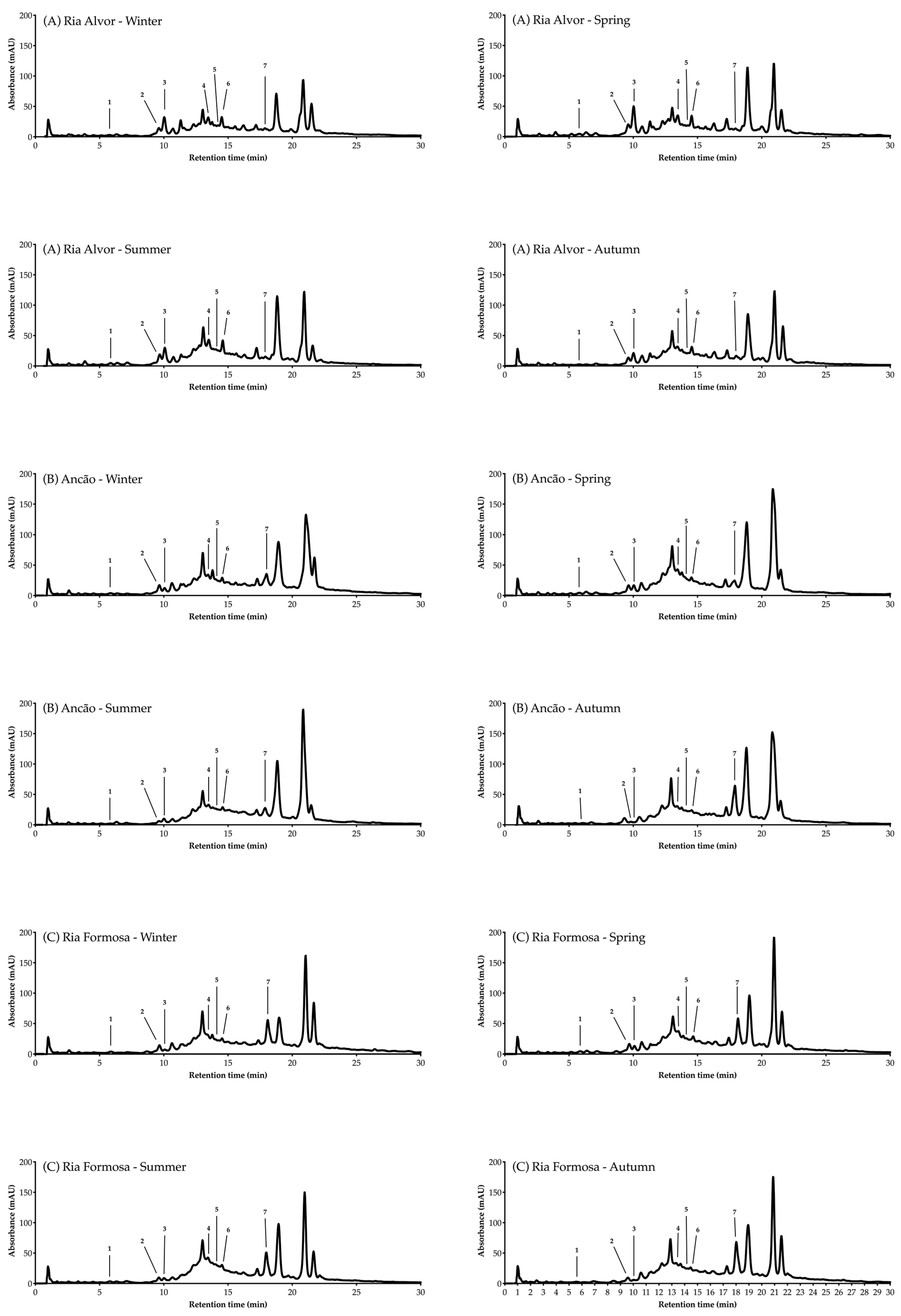
3.4.2. Phenolic Profile via High-Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD)
3.5. Bioactivity Profiling
3.5.1. In Vitro Antioxidant Activity
3.5.2. In Vitro Anti-Inflammatory Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millennium Ecosystem Assessment (MEA). Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- McLachlan, A.; Defeo, O. Human Impacts. In The Ecology of Sandy Shores, 3rd ed.; McLachlan, A., Brown, A.C., Eds.; Academic Press: London, UK, 2018; pp. 375–420. [Google Scholar]
- Drius, M.; Jones, L.; Marzialetti, F.; De Francesco, M.C.; Stanisci, A.; Carranza, M.L. Not just a sandy beach. The multi-service value of Mediterranean coastal dunes. Sci. Total Environ. 2019, 668, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Maximo, P.; Ferreira, L.M.; Branco, P.S.; Lourenço, A. Invasive plants: Turning enemies into value. Molecules 2020, 25, 3529. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Malanson, G.P.; Walsh, S.J. A geographical approach to optimization of response to invasive species. In Science and Conservation in the Galapagos Islands, 1st ed.; Walsh, S.J., Mena, C.F., Eds.; Springer: New York, NY, USA, 2013; Volume 1, pp. 199–215. [Google Scholar]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive plant species biomass—Evaluation of functional value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef]
- Roiloa, S.R. Clonal traits and plant invasiveness: The case of Carpobrotus N.E.Br. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125479. [Google Scholar] [CrossRef]
- Akinyede, K.A.; Ekpo, O.E.; Oguntibeju, O.O. Ethnopharmacology, therapeutic properties and nutritional potentials of Carpobrotus edulis: A comprehensive review. Sci. Pharm. 2020, 88, 39. [Google Scholar] [CrossRef]
- Campoy, J.G.; Acosta, A.T.R.; Affre, L.; Barreiro, R.; Brundu, G.; Buisson, E.; González, L.; Lema, M.; Novoa, A.; Retuerto, R.; et al. Monographs of invasive plants in Europe: Carpobrotus. Bot. Lett. 2018, 165, 440–475. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.; Zengin, G.; Uysal, A.; Jeko, J.; Cziáky, Z.; Reis, C.P.; Gaspar, M.M.; et al. If you cannot beat them, join them: Exploring the fruits of the invasive species Carpobrotus edulis (L.) N.E. Br as a source of bioactive products. Ind. Crop. Prod. 2020, 144, 112005. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L.) N.E.Br. extract as a skin preserving agent: From traditional medicine to scientific validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef]
- Neves, M.; Antunes, M.; Fernandes, W.; Campos, M.J.; Azevedo, Z.M.; Freitas, V.; Rocha, J.M.; Tecelão, C. Physicochemical and nutritional profile of leaves, flowers, and fruits of the edible halophyte chorão-da-praia (Carpobrotus edulis) on Portuguese west shores. Food Biosci. 2021, 43, 101288. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From tradition to health: Chemical and bioactive characterization of five traditional plants. Molecules 2022, 27, 6495. [Google Scholar] [CrossRef]
- Rocha, M.I.; Rodrigues, M.J.; Pereira, C.; Pereira, H.; da Silva, M.M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Biochemical profile and in vitro neuroprotective properties of Carpobrotus edulis L., a medicinal and edible halophyte native to the coast of South Africa. S. Afr. J. Bot. 2017, 111, 222–231. [Google Scholar] [CrossRef]
- Custódio, L.; Ferreira, A.C.; Pereira, H.; Silvestre, L.; Vizetto-Duarte, C.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. The marine halophytes Carpobrotus edulis L. and Arthrocnemum macrostachyum L. are potential sources of nutritionally important PUFAs and metabolites with antioxidant, metal chelating and anticholinesterase inhibitory activities. Bot. Mar. 2012, 55, 281–288. [Google Scholar] [CrossRef]
- Chokoe, P.K.; Masoko, P.; Mokgotho, M.P.; Howard, R.L.; Mampuru, L.J. Does seasonal variation influence the phytochemical and antibacterial properties of Carpobrotus edulis? Afr. J. Biotechnol. 2008, 7, 4164–4171. [Google Scholar]
- Zaier, M.M.; Ciudad-Mulero, M.; Cámara, M.; Pereira, C.; Ferreira, I.C.; Achour, L.; Kacem, A.; Morales, P. Revalorization of Tunisian wild Amaranthaceae halophytes: Nutritional composition variation at two different phenotypes stages. J. Food Compos. Anal. 2020, 89, 103463. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Parida, A.; Das, A.B.; Das, P. NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J. Plant Biol. 2002, 45, 28–36. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef]
- Zou, L.; Tan, W.K.; Du, Y.; Lee, H.W.; Liang, X.; Lei, J.; Striegel, L.; Weber, N.; Rychlik, M.; Ong, C.N. Nutritional metabolites in Brassica rapa subsp. chinensis var. parachinensis (choy sum) at three different growth stages: Microgreen, seedling and adult plant. Food Chem. 2021, 357, 129535. [Google Scholar] [CrossRef]
- Rayburn, E.B.; Sharpe, P. Chapter 5—Introduction to Pasture Ecology. In Horse Pasture Management; Sharpe, P., Ed.; Academic Press: London, UK, 2019; pp. 81–91. [Google Scholar] [CrossRef]
- Mateus, M.; Almeida, D.; Simonson, W.; Felgueiras, M.; Banza, P.; Batty, L. Conflictive uses of coastal areas: A case study in a southern European coastal lagoon (Ria de Alvor, Portugal). Ocean Coast. Manag. 2016, 132, 90–100. [Google Scholar] [CrossRef]
- Cravo, A.; Rosa, A.; Jacob, J.; Correira, C. Dissolved oxygen dynamics in Ria Formosa Lagoon (South Portugal)—A real time monitoring station observatory. Mar. Chem. 2020, 223, 103806. [Google Scholar] [CrossRef]
- Oelberg, K. Factors affecting the nutritive value of range forage. Rangel. Ecol. Manag. J. Range Manag. Arch. 1956, 9, 220–225. [Google Scholar]
- Oliveira, M.; Rodrigues, M.J.; Neng, N.R.; Nogueira, J.M.F.; Bessa, R.J.B.; Custódio, L. Seasonal variations of the nutritive value and phytotherapeutic potential of Cladium mariscus L. (Pohl.) targeting ruminant’s production. Plants 2021, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- IPMA, Instituto Português do Mar e da Atmosfera, Divisão de Clima e Alterações Climáticas. Boletim Climatológico Mensal (Janeiro, Maio, Julho, Novembro) 2020. Available online: https://www.ipma.pt/pt/media/noticias/index.jsp?year=2020&p=2 (accessed on 13 June 2023).
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Kader, J.C., Derseny, M., Eds.; Academic Press: London, UK, 2010; Volume 55, pp. 179–225. [Google Scholar] [CrossRef]
- Broomhead, N.K.; Moodley, R.; Jonnalagadda, S.B. Chemical and elemental analysis of the edible fruit of five Carpobrotus species from South Africa: Assessment of nutritional value and potential metal toxicity. Int. J. Environ. Health Res. 2020, 30, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Nutritional profile of vegetables and its significance to human health. In Handbook of Vegetables & Vegetable Processing; Sinha, N.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 107–123. [Google Scholar]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Rabbimov, A.; Bekchanov, B.; Mukimov, T. Chemical composition and palatability of some species of halophytes. Arid Ecosyst. 2011, 1, 104–109. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs. cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture—USDA. Composition of Foods Raw, Processed, Prepared; USDA Nutrient Database for Standard Reference: Washington, DC, USA, 2001. [Google Scholar]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The Effects of Biostimulants, Biofertilizers and Water-Stress on Nutritional Value and Chemical Composition of Two Spinach Genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef]
- Akinshina, N.; Azizov, A.; Karasyova, T.; Klose, E. On the issue of halophytes as energy plants in saline environment. Biomass Bioenerg. 2016, 91, 306–311. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef] [PubMed]
- Debez, A.; Belghith, I.; Friesen, J.; Montzka, C.; Elleuche, S. Facing the challenge of sustainable bioenergy production: Could halophytes be part of the solution? J. Biol. Eng. 2017, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.; Kawatra, A. Fibre constituents of some foods. Plant Foods Hum. Nutr. 1994, 45, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Díaz, F.J.; Benes, S.E.; Grattan, S.R. Field performance of halophytic species under irrigation with saline drainage water in the San Joaquin Valley of California. Agric. Water Manag. 2013, 118, 59–69. [Google Scholar] [CrossRef]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; Koubaier, H.B.H.; Casabianca, H.; Abe, N.; Omri, A.E.; Chaabouni, M.M.; Bouzouita, N. Phytochemical investigation of Tunisian Salicornia herbacea L., antioxidant, antimicrobial and cytochrome P450 (CYPs) inhibitory activities of its methanol extract. Food Control 2013, 32, 125–133. [Google Scholar] [CrossRef]
- McCance, R.A.; Widdowson, E.M. McCance and Widdowson’s the Composition of Foods, 6th ed.; Food Standards Agency, Royal Society of Chemistry: Cambridge, UK, 2002; pp. 29–399. [Google Scholar]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture—USDA. Nutrient Data Laboratory; Agricultural Research Service; USDA Nutrient Database for Standard Reference: Washington, DC, USA, 2014. [Google Scholar]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; 1329p. [Google Scholar]
- EC Regulation, 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&qid=1690830357576 (accessed on 17 May 2023).
- Kähkönen, M.P.; Hopia, A.I.; Heikki, J.V.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crop. Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Phenol-Explorer, Database on Polyphenol Content in Foods, Version 3.6. 2023. Available online: http://phenol-explorer.eu (accessed on 11 January 2023).
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Singh, P.K.; Gautam, S. Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol. Plant. 2013, 35, 2345–2353. [Google Scholar] [CrossRef]
- Vicente, M.R.S.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pak. J. Agric. Res. 2019, 32, 8. [Google Scholar] [CrossRef]
- Belles, J.M.; Garro, R.; Fayos, J.; Navarro, P.; Primo, J.; Conejero, V. Gentisic acid as a pathogen-inducible signal, additional to salicylic acid for activation of plant defences in tomato. Mol. Plant-Microbe Interact. 1999, 12, 227–235. [Google Scholar] [CrossRef]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.; Finger-Teixeira, A.; Mota, T.R.; Salvador, V.H.; Moreira-Vilar, F.C.; Correa Molinari, H.B.; Craig Mitchell, R.A.; Marchiosi, R.; Ferrarese-Filho, O.; dos Santos, W.D. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A Review of Analytical Methods for p-Coumaric Acid in Plant-Based Products, Beverages, and Biological Matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Soviguidi, D.R.J.; Pan, R.; Liu, Y.; Rao, L.; Zhang, W.; Yang, X. Chlorogenic acid metabolism: The evolution and roles in plant response to abiotic stress. Phyton 2022, 91, 239. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Bouyahya, A.; Taha, D.; Benali, T.; Zengin, G.; El Omari, N.; El Hachlafi, N.; Khalid, A.; Abdalla, A.N.; Ardianto, C.; Tan, C.S.; et al. Natural sources, biological effects, and pharmacological properties of cynaroside. Biomed. Pharmacother. 2023, 161, 114337. [Google Scholar] [CrossRef]
- Yasuda, M.T.; Fujita, K.; Hosoya, T.; Imai, S.; Shimoi, K. Absorption and Metabolism of Luteolin and Its Glycosides from the Extract of Chrysanthemum morifolium Flowers in Rats and Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 7693–7699. [Google Scholar] [CrossRef]
- Beekmann, K.; Actis-Goretta, L.; van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I.M. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008–1018. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef] [PubMed]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Kadar, N.N.M.A.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Pilluzza, G.; Bullita, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Custódio, L.; Mecha, D.; Zengin, G.; Cziáky, Z.; Sotkó, G.; Pereira, C.G. Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants 2022, 11, 2910. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Chlorogenic Acid Electroanalysis. Int. J. Mol. Sci. 2021, 22, 13138. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 18th ed.; Method 942.05; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Uslu, L.; Durmaz, Y.; Duyar, H.A.; Bandarra, N.M. Fatty acids, α-tocopherol and proximate composition of four red macroalgae in the Sinop Bay (Turkey). J. Anim. Vet. Adv. 2013, 12, 29–33. [Google Scholar]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61–73. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Neng, N.R.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizzeto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from Southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef]


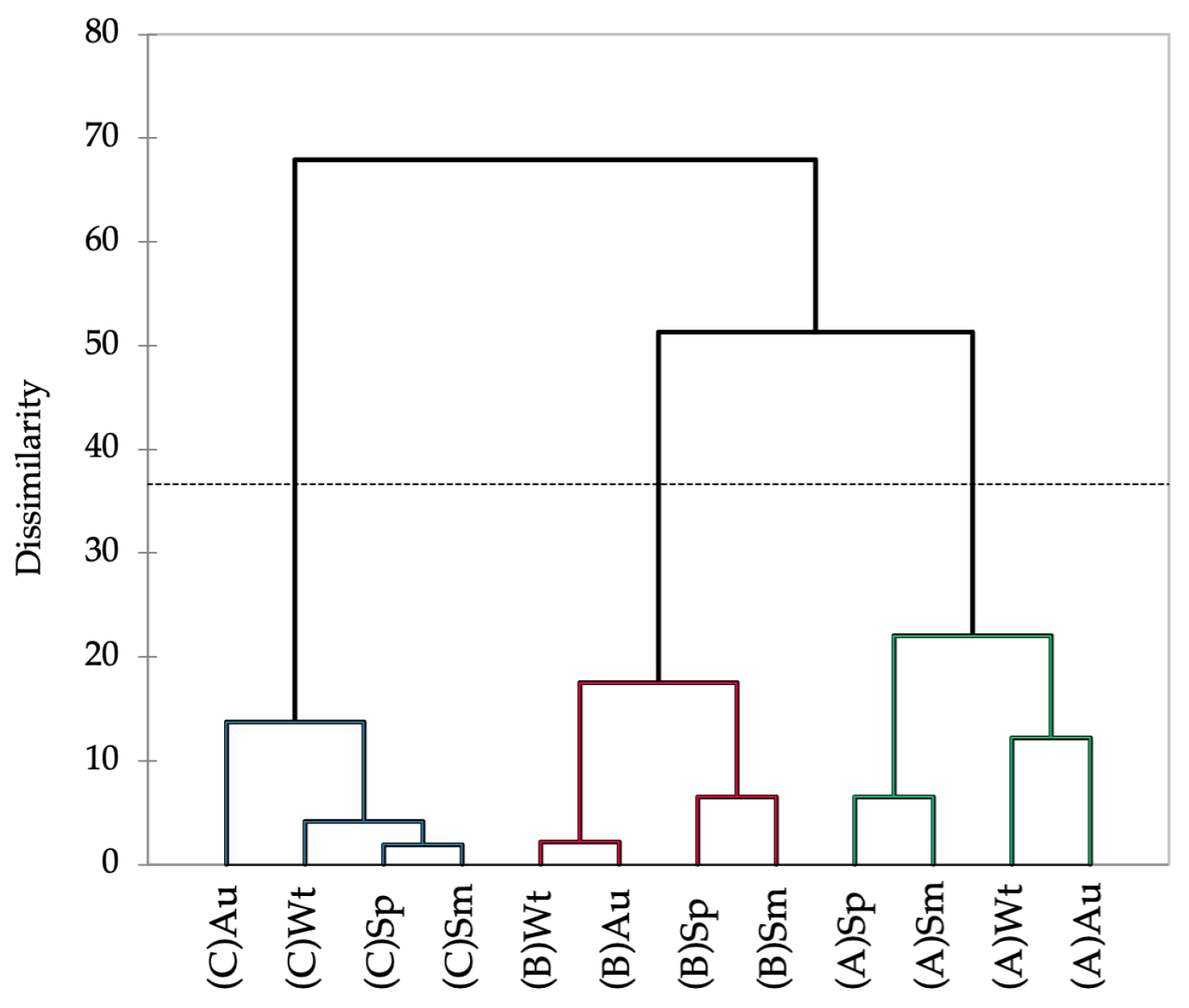



| Location | (A): Ria de Alvor Lagoon | (B): Ancão Beach | (C): Ria Formosa Lagoon | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | ||
| Proximate composition (g/100 g DW) | ||||||||||||||
| Moisture | 93.3 ± 0.4 ab | 90.4 ± 0.9 bc | 89.2 ± 0.6 c | 94.3 ± 0.5 a | 88.5 ± 1.2 cd | 87.9 ± 2.4 cd | 85.2 ± 0.2 d | 88.9 ± 0.4 c | 89.4 ± 0.8 c | 89.2 ± 1.9 c | 87.5 ± 1.0 cd | 88.5 ± 0.0 cd | ||
| Ash | 22.7 ± 0.6 bc | 21.6 ± 0.6 bc | 23.9 ± 0.2 b | 27.5 ± 0.2 a | 23.7 ± 0.3 b | 18.6 ± 0.7 d | 19.0 ± 0.8 d | 23.0 ± 0.1 bc | 21.8 ± 0.4 bc | 23.3 ± 0.2 b | 20.7 ± 0.2 cd | 27.0 ± 2.5 a | ||
| Crude protein | 6.2 ± 0.5 a | 3.8 ± 0.3 c | 3.2 ± 0.3 cd | 5.0 ± 0.5 b | 2.4 ± 0.4 defg | 1.8 ± 0.2 efg | 1.8 ± 0.2 fg | 1.6 ± 0.2 g | 2.6 ± 0.5 defg | 2.6 ± 0.4 def | 2.8 ± 0.3 de | 3.2 ± 0.2 cd | ||
| Crude fat | 1.0 ± 0.0 cd | 1.2 ± 0.0 abcd | 0.8 ± 0.1 fg | 1.3 ± 0.0 ab | 1.1 ± 0.1 bcd | 1.0 ± 0.1 def | 1.0 ± 0.0 cde | 1.3 ± 0.1 a | 0.8 ± 0.1 efg | 0.8 ± 0.1 g | 0.6 ± 0.1 g | 1.2 ± 0.1 abc | ||
| Carbohydrates | 70.0 ± 0.4 | 73.4 ± 0.4 | 72.0 ± 0.2 | 66.2 ± 0.3 | 72.8 ± 0.3 | 78.5 ± 0.4 | 78.2 ± 0.5 | 74.1 ± 0.2 | 74.8 ± 0.4 | 73.3 ± 0.2 | 75.9 ± 0.2 | 68.6 ± 1.5 | ||
| Fiber, NDF | 22.3 | 24.6 | 26.0 | 20.3 | 17.8 | 19.2 | 24.9 | 17.1 | 18.9 | 22.1 | 23.1 | 20.2 | ||
| Minerals—Macro elements (g/100 g DW) | ||||||||||||||
| Ca | 3.7 ± 0.0 bc | 3.5 ± 0.0 cd | 2.7 ± 0.0 fgh | 2.9 ± 0.0 f | 4.3 ± 0.1 a | 3.4 ± 0.1 d | 3.3 ± 0.1 de | 3.7 ± 0.0 b | 2.6 ± 0.0 gh | 3.1 ± 0.1 e | 2.5 ± 0.0 h | 2.8 ± 0.1 fg | ||
| K | 2.0 ± 0.0 d | 1.7 ± 0.1 e | 1.5 ± 0.0 f | 1.6 ± 0.0 ef | 0.5 ± 0.0 g | 0.6 ± 0.0 g | 0.6 ± 0.0 g | 0.3 ± 0.0 h | 2.6 ± 0.1 c | 3.0 ± 0.1 a | 2.9 ± 0.0 ab | 2.8 ± 0.0 b | ||
| Mg | 0.8 ± 0.0 d | 0.7 ± 0.0 ef | 0.8 ± 0.0 d | 0.8 ± 0.0 d | 0.6 ± 0.0 g | 0.5 ± 0.0 h | 0.7 ± 0.0 fg | 0.8 ± 0.0 de | 1.1 ± 0.0 a | 0.9 ± 0.0 c | 0.9 ± 0.0 c | 1.0 ± 0.0 b | ||
| Na | 3.3 ± 0.1 d | 2.5 ± 0.0 f | 3.9 ± 0.1 c | 4.8 ± 0.1 a | 3.8 ± 0.2 c | 2.3 ± 0.0 f | 2.4 ± 0.1 f | 4.2 ± 0.1 b | 2.9 ± 0.1 e | 2.3 ± 0.1 f | 2.3 ± 0.0 f | 3.8 ± 0.0 c | ||
| Trace elements (mg/100 g DW) | ||||||||||||||
| Fe | 5.0 ± 0.1 a | 2.5 ± 0.0 f | 2.3 ± 0.0 f | 3.3 ± 0.1 de | 4.8 ± 0.4 ab | 3.5 ± 0.0 cde | 4.1 ± 0.6 bc | 5.3 ± 0.3 a | 3.0 ± 0.2 ef | 3.0 ± 0.0 ef | 3.9 ± 0.0 cd | 3.6 ± 0.1 cde | ||
| Mn | 2.9 ± 0.1 g | 2.3 ± 0.1 g | 2.8 ± 0.0 g | 2.5 ± 0.0 g | 10.6 ± 0.2 d | 7.2 ± 0.2 f | 8.4 ± 0.2 e | 12.1 ± 0.1 c | 16.3 ± 1.1 b | 24.8 ± 0.2 a | 16.2 ± 0.3 b | 15.6 ± 0.1 b | ||
| Zn | 2.0 ± 0.1 ef | 1.5 ± 0.0 g | 1.1 ± 0.0 h | 1.3 ± 0.0 gh | 2.3 ± 0.1 e | 1.9 ± 0.1 f | 2.1 ± 0.1 ef | 3.1 ± 0.0 d | 7.4 ± 0.2 b | 8.7 ± 0.1 a | 7.2 ± 0.1 b | 6.1 ± 0.1 c | ||
| Cu | 0.3 ± 0.0 c | 0.3 ± 0.0 c | 0.2 ± 0.0 d | 0.4 ± 0.0 c | 0.3 ± 0.0 c | 0.3 ± 0.0 c | 0.4 ± 0.0 c | 0.3 ± 0.0 c | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.9 ± 0.0 a | 0.6 ± 0.0 b | ||
| Cr | 0.1 ± 0.0 b | 0.0 ± 0.0 de | 0.0 ± 0.0 de | 0.1 ± 0.0 c | 0.1 ± 0.0 b | 0.0 ± 0.0 cd | 0.1 ± 0.0 a | 0.0 ± 0.0 cd | 0.1 ± 0.0 b | 0.0 ± 0.0 de | 0.0 ± 0.0 cde | 0.0 ± 0.0 e | ||
| Ni | 0.2 ± 0.0 ab | 0.1 ± 0.0 efg | 0.1 ± 0.0 ef | 0.2 ± 0.0 de | 0.1 ± 0.0 gh | 0.1 ± 0.0 h | 0.1 ± 0.0 fg | 0.1 ± 0.0 h | 0.2 ± 0.0 cd | 0.2 ± 0.0 ab | 0.2 ± 0.0 a | 0.2 ± 0.0 bc | ||
| Cd | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | <LOQ 1 | ||
| Pb | 0.1 ± 0.0 d | 0.2 ± 0.0 abcd | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.2 ± 0.0 bcd | 0.2 ± 0.0 cd | 0.3 ± 0.0 abc | 0.1 ± 0.0 cd | 0.3 ± 0.0 abc | 0.3 ± 0.0 ab | 0.3 ± 0.0 abc | 0.1 ± 0.0 d | ||
| Season | Month | min. temp. * | max. temp. * | Total Precipitation |
|---|---|---|---|---|
| Winter | January | 8.9 °C | 16.6 °C | 29.6 mm |
| Spring | May | 17.0 °C | 24.7 °C | 37.5 mm |
| Summer | July | 21.6 °C | 30.3 °C | 0.0 mm |
| Autumn | November | 13.7 °C | 20.0 °C | 155.8 mm |
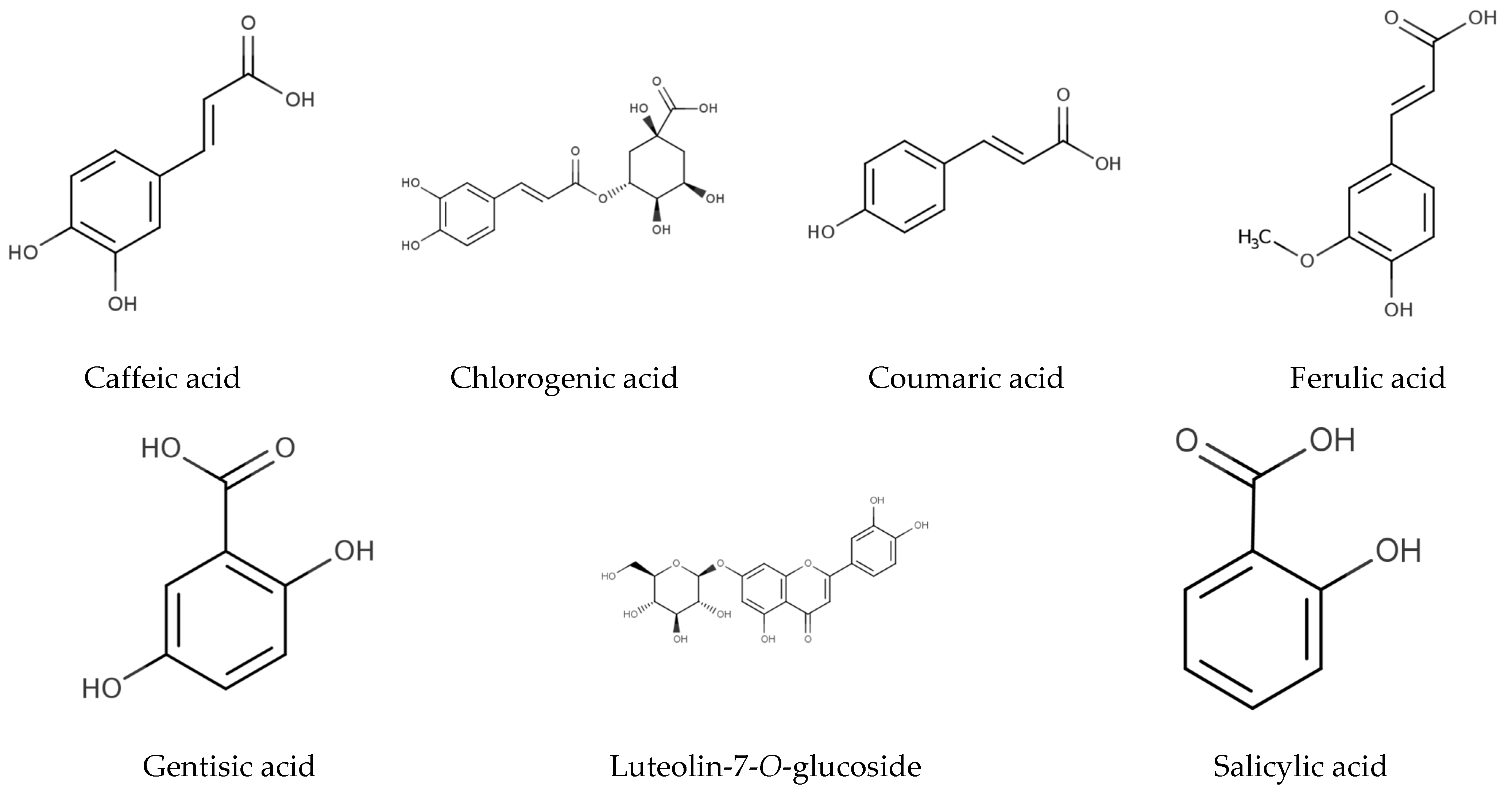
| Peak | RT | Compound | (A): Ria de Alvor Lagoon | (B): Ancão Beach | (C): Ria Formosa Lagoon | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (min) | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | |
| Phenolic acids | ||||||||||||||
| Hydroxybenzoic acids | ||||||||||||||
| 1 | 5.9 | Gentisic acid | 0.03 | 0.07 | 0.09 | 0.03 | 0.04 | 0.07 | 0.01 | 0.03 | 0.07 | 0.09 | 0.05 | 0.00 |
| 6 | 14.6 | Salicylic acid | 1.68 | 1.56 | 2.14 | 1.35 | 1.31 | 1.44 | 2.34 | 1.32 | 1.05 | 1.78 | 0.88 | 1.05 |
| Hydroxycinnamic acids | ||||||||||||||
| 2 | 9.6 | Chlorogenic acid | 0.24 | 0.33 | 0.30 | 0.24 | 0.23 | 0.25 | 0.11 | 0.04 | 0.17 | 0.25 | 0.12 | 0.13 |
| 3 | 10.2 | Caffeic acid | 0.51 | 0.73 | 0.48 | 0.40 | 0.30 | 0.35 | 0.29 | 0.28 | 0.21 | 0.31 | 0.23 | – |
| 4 | 13.4 | Coumaric acid | 0.35 | 0.34 | 0.49 | – | 0.30 | 0.38 | 0.31 | 0.30 | 0.34 | 0.38 | 0.38 | 0.45 |
| 5 | 14.3 | Ferulic acid | 0.10 | 0.13 | 0.12 | 0.14 | 0.13 | 0.17 | – | 0.24 | 0.09 | 0.24 | 0.22 | 0.12 |
| Flavonoids | ||||||||||||||
| Flavones | ||||||||||||||
| 7 | 17.9 | Luteolin-7-O-glucoside | 0.43 | 0.55 | 0.42 | 0.43 | 1.56 | 0.76 | 1.37 | 2.68 | 2.10 | 2.36 | 1.26 | 2.44 |
| Σ phenolics | 3.34 | 3.71 | 4.04 | 2.59 | 3.87 | 3.42 | 4.43 | 4.89 | 4.03 | 5.41 | 3.14 | 4.19 | ||
| Location | Season | DPPH• | ABTS•+ | NO | FRAP | CCA | ICA |
|---|---|---|---|---|---|---|---|
| (A): Ria de Alvor | Winter | 0.22 ± 0.00 d | 0.08 ± 0.01 abc | 2.17 ± 0.02 f | 0.50 ± 0.03 f | 4.23 ± 0.18 f | >10 |
| Spring | 0.07 ± 0.01 ab | 0.11 ± 0.01 bc | 0.26 ± 0.02 ab | 0.29 ± 0.03 d | 3.50 ± 0.20 e | >10 | |
| Summer | 0.08 ± 0.01 ab | 0.09 ± 0.01 abc | 1.24 ± 0.01 d | 0.56 ± 0.02 g | 2.68 ± 0.15 d | >10 | |
| Autumn | 0.18 ± 0.02 c | 0.19 ± 0.02 d | 0.85 ± 0.07 c | 0.12 ± 0.00 ab | 2.57 ± 0.21 d | >10 | |
| (B): Ancão beach | Winter | 0.17 ± 0.00 c | 0.08 ± 0.02 abc | 0.41 ± 0.03 b | 0.41 ± 0.02 e | 4.88 ± 0.31 g | >10 |
| Spring | 0.06 ± 0.01 ab | 0.06 ± 0.00 a | 0.21 ± 0.01 a | 0.18 ± 0.00 bc | 1.99 ± 0.02 bc | >10 | |
| Summer | 0.10 ± 0.00 b | 0.11 ± 0.01 bc | 0.98 ± 0.03 c | 0.56 ± 0.01 g | 3.38 ± 0.04 e | >10 | |
| Autumn | 0.29 ± 0.02 e | 0.25 ± 0.03 e | 1.67 ± 0.13 e | 0.12 ± 0.01 ab | 2.46 ± 0.20 d | >10 | |
| (C): Ria Formosa | Winter | 0.22 ± 0.01 d | 0.12 ± 0.01 c | 0.16 ± 0.01 a | 0.64 ± 0.05 h | 4.73 ± 0.09 g | >10 |
| Spring | 0.05 ± 0.00 a | 0.07 ± 0.00 ab | 0.18 ± 0.01 a | 0.21 ± 0.02 c | 1.84 ± 0.08 b | >10 | |
| Summer | 0.09 ± 0.00 ab | 0.05 ± 0.00 a | 1.25 ± 0.02 d | 0.44 ± 0.01 ef | 2.51 ± 0.04 d | >10 | |
| Autumn | 0.18 ± 0.01 c | 0.68 ± 0.02 g | 1.66 ± 0.09 e | 0.10 ± 0.00 a | 2.37 ± 0.14 cd | >10 | |
| Positive controls | BHA | 0.60 ± 0.03 f | 0.33 ± 0.02 f | 0.16 ± 0.01 abc | |||
| EDTA | 0.16 ± 0.00 a | 0.03 ± 0.00 a | |||||
| Asc. acid | 1.71 ± 0.02 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.G.; Neng, N.R.; Custódio, L. From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability. Mar. Drugs 2023, 21, 436. https://doi.org/10.3390/md21080436
Pereira CG, Neng NR, Custódio L. From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability. Marine Drugs. 2023; 21(8):436. https://doi.org/10.3390/md21080436
Chicago/Turabian StylePereira, Catarina Guerreiro, Nuno R. Neng, and Luísa Custódio. 2023. "From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability" Marine Drugs 21, no. 8: 436. https://doi.org/10.3390/md21080436
APA StylePereira, C. G., Neng, N. R., & Custódio, L. (2023). From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability. Marine Drugs, 21(8), 436. https://doi.org/10.3390/md21080436








