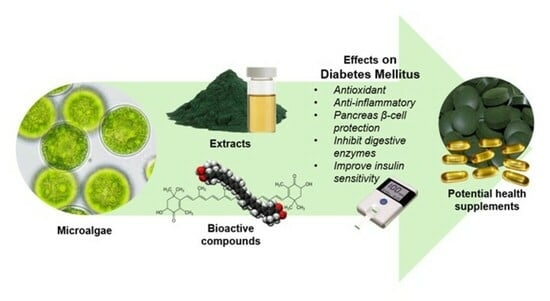
Therapeutic Potentials of Microalgae and Their Bioactive Compounds on Diabetes Mellitus
Abstract
:1. Introduction
2. Effect of Microalgae on Diabetes Mellitus
2.1. In Vitro Study
2.1.1. Microalgae Extracts
2.1.2. Protein Hydrolysate
2.1.3. Oxo-Fatty Acids
2.2. In Vivo Studies
2.2.1. Arthrospira platensis
2.2.2. Nannochloropsis oculata
2.2.3. Nannochloropsis gaditana
2.2.4. Chlorella pyrenoidosa
2.2.5. Porphyridium cruentum
2.2.6. Dunaliella salina
2.2.7. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA)
2.2.8. Polysaccharides
2.2.9. Astaxanthin
2.3. Human Studies
Microalgae Extract
3. Limitations
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmet, P.; George Alberti, K.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the Anti-Inflammatory and Antioxidant Effects of the Microalgae Nannochloropsis Gaditana in Streptozotocin-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Hassan, M.R.; Jamhari, M.N.; Hayati, F.; Ahmad, N.; Zamzuri, M.A.I.A.; Nawi, A.M.; Sharif, K.Y.; Sufri, M.; Ahmad, S.B.; Ismail, N.; et al. Determinants of Glycaemic Control among Type 2 Diabetes Mellitus Patients in Northern State of Kedah, Malaysia: A Cross-Sectional Analysis of 5 Years National Diabetes Registry 2014–2018. Pan Afr. Med. J. 2021, 39, 206. [Google Scholar] [CrossRef]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The Pathogenesis and Pathophysiology of Type 1 and Type 2 Diabetes Mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Amsah, N.; Isa, M.Z.; Kassim, Z. Poor Glycaemic Control and Its Associated Factors among Type 2 Diabetes Mellitus Patients in Southern Part of Peninsular Malaysia: A Registry-Based Study. Open Access Maced. J. Med. Sci. 2022, 10, 422–427. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Hazmatulhaq, F.; Gumilar, G.G.; Pratiwi, R.N.; Kurniawan, I.; Ningrum, A.; Hidayati, N.A.; Koyande, A.K.; Kumar, P.S.; Show, P.L. Microalgae as a Potential Sustainable Solution to Environment Health. Chemosphere 2022, 295, 133740. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Grant, I.D.; Ozanne, S.E.; Reynolds, R.M.; Aiken, C.E. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021, 12, 1901–1914. [Google Scholar] [CrossRef]
- Lambrinou, E.; Hansen, T.B.; Beulens, J.W. Lifestyle Factors, Self-Management and Patient Empowerment in Diabetes Care. Eur. J. Prev. Cardiol. 2019, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Khandelwal, D.; Lal, P.R.; Gupta, Y.; Kalra, S.; Dutta, D. Factors Determining the Success of Therapeutic Lifestyle Interventions in Diabetes—Role of Partner and Family Support. Eur. Endocrinol. 2019, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Wai, S.T.C.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; San Cheang, W.; Liu, B.; Carpéné, C.; et al. Regulation of Glucose Metabolism by Bioactive Phytochemicals for the Management of Type 2 Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2018, 59, 830–847. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Desjardins, S.M.; Senhorinho, G.N.A.; Scott, J.A. Harvesting Microalgae for Health Beneficial Dietary Supplements. Algal Res. 2021, 54, 102189. [Google Scholar] [CrossRef]
- Ibrahim, T.N.B.T.; Feisal, N.A.S.; Kamaludin, N.H.; Cheah, W.Y.; How, V.; Bhatnagar, A.; Ma, Z.; Show, P.L. Biological Active Metabolites from Microalgae for Healthcare and Pharmaceutical Industries: A Comprehensive Review. Bioresour. Technol. 2023, 372, 128661. [Google Scholar] [CrossRef] [PubMed]
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; de Carvalho, J.C.M.; Danesi, E.D.G. Microalgae Trends toward Functional Staple Food Incorporation: Sustainable Alternative for Human Health Improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as Source of Functional Ingredients in New-Generation Foods: Challenges, Technological Effects, Biological Activity, and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Rajauria, G.; Yuan, Y.V. Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Wiley Blackwell: Hoboken, NJ, USA, 2021; ISBN 9781119542582. [Google Scholar]
- Krohn, I.; Menanteau-Ledouble, S.; Hageskal, G.; Astafyeva, Y.; Jouannais, P.; Nielsen, J.L.; Pizzol, M.; Wentzel, A.; Streit, W.R. Health Benefits of Microalgae and Their Microbiomes. Microb. Biotechnol. 2022, 15, 1966–1983. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive Peptides from Microalgae: Focus on Anti-Cancer and Immunomodulating Activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of Green Process through Microwave-Assisted Extraction of Bioactive Metabolites from Arthrospira Platensis and Bioactivity Evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.F.; García-Alamilla, P.; Palma-Ramírez, D.; Álvarez-González, C.A.; Paredes-Rojas, J.C.; Márquez-Rocha, F.J. Continuous Microalgal Cultivation for Antioxidants Production. Molecules 2020, 25, 4171. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Swick, J.; Ronnenberg, A.G. Vitamin E and Adiponectin: Proposed Mechanism for Vitamin E-Induced Improvement in Insulin Sensitivity. Nutr. Rev. 2011, 69, 155–161. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Millao, S.; Uquiche, E. Antioxidant Activity of Supercritical Extracts from Nannochloropsis Gaditana: Correlation with Its Content of Carotenoids and Tocopherols. J. Supercrit. Fluids 2016, 111, 143–150. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira Platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of Microalgal Biomass Profiles as Novel Functional Ingredient for Food Products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and Other Nutrients from Haematococcus Pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G.; Noroozi, M. Manipulation of Chlorella Vulgaris Polyunsaturated ω-3 Fatty Acid Profile by Supplementation with Vegetable Amino Acids and Fatty Acids. Phycol. Res. 2021, 69, 116–123. [Google Scholar] [CrossRef]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic Acid, Glycemic Control and Type 2 Diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30–33. [Google Scholar] [CrossRef]
- Hamilton, J.S.; Klett, E.L. Linoleic Acid and the Regulation of Glucose Homeostasis: A Review of the Evidence. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef] [PubMed]
- Gkioni, M.D.; Andriopoulos, V.; Koutra, E.; Hatziantoniou, S.; Kornaros, M.; Lamari, F.N. Ultrasound-Assisted Extraction of Nannochloropsis Oculata with Ethanol and Betaine: 1,2-Propanediol Eutectic Solvent for Antioxidant Pigment-Rich Extracts Retaining Nutritious the Residual Biomass. Antioxidants 2022, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Vallejo, R.A.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis Gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. [Google Scholar] [CrossRef]
- Hu, H.; Wang, H.F.; Ma, L.L.; Shen, X.F.; Zeng, R.J. Effects of Nitrogen and Phosphorous Stress on the Formation of High Value LC-PUFAs in Porphyridium Cruentum. Appl. Microbiol. Biotechnol. 2018, 102, 5763–5773. [Google Scholar] [CrossRef]
- Guil-Gucrrcro, J.L.; Belarbi, E.H.; Rebolloso-Fuentes, M.M. Eicosapentaenoic and Arachidonic Acids Purification from the Red Microalga Porphyridium Cruentum. Bioseparation 2000, 9, 299–306. [Google Scholar] [CrossRef]
- Pantami, H.A.; Bustamam, M.S.A.; Lee, S.Y.; Ismail, I.S.; Faudzi, S.M.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella Vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Amelioration of Streptozotocin-Induced Type 2 Diabetes Mellitus in Wistar Rats by Arachidonic Acid. Biochem. Biophys. Res. Commun. 2018, 496, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Suresh, Y.; Das, U.N. Protective Action of Arachidonic Acid against Alloxan-Induced Cytotoxicity and Diabetes Mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2001, 64, 37–52. [Google Scholar] [CrossRef]
- Sarbolouki, S.; Javanbakht, M.H.; Derakhshanian, H.; Hosseinzadeh, P.; Zareei, M.; Hashemi, S.B.; Dorosty, A.R.; Eshraghian, M.R.; Djalali, M. Eicosapentaenoic Acid Improves Insulin Sensitivity and Blood Sugar in Overweight Type 2 Diabetes Mellitus Patients: A Double-Blind Randomised Clinical Trial. Singap. Med. J. 2013, 54, 387–390. [Google Scholar] [CrossRef]
- Wan, X.; Li, T.; Zhong, R.; Chen, H.; Xia, X.; Gao, L.; Gao, X.; Liu, B.; Zhang, H.; Zhao, C. Anti-Diabetic Activity of PUFAs-Rich Extracts of Chlorella Pyrenoidosa and Spirulina Platensis in Rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, R.; Yao, Q.; Xu, Y.; Luo, Z.; Luo, X.; Wang, N. Docosahexaenoic Acid Attenuates Adipose Tissue Angiogenesis and Insulin Resistance in High Fat Diet-Fed Middle-Aged Mice via a Sirt1-Dependent Mechanism. Mol. Nutr. Food Res. 2016, 60, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Ashraf ElFar, O.; Billa, N.; Ren Lim, H.; Wayne Chew, K.; Yan Cheah, W.; Siti Halimatul Munawaroh, H.; Balakrishnan, D.; Loke, P. Advances in Delivery Methods of Arthrospira Platensis (Spirulina) for Enhanced Therapeutic Outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic Potential of Phycocyanin: Effects on KKAy Mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, F.; Li, Q.; Yang, Q.; Zhang, W. Phycocyanin Protects against High Glucose High Fat Diet Induced Diabetes in Mice and Participates in AKT and AMPK Signaling. Foods 2022, 11, 3183. [Google Scholar] [CrossRef]
- Ou, Y.; Ren, Z.; Wang, J.; Yang, X. Phycocyanin Ameliorates Alloxan-Induced Diabetes Mellitus in Mice: Involved in Insulin Signaling Pathway and GK Expression. Chem. Biol. Interact. 2016, 247, 49–54. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Xiang, W.; Wu, H.; Wang, N.; Wu, J.; Li, T. Comparison of Production and Fluorescence Characteristics of Phycoerythrin from Three Strains of Porphyridium. Foods 2022, 11, 2069. [Google Scholar] [CrossRef]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of Diabetic Complications by C-Phycoerythrin in Rats: Antioxidant Activity of C-Phycoerythrin Including Copper-Induced Lipoprotein and Serum Oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Molecules Impact of Different Storage Methods on Bioactive Compounds in Arthrospira Platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Ennaji, H.; Bourhia, M.; Taouam, I.; Falaq, A.; Bellahcen, T.O.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Ullah, R.; Ibenmoussa, S.; et al. Physicochemical Evaluation of Edible Cyanobacterium Arthrospira Platensis Collected from the South Atlantic Coast of Morocco: A Promising Source of Dietary Supplements. Evid.-Based Complement. Altern. Med. 2021, 2021, 3337231. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Del Prete, F.; Aquino, R.P.; Campiglia, P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of Their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin Protects Beta-Cells against Glucose Toxicity in Diabetic Db/Db Mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Magoulas, K.; Voutsas, E. Experimental Design and Optimization of Recovering Bioactive Compounds from Chlorella Vulgaris through Conventional Extraction. Molecules 2022, 27, 29. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction and Characterization of Bioactive Compounds from Haematococcus Pluvialis Microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef]
- Todorović, B.; Grujić, V.J.; Krajnc, A.U.; Kranvogl, R.; Ambrožič-Dolinšek, J. Identification and Content of Astaxanthin and Its Esters from Microalgae Haematococcus Pluvialis by HPLC-DAD and LC-QTOF-MS after Extraction with Various Solvents. Plants 2021, 10, 2413. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Total Lipids Content, Lipid Class and Fatty Acid Composition of Ten Species of Microalgae. J Oleo Sci 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Tsou, Y.C.; Chen, Y.T.; Lu, W.J.; Hwang, P.A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The Hypoglycemic, Hypolipidemic, and Anti-Diabetic Nephritic Activities of Zeaxanthin in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955. [Google Scholar] [CrossRef]
- Juin, C.; Bonnet, A.; Nicolau, E.; Bérard, J.B.; Devillers, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. UPLC-MSE Profiling of Phytoplankton Metabolites: Application to the Identification of Pigments and Structural Analysis of Metabolites in Porphyridium Purpureum. Mar. Drugs 2015, 13, 2541. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved High Performance Liquid Chromatographic Method for Determination of Carotenoids in the Microalga Chlorella Pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Vaz, B.d.S.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Fang, M.; Zheng, J.H.; Ren, D.F.; Lu, J. Optimised Extraction of β-Carotene from Spirulina Platensis and Hypoglycaemic Effect in Streptozotocin-Induced Diabetic Mice. J. Sci. Food Agric. 2016, 96, 1783–1789. [Google Scholar] [CrossRef]
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro-Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective Potential of Phycobiliproteins Extracted from Porphyridium Cruentum. Metabolites 2023, 13, 366. [Google Scholar] [CrossRef]
- El-Adl, M.F.; Deyab, M.A.; Ghazal, M.A.; Elsadany, A.Y. Impact of the Microalga Dunaliella Salina (Dunal) Teodoresco Culture and Its β-Carotene Extract on the Development of Salt-Stressed Squash (Cucurbita pepo L. Cv. Mabrouka). Physiol. Mol. Biol. Plants 2022, 28, 749. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Shen, S.; Ji, X.; Chen, F.; Liao, X.; Zhang, H.; Zhang, Y. Inhibitory Effects of Chlorophylls and Its Derivative on Starch Digestion in Vitro. Food Chem. 2023, 413, 135377. [Google Scholar] [CrossRef]
- Semaan, D.G.; Igoli, J.O.; Young, L.; Gray, A.I.; Rowan, E.G.; Marrero, E. In Vitro Anti-Diabetic Effect of Flavonoids and Pheophytins from Allophylus Cominia Sw. on the Glucose Uptake Assays by HepG2, L6, 3T3-L1 and Fat Accumulation in 3T3-L1 Adipocytes. J. Ethnopharmacol. 2018, 216, 8–17. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.J.; Han, J.S. Pheophorbide A from Gelidium Amansii Improves Postprandial Hyperglycemia in Diabetic Mice through α-Glucosidase Inhibition. Phytother. Res. 2019, 33, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Higuchi, O.; Sasaki, M.; Ota, M.; Aida, T.; Takekoshi, H.; Inomata, H.; Miyazawa, T. Removal of Chlorophyll and Pheophorbide from Chlorella Pyrenoidosa by Supercritical Fluid Extraction: Potential of Protein Resource. Biosci. Biotechnol. Biochem. 2021, 85, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of Caffeine and Lycopene in Experimentally Induced Diabetes Mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef]
- Sharavana, G.; Joseph, G.S.; Baskaran, V. Lutein Attenuates Oxidative Stress Markers and Ameliorates Glucose Homeostasis through Polyol Pathway in Heart and Kidney of STZ-Induced Hyperglycemic Rat Model. Eur. J. Nutr. 2017, 56, 2475–2485. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Martín-Pozuelo, G.; González-Barrio, R.; Navarro-González, I.; Pallarés, F.J.; Santaella, M.; García-Alonso, J.; Sevilla, Á.; Periago-Castón, M.J. Ameliorative Effect of Spinach on Non-Alcoholic Fatty Liver Disease Induced in Rats by a High-Fat Diet. Int. J. Mol. Sci. 2019, 20, 1662. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.; Wang, C.; Zhu, Z.; Yu, L.; Yang, W.; Ren, X.; Liu, X. Characterization of Selenium-Containing Polysaccharide from Spirulina Platensis and Its Protective Role against Cd-Induced Toxicity. Int. J. Biol. Macromol. 2020, 164, 2465–2476. [Google Scholar] [CrossRef]
- Li, T.T.; Huang, Z.R.; Jia, R.B.; Lv, X.C.; Zhao, C.; Liu, B. Spirulina Platensis Polysaccharides Attenuate Lipid and Carbohydrate Metabolism Disorder in High-Sucrose and High-Fat Diet-Fed Rats in Association with Intestinal Microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Setyaningsih, I.; Prasetyo, H.; Agungpriyono, D.R.; Tarman, K. Antihyperglycemic Activity of Porphyridium Cruentum Biomass and Extra-Cellular Polysaccharide in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Macromol. 2020, 156, 1381–1386. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Sun, L.; Gao, Y. Characterization and Anti-Diabetic Evaluation of Sulfated Polysaccharide from Spirulina Platensis. J. Funct. Foods 2022, 95, 105155. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium Cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Wu, L.; Tong, A.; Zhao, L.; Liu, B.; Zhao, C. Physicochemical Characterization of Polysaccharides from Chlorella Pyrenoidosa and Its Anti-Ageing Effects in Drosophila Melanogaster. Carbohydr. Polym. 2018, 185, 120–126. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Collins, C.; Saha, S.K.; Major, I.; Murray, P. Sustainable Production and Pharmaceutical Applications of β-Glucan from Microbial Sources. Microbiol. Res. 2023, 274, 127424. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-Glucans in the Treatment of Diabetes and Associated Cardiovascular Risks. Vasc. Health Risk Manag. 2008, 4, 1265. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.J.; D’Souza, P.P.; Fazal, F.; Kumar, A.; Bhat, H.P.; Baliga, M.S. Anti-Diabetic Effects of the Indian Indigenous Fruit Emblica Officinalis Gaertn: Active Constituents and Modes of Action. Food Funct. 2014, 5, 635–644. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmed, Z.A.; Mahwi, T.O.; Aziz, T.A. Effect of Quercetin on Postprandial Glucose Excursion after Mono- and Disaccharides Challenge in Normal and Diabetic Rats. J. Diabetes Mellit. 2012, 2012, 82–87. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef]
- Dhanya, R.; Kartha, C.C. Quercetin Improves Oxidative Stress-Induced Pancreatic Beta Cell Alterations via MTOR-Signaling. Mol. Cell. Biochem. 2021, 476, 3879–3887. [Google Scholar] [CrossRef]
- Bellahcen, T.O.; Aamiri, A.; Touam, I.; Hmimid, F.; El Amrani, A.; Cherif, A.; Cherki, M. Evaluation of Moroccan Microalgae: Spirulina platensis as a Potential Source of Natural Antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Q.M.; Hu, H.J.; Yang, L.; Yang, Y.B.; Chou, G.X.; Wang, Z.T. Bioactive Diterpenoids and Flavonoids from the Aerial Parts of Scoparia Dulcis. J. Nat. Prod. 2014, 77, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.C.; Pizzolatti, M.G.; Silva, F.R.M.B. Mechanism of Action of the Stimulatory Effect of Apigenin-6-C-(2′-O-Alpha-l-Rhamnopyranosyl)-Beta-L-Fucopyranoside on 14C-Glucose Uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Cho, Y.Y.; Choi, M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef]
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin Attenuates Streptozotocin-Induced Pancreatic β Cell Damage by Its Protective Effects on Cellular Antioxidant Defense. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 554–563. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, X.; Sun, W.L.; Xing, Y.; Xiu, Z.L.; Zhuang, C.L.; Dong, Y.S. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Shanak, S.; Bassalat, N.; Albzoor, R.; Kadan, S.; Zaid, H. In Vitro and In Silico Evaluation for the Inhibitory Action of O. basilicum Methanol Extract on α-Glucosidase and α-Amylase. Evid.-Based Complement. Altern. Med. 2021, 2021, 5515775. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Tibullo, D.; Godos, J.; Pluchinotta, F.R.; Di Giacomo, C.; Sorrenti, V.; Acquaviva, R.; Russo, A.; Li Volti, G.; Barbagallo, I. Caffeic Acid Phenethyl Ester Regulates PPAR’s Levels in Stem Cells-Derived Adipocytes. PPAR Res. 2016, 2016, 7359521. [Google Scholar] [CrossRef]
- Un, J.J.; Lee, M.K.; Yong, B.P.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in Db/Db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and Antihyperlipidemic Activity of P-Coumaric Acid in Diabetic Rats, Role of Pancreatic GLUT 2: In Vivo Approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of Hyperglycemia and Dyslipidemia in Experimental Type 2 Diabetes by Gallic Acid and P-Coumaric Acid: The Role of Adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition Mechanism of Ferulic Acid against α-Amylase and α-Glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic Acid Exerts Its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Zhang, N.; Ji, Z.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Ferulic Acid Attenuates Diabetes-Induced Cognitive Impairment in Rats via Regulation of PTP1B and Insulin Signaling Pathway. Physiol. Behav. 2017, 182, 93–100. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H. Ferulic Acid Confers Protection on Islet β Cells and Placental Tissues of Rats with Gestational Diabetes Mellitus. Cell. Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. Biomed. Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative Effect of Kaempferol, a Flavonoid, on Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D. Flavonol Kaempferol Improves Chronic Hyperglycemia-Impaired Pancreatic Beta-Cell Viability and Insulin Secretory Function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, W.; Maechler, P.; Liu, D. Small Molecule Kaempferol Modulates PDX-1 Protein Expression and Subsequently Promotes Pancreatic β-Cell Survival and Function via CREB. J. Nutr. Biochem. 2013, 24, 638–646. [Google Scholar] [CrossRef]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, H.Y.; Shih, C.C. Tormentic Acid, a Major Component of Suspension Cells of Eriobotrya Japonica, Suppresses High-Fat Diet-Induced Diabetes and Hyperlipidemia by Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic Acid Reduces Blood Glucose in KK-Ay Mice. Biol. Pharm. Bull. 2007, 30, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Chen, Y.P.; Mao, L.F.; Shang, J.; Sun, H.B.; Zhang, L.Y. Maslinic Acid Modulates Glycogen Metabolism by Enhancing the Insulin Signaling Pathway and Inhibiting Glycogen Phosphorylase. Chin. J. Nat. Med. 2014, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory Effect of Corosolic Acid on α-Glucosidase: Kinetics, Interaction Mechanism, and Molecular Simulation. J. Sci. Food Agric. 2019, 99, 5881–5889. [Google Scholar] [CrossRef]
- Miura, T.; Ueda, N.; Yamada, K.; Fukushima, M.; Ishida, T.; Kaneko, T.; Matsuyama, F.; Seino, Y. Antidiabetic Effects of Corosolic Acid in KK-Ay Diabetic Mice. Biol. Pharm. Bull. 2006, 29, 585–587. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia Indica. BioMed Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef]
- Gomes Castro, A.J.; Silva Frederico, M.J.; Cazarolli, L.H.; Bretanha, L.C.; Tavares, L.D.C.; Buss, Z.D.S.; Dutra, M.F.; Pacheco De Souza, A.Z.; Pizzolatti, M.G.; Silva, F.R.M.B. Betulinic Acid and 1,25(OH)2 Vitamin D₃ Share Intracellular Signal Transduction in Glucose Homeostasis in Soleus Muscle. Int. J. Biochem. Cell Biol. 2014, 48, 18–27. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.R.; Fonseca, S.G.C.; Bizerra, A.M.C.; Lemos, T.L.G.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic Acid, a Natural Triterpenoid Improves Blood Glucose Tolerance in Normal Mice and Ameliorates Visceral Obesity in Mice Fed a High-Fat Diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef]
- Salah El Dine, R.; Ma, Q.; Kandil, Z.A.; El-Halawany, A.M. Triterpenes as Uncompetitive Inhibitors of α-Glucosidase from Flowers of Punica Granatum L. Nat. Prod. Res. 2014, 28, 2191–2194. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic Acid Improves Hepatic Insulin Resistance via Antioxidant, Hypolipidemic and Anti-Inflammatory Effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic Acid Enhances Insulin Secretion in Pancreatic Beta-Cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef]
- Nataraju, A.; Saini, D.; Ramachandran, S.; Benshoff, N.; Liu, W.; Chapman, W.; Mohanakumar, T. Oleanolic Acid, a Plant Triterpenoid, Significantly Improves Survival and Function of Islet Allograft. Transplantation 2009, 88, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Etsassala, N.G.E.R.; Badmus, J.A.; Waryo, T.T.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A.; Iwuoha, E.I. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia Africana-Lutea. Antioxidants 2019, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic Acid Enhances the Cellular Immune System and Pancreatic Beta-Cell Function in Streptozotocin-Induced Diabetic Mice Fed a High-Fat Diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Villaró, S.; Jiménez-Márquez, S.; Musari, E.; Bermejo, R.; Lafarga, T. Production of Enzymatic Hydrolysates with in Vitro Antioxidant, Antihypertensive, and Antidiabetic Properties from Proteins Derived from Arthrospira Platensis. Food Res. Int. 2023, 163, 112270. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella Vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Fassi, E.M.A.; Boschin, G.; Bartolomei, M.; Bollati, C.; Roda, G.; Arnoldi, A.; Grazioso, G.; Lammi, C. Investigation of Chlorella Pyrenoidosa Protein as a Source of Novel Angiotensin I-Converting Enzyme (ACE) and Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides. Nutrients 2021, 13, 1624. [Google Scholar] [CrossRef]
- He, W.; Xie, J.; Xia, Z.; Chen, X.; Xiao, J.; Cao, Y.; Liu, X. A Novel Peptide Derived from Haematococcus Pluvialis Residue Exhibits Anti-Aging Activity in Caenorhabditis Elegans via the Insulin/IGF-1 Signaling Pathway. Food Funct. 2023, 14, 5576–5588. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Ai, W.; Zhang, F.; Yang, K.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; Jiang, Q.; et al. Phytol Increases Adipocyte Number and Glucose Tolerance through Activation of PI3K/Akt Signaling Pathway in Mice Fed High-Fat and High-Fructose Diet. Biochem. Biophys. Res. Commun. 2017, 489, 432–438. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Sæther, T.; Uhlig, S.; Nebb, H.I.; Larsen, T.; Eilertsen, H.C.; Paulsen, S.M. Two Isomeric C16 Oxo-Fatty Acids from the Diatom Chaetoceros Karianus Show Dual Agonist Activity towards Human Peroxisome Proliferator-Activated Receptors (PPARs) α/γ. Mar. Drugs 2017, 15, 148. [Google Scholar] [CrossRef]
- Sæther, T.; Paulsen, S.M.; Tungen, J.E.; Vik, A.; Aursnes, M.; Holen, T.; Hansen, T.V.; Nebb, H.I. Synthesis and Biological Evaluations of Marine Oxohexadecenoic Acids: PPARα/γ Dual Agonism and Anti-Diabetic Target Gene Effects. Eur. J. Med. Chem. 2018, 155, 736–753. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kim, Y.I.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-Oxo-12(Z)-Octadecenoic Acid, a Linoleic Acid Metabolite Produced by Gut Lactic Acid Bacteria, Potently Activates PPARγ and Stimulates Adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Gradíssimo, D.G.; da Silva, V.C.O.; Xavier, L.P.; Do Nascimento, S.V.; Valadares, R.B.d.S.; Faustino, S.M.M.; Schneider, M.P.C.; Santos, A.V. Glucosidase Inhibitors Screening in Microalgae and Cyanobacteria Isolated from the Amazon and Proteomic Analysis of Inhibitor Producing Synechococcus sp. GFB01. Microorganisms 2021, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory Activities of Microalgal Fucoxanthin against α-Amylase, α-Glucosidase, and Glucose Oxidase in 3T3-L1 Cells Linked to Type 2 Diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Joventino, I.P.; Alves, H.G.R.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.A.M.; Neves, S.A.; Ferreira, F.V.; Brito, G.A.C.; Viana, G.B. The Microalga Spirulina Platensis Presents Anti-Inflammatory Action as Well as Hypoglycemic and Hypolipidemic Properties in Diabetic Rats. J. Complement. Integr. Med. 2012, 9, 17. [Google Scholar] [CrossRef]
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the Role of Myeloperoxidase and Angiopoietin-like Protein 6 in Obesity and Diabetes. Sci. Rep. 2020, 10, 6170. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyande, A.K.; et al. In-Vitro Molecular Docking Analysis of Microalgae Extracted Phycocyanin as an Anti-Diabetic Candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Bioactive Polysaccharides and Their Derivatives from Microalgae: Biosynthesis, Applications, and Challenges. Stud. Nat. Prod. Chem. 2021, 71, 67–85. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Kim, Y.; Kim, E.Y. Effect of DPP-IV Inhibitors on Glycemic Variability in Patients with T2DM: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 13296. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Jadhav, P.B.; Jadhav, S.B.; Zehravi, M.; Mubarak, M.S.; Islam, F.; Jeandet, P.; Khan, S.L.; Hossain, N.; Rashid, S.; Ming, L.C.; et al. Virtual Screening, Synthesis, and Biological Evaluation of Some Carbohydrazide Derivatives as Potential DPP-IV Inhibitors. Molecules 2023, 28, 149. [Google Scholar] [CrossRef]
- Chong, S.C.; Sukor, N.; Robert, S.A.; Ng, K.F.; Kamaruddin, N.A. Endogenous GLP-1 Levels Play an Important Role in Determining the Efficacy of DPP-IV Inhibitors in Both Prediabetes and Type 2 Diabetes. Front. Endocrinol. 2022, 13, 1012412. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Amodeo, P.; Vitale, R.M. Marine Natural and Nature-Inspired Compounds Targeting Peroxisome Proliferator Activated Receptors (PPARs). Mar. Drugs. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish Oil Supplementation and Insulin Sensitivity: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Batten, K.; Huffman, K.E.; Liu, J.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef]
- Kersten, S. Role and Mechanism of the Action of Angiopoietin-like Protein ANGPTL4 in Plasma Lipid Metabolism. J. Lipid Res. 2021, 62, 100150. [Google Scholar] [CrossRef]
- Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Boo, R.L.C.; Xu, J.Y.; Chen, B.; Chow, W.S.; Tso, A.W.K.; et al. Angiopoietin-like Protein 4 Decreases Blood Glucose and Improves Glucose Tolerance but Induces Hyperlipidemia and Hepatic Steatosis in Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. [Google Scholar] [CrossRef]
- Lagathu, C.; Yvan-Charvet, L.; Bastard, J.P.; Maachi, M.; Quignard-Boulangé, A.; Capeau, J.; Caron, M. Long-Term Treatment with Interleukin-1β Induces Insulin Resistance in Murine and Human Adipocytes. Diabetologia 2006, 49, 2162–2173. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, A.; Khaleghian, A.; Mousavi-Niri, N.; Moradikor, N. The Effects of Supplementation of Nannochloropsis Oculata Microalgae on Biochemical, Inflammatory and Antioxidant Responses in Diabetic Rats. Biomol. Concepts 2022, 13, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Gao, X.; Chen, R.; Lu, S.; Wan, X.; Farag, M.A.; Zhao, C. Metabolomics and Biochemical Insights on the Regulation of Aging-Related Diabetes by a Low-Molecular-Weight Polysaccharide from Green Microalga Chlorella Pyrenoidosa. Food Chem. X 2022, 14, 100316. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Valdés-Ramos, R. Effect on Adipose Tissue of Diabetic Mice Supplemented with N-3 Fatty Acids Extracted from Microalgae. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, F.; Sarir, H.; Moradi-Kor, N. Antihyperglycemic and Antihyperlipidemic Activities of Nannochloropsis Oculata Microalgae in Streptozotocin-Induced Diabetic Rats. Biomol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina Platensis Microalgae on Antioxidant and Anti-Inflammatory Factors in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2018, 11, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Arciniega-Martínez, I.M.; Escoto-Herrera, J.A.; Rosales-Gómez, C.A.; Valdés-Ramos, R. Effect of Supplementation with N-3 Fatty Acids Extracted from Microalgae on Inflammation Biomarkers from Two Different Strains of Mice. J. Lipids 2018, 2018, 4765358. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina Platensis Prevents Hyperglycemia in Rats by Modulating Gluconeogenesis and Apoptosis via Modification of Oxidative Stress and MAPK-Pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Ruperez, F.J.; Garcia-Martinez, D.; Baena, B.; Maeso, N.; Vallejo, M.; Angulo, S.; Garcia, A.; Ibañez, E.; Señorans, F.J.; Cifuentes, A.; et al. Dunaliella Salina Extract Effect on Diabetic Rats: Metabolic Fingerprinting and Target Metabolite Analysis. J. Pharm. Biomed. Anal. 2009, 49, 786–792. [Google Scholar] [CrossRef]
- Bitam, A.; Aissaoui, O. Spirulina Platensis, Oxidative Stress, and Diabetes; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128157763. [Google Scholar]
- Bohórquez-Medina, S.L.; Bohórquez-Medina, A.L.; Benites Zapata, V.A.; Ignacio-Cconchoy, F.L.; Toro-Huamanchumo, C.J.; Bendezu-Quispe, G.; Pacheco-Mendoza, J.; Hernandez, A.V. Impact of Spirulina Supplementation on Obesity-Related Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. NFS J. 2021, 25, 21–30. [Google Scholar] [CrossRef]
- Godavari, A.; Manickamoorthi, N. Role of Micronutrients and Trace Elements in Diabetes Mellitus: A Review. In Structure and Health Effects of Natural Products on Diabetes Mellitus; Springer: Singapore, 2021; pp. 297–307. [Google Scholar] [CrossRef]
- Akhuemokhan, I.K.; Eregie, A.; Fasanmade, O.A. Diabetes Prevention and Management: The Role of Trace Minerals. Afr. J. Diabetes Med. 2013, 21, 37–41. [Google Scholar]
- Battin, E.E.; Brumaghim, J.L. Antioxidant Activity of Sulfur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase, and Metal-Binding Antioxidant Mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Chen, M.D.; Liou, S.J.; Lin, P.I.Y.; Yang, V.C.; Alexander, P.S.; Lin, W.H. Effects of Zinc Supplementation on the Plasma Glucose Level and Insulin Activity in Genetically Obese (Ob/Ob) Mice. Biol. Trace Elem. Res. 1998, 61, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nazem, M.R.; Asadi, M.; Adelipour, M.; Jabbari, N.; Allameh, A. Zinc Supplementation Ameliorates Type 2 Diabetes Markers through the Enhancement of Total Antioxidant Capacity in Overweight Patients. Postgrad. Med. J. 2022, 99, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Viktorínová, A.; Tošerová, E.; Križko, M.; Ďuračková, Z. Altered Metabolism of Copper, Zinc, and Magnesium Is Associated with Increased Levels of Glycated Hemoglobin in Patients with Diabetes Mellitus. Metabolism 2009, 58, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zhang, X.H.; Russell, J.C.; Hulver, M.; Cefalu, W.T. Chromium Picolinate Enhances Skeletal Muscle Cellular Insulin Signaling In Vivo in Obese, Insulin-Resistant JCR:LA-Cp Rats. J. Nutr. 2006, 136, 415–420. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.S. Diabetes, Glucose Toxicity, and Oxidative Stress: A Case of Double Jeopardy for the Pancreatic Islet β Cell. Free Radic. Biol. Med. 2006, 41, 177–184. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, Hypoglycaemic and Hypolipidaemic Effects of Bioactive Compounds from Microalgae and Marine Micro-Organisms. Int. J. Food Sci. Technol. 2015, 50, 1705–1717. [Google Scholar] [CrossRef]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate Immunity and Intestinal Microbiota in the Development of Type 1 Diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Kiesel, V.A.; Sheeley, M.P.; Coleman, M.F.; Cotul, E.K.; Donkin, S.S.; Hursting, S.D.; Wendt, M.K.; Teegarden, D. Pyruvate Carboxylase and Cancer Progression. Cancer Metab. 2021, 9, 20. [Google Scholar] [CrossRef]
- Hughey, C.C.; Crawford, P.A. Pyruvate Carboxylase Wields a Double-Edged Metabolic Sword. Cell Metab. 2019, 29, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Jörns, A.; Tiedge, M.; Lenzen, S.; Munday, R. Effect of Superoxide Dismutase, Catalase, Chelating Agents, and Free Radical Scavengers on the Toxicity of Alloxan to Isolated Pancreatic Islets in Vitro. Free Radic. Biol. Med. 1999, 26, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Production of Antioxidants and High Value Biomass from Nannochloropsis Oculata: Effects of PH, Temperature and Light Period in Batch Photobioreactors. Mar. Drugs 2022, 20, 552. [Google Scholar] [CrossRef]
- Casanova, P.; Monleon, D. Role of Selenium in Type 2 Diabetes, Insulin Resistance and Insulin Secretion. World J. Diabetes 2023, 14, 147. [Google Scholar] [CrossRef]
- Kosmalski, M.; Pękala-Wojciechowska, A.; Sut, A.; Pietras, T.; Luzak, B. Dietary Intake of Polyphenols or Polyunsaturated Fatty Acids and Its Relationship with Metabolic and Inflammatory State in Patients with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 1083. [Google Scholar] [CrossRef]
- Wu, G.; Bai, Z.; Wan, Y.; Shi, H.; Huang, X.; Nie, S. Antidiabetic Effects of Polysaccharide from Azuki Bean (Vigna angularis) in Type 2 Diabetic Rats via Insulin/PI3K/AKT Signaling Pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a Citrus Flavonoid Ameliorates Hyperglycemia by Regulating Key Enzymes of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Pan, S.; Nie, S. Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 5278–5288. [Google Scholar] [CrossRef]
- Kreilmeier-Berger, T.; Zeugswetter, F.K.; Blohm, K.O.; Schwendenwein, I.; Baszler, E.; Ploderer, B.; Burgener, I.A.; Künzel, F. Successful Insulin Glargine Treatment in Two Pet Guinea Pigs with Suspected Type 1 Diabetes Mellitus. Animals 2021, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Teng, J.; Dong, R.; Ban, Q.; Yang, L.; Du, K.; Wang, Y.; Pu, H.; Yang, C.S.; Ren, Z. Alleviating Effects and Mechanisms of Action of Large-Leaf Yellow Tea Drinking on Diabetes and Diabetic Nephropathy in Mice. Food Sci. Hum. Wellness 2023, 12, 1660–1673. [Google Scholar] [CrossRef]
- Yaas, A.A.; Al-Shakour, A.A.; Mansour, A.A. Assessment of Serum Level of Protein Carbonyl as a Marker of Protein Oxidation in Patients with Type 2 Diabetes Mellitus. AL-Kindy Coll. Med. J. 2022, 18, 190–195. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A. Streptozotocin-Induced Cytotoxicity, Oxidative Stress and Mitochondrial Dysfunction in Human Hepatoma HepG2 Cells. Int. J. Mol. Sci. 2012, 13, 5751–5767. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Kiasalari, Z.; Roghani, M. Paeonol Ameliorates Cognitive Deficits in Streptozotocin Murine Model of Sporadic Alzheimer’s Disease via Attenuation of Oxidative Stress, Inflammation, and Mitochondrial Dysfunction. J. Mol. Neurosci. 2022, 72, 336–348. [Google Scholar] [CrossRef]
- Bendaoud, A.; Baba Ahmed, F.Z.; Merzouk, H.; Bouanane, S.; Bendimerad, S. Effects of Dietary Microalgae Nannochloropsis Gaditana on Serum and Redox Status in Obese Rats Subjected to a High Fat Diet. Phytothérapie 2019, 17, 177–187. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Suárez-Montenegro, Z.J.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Anti-Inflammatory and Neuroprotective Evaluation of Diverse Microalgae Extracts Enriched in Carotenoids. Algal Res. 2022, 67, 102830. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The Role of Gut Microbiota in Obesity, Diabetes Mellitus, and Effect of Metformin: New Insights into Old Diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J.; Shah, V.O. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y.L.; Kozik, A.; Nakatsu, C. Correction: Ablation of Tumor Necrosis Factor Is Associated with Decreased Inflammation and Alterations of the Microbiota in a Mouse Model of Inflammatory Bowel Disease. PLoS ONE 2015, 10, e0125309. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Bourwis, N.; Dolan, S.; Lang, S.; Spencer, J.; Craft, J.A. Characterisation of Gut Microbiota of Obesity and Type 2 Diabetes in a Rodent. Biosci. Microbiota Food Health 2021, 40, 65–74. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated Oligosaccharides and Ferulic Acid Alter Gut Microbiome to Alleviate Diabetic Syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Wan, X.; Li, T.; Liu, D.; Chen, Y.; Liu, Y.; Liu, B.; Zhang, H.; Zhao, C. Effect of Marine Microalga Chlorella Pyrenoidosa Ethanol Extract on Lipid Metabolism and Gut Microbiota Composition in High-Fat Diet-Fed Rats. Mar. Drugs 2018, 16, 498. [Google Scholar] [CrossRef]
- Widyaningrum, D.; Prianto, A.D. Chlorella as a Source of Functional Food Ingredients: Short Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012148. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim. Biophys. Acta-Mol. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic Opportunities for Pancreatic β-Cell ER Stress in Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and Therapeutic Effects of Natural Products against Diabetes Mellitus via Regenerating Pancreatic β-Cells and Restoring Their Dysfunction. Phytother. Res. 2021, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, U.H.; Lee, S.B.; Jeong, G.T.; Kim, S.K. Improvement of Unsaturated Fatty Acid Production from Porphyridium Cruentum Using a Two-Phase Culture System in a Photobioreactor with Light-Emitting Diodes (LEDs). J. Microbiol. Biotechnol. 2021, 31, 456–463. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Ferraro, G.; Casillo, A.; Illiano, A.; Pinto, G.; Di Meo, M.C.; Alvarez-Rivera, G.; et al. Inside out Porphyridium Cruentum: Beyond the Conventional Biorefinery Concept. ACS Sustain. Chem. Eng. 2023, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Howard, C.P. Improper Insulin Compliance May Lead to Hepatomegaly and Elevated Hepatic Enzymes in Type 1 Diabetic Patients. Diabetes Care 2004, 27, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Salgıntaş, H.H.; Dönmez, N.; Özsan, M. The Effect of Curcumin on the Antioxidant System in Diabetic Rats. J. Hell. Vet. Med. Soc. 2022, 72, 3279–3284. [Google Scholar] [CrossRef]
- Arab Sadeghabadi, Z.; Abbasalipourkabir, R.; Mohseni, R.; Ziamajidi, N. Investigation of Oxidative Stress Markers and Antioxidant Enzymes Activity in Newly Diagnosed Type 2 Diabetes Patients and Healthy Subjects, Association with IL-6 Level. J. Diabetes Metab. Disord. 2019, 18, 437–443. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; Norma, M.A.; Georgiana, M.G.S.; Rosemarie, A.W.P.; Jahoor, F.; Boyne, M.S. Glutathione Metabolism in Type 2 Diabetes and Its Relationship with Microvascular Complications and Glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Zafar, H.; Mirza, I.A.; Hussain, W.; Fayyaz, M. Comparative Efficacy of Tocotrienol and Tocopherol for Their Anti Diabetic Effects Coupling And Condensation of Azo Compounds and Other Aromatic Compounds View Project Candida Auris Outbreak Report from Pakistan: A Success Story of Infection Control in Tertiary Care Hospital View Project Comparative Efficacy of Tocotrienol and Tocopherol for Their Anti Diabetic Effects. Biomed. J. Sci. Tech. Res. 2021, 38, 30835–30840. [Google Scholar] [CrossRef]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-Obesity Effects of DHA and EPA in High Fat-Induced Insulin Resistant Mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Song, X.; Tian, S.; Liu, Y.; Shan, Y. Effects of Omega-3 PUFA Supplementation on Insulin Resistance and Lipid Metabolism in Patients with T2DM: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2020, 4, 77. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine N−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The Effects of Omega-3 Fatty Acids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Mishra, N.; Gupta, E.; Singh, P. Application of Microalgae Metabolites in Food and Pharmaceutical Industry Screening of Stevia Rebaundiana, Therapeutic Benefits beyond Sweetness, by Using In Vitro Systems View Project Potential Use of Brown Algae Isochrysis Galbana in Bakery Products View Project; Academic Press: Cambridge, MA, USA, 2021; ISBN 9780128202845. [Google Scholar]
- Haimeur, A.; Mimouni, V.; Ulmann, L.; Martineau, A.S.; Messaouri, H.; Pineau-Vincent, F.; Tremblin, G.; Meskini, N. Fish Oil and Microalga Omega-3 as Dietary Supplements: A Comparative Study on Cardiovascular Risk Factors in High-Fat Fed Rats. Lipids 2016, 51, 1037–1049. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, X.; Chen, D.; Li, Z. The Controversial Role of Adiponectin in Appetite Regulation of Animals. Nutrients 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, P.; Calvo, P.; Pujol, A.; Rivera, R.; Berga, F.; Fortuny, R.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Daily Phytate Intake Increases Adiponectin Levels among Patients with Diabetes Type 2: A Randomized Crossover Trial. Nutr. Diabetes 2023, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.F.; Haines, D.; Dashti, A.A.; El-Shazly, S.; Al-Najjar, F. Correlation between Heat Shock Proteins, Adiponectin, and T Lymphocyte Cytokine Expression in Type 2 Diabetics. Cell Stress Chaperones 2018, 23, 955–965. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.E.; Dragun, D.; Skurk, T.; et al. T-Lymphocyte Infiltration in Visceral Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J.L. Carbohydrates and Dietary Fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, Isolation and Bioactive Estimation of Extracellular Polysaccharides of Green Microalga Neochloris Oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, Pancreatic Dysfunction and Type 2 Diabetes. Pancreatology 2019, 19, 617–622. [Google Scholar] [CrossRef]
- Benchoula, K.; Arya, A.; Parhar, I.S.; Hwa, W.E. FoxO1 Signaling as a Therapeutic Target for Type 2 Diabetes and Obesity. Eur. J. Pharmacol. 2021, 891, 173758. [Google Scholar] [CrossRef]
- Malhotra, K.; Katsanos, A.H.; Lambadiari, V.; Goyal, N.; Palaiodimou, L.; Kosmidou, M.; Krogias, C.; Alexandrov, A.V.; Tsivgoulis, G. GLP-1 Receptor Agonists in Diabetes for Stroke Prevention: A Systematic Review and Meta-Analysis. J. Neurol. 2020, 267, 2117–2122. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Mahmoud-Ghoneim, D.; Adeghate, E.; Al-Akhras, M.A.; Al-Saadi, M.; Al-Rahmoun, S.; Hameed, R. Pancreas-Protective Effects of Chlorella in STZ-Induced Diabetic Animal Model: Insights into the Mechanism. J. Diabetes Mellit. 2011, 1, 36–45. [Google Scholar] [CrossRef]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-Diabetic Activity of Crude Polysaccharide and Rhamnose-Enriched Polysaccharide from G. lithophila on Streptozotocin (STZ)-Induced in Wistar Rats. Sci. Rep. 2020, 10, 556. [Google Scholar] [CrossRef]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Baccari, M.C.; Vannucchi, M.G.; Idrizaj, E. Glucagon-Like Peptide-2 in the Control of Gastrointestinal Motility: Physiological Implications. Curr. Protein Pept. Sci. 2022, 23, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Feng, M.; Zhu, X.; Long, J.; Zhou, Z.; Liu, S. WR-GLP2, a Glucagon-like Peptide 2 from Hybrid Crucian Carp That Protects Intestinal Mucosal Barrier and Inhibits Bacterial Infection. Fish Shellfish Immunol. 2022, 122, 29–37. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.W. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, 2100252. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Tambat, V.S.; Chen, C.W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.Y.; Dong, C.D.; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194. [Google Scholar] [CrossRef]
- Sakayanathan, P.; Loganathan, C.; Iruthayaraj, A.; Periyasamy, P.; Poomani, K.; Periasamy, V.; Thayumanavan, P. Biological Interaction of Newly Synthesized Astaxanthin-s-Allyl Cysteine Biconjugate with Saccharomyces Cerevisiae and Mammalian α-Glucosidase: In Vitro Kinetics and in Silico Docking Analysis. Int. J. Biol. Macromol. 2018, 118, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative Assessment of Antioxidant Properties of Natural Colorants and Phytochemicals: Carotenoids, Flavonoids, Phenols and Indigoids. The Role of β-Carotene in Antioxidant Functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Kaur, K.; Sachdeva, R.; Grover, K. Effect of Supplementation of Spirulina on Blood Glucose and Lipid Profile of the Non-Insulin Dependent Diabetic Male Subjects. J. Dairy. Foods Home Sci. 2008, 27, 202–208. [Google Scholar]
- Anitha, L.; Chandralekha, K. Effect of Supplementation of Spirulina on Blood Glucose, Glycosylated Hemoglobin and Lipid Profile of Male Non-Insulin Dependent Diabetics. Asian J. Exp. Biol. Sci. 2010, 1, 36–46. [Google Scholar]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the Long-Term Effect of Spirulina Supplementation on Serum Lipid Profile and Glycated Proteins in NIDDM Patients. J. Nutraceuticals Funct. Med. Foods 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Alam, A.; Quamri, S.; Fatima, S.; Roqaiya, M.; Ahmad, Z. Efficacy of Spirulina (Tahlab) in Patients of Type 2 Diabetes Mellitus (Ziabetus Shakri)—A Randomized Controlled Trial. J. Diabetes Metab. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Beihaghi, M.; Ghodrati Azadi, H.; Taherzadeh, Z.; Bahrami, H. reza The Effects of Oral Administration of Spirulina Platensis (Cultured Iranian) on Blood Glucose and Glycosylated Hemoglobin Blood in Type Ii Diabetes Mellitus Patients. Iran. J. Diabetes Metab. 2017, 16, 183–190. [Google Scholar]
- Karizi, S.R.; Armanmehr, F.; Azadi, H.G.; Zahroodi, H.S.; Ghalibaf, A.A.M.; Bazzaz, B.S.F.; Abbaspour, M.; Boskabadi, J.; Eslami, S.; Taherzadeh, Z. A Randomized, Double-Blind Placebo-Controlled Add-on Trial to Assess the Efficacy, Safety, and Anti-Atherogenic Effect of Spirulina Platensis in Patients with Inadequately Controlled Type 2 Diabetes Mellitus. Phytother. Res. 2023, 37, 1435–1448. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Niccolai, A.; Tredici, M.R.; Biondi, N.; Rodolfi, L.; Lodovici, M.; D’Ambrosio, M.; Mori, G.; Luceri, C. Safety Evaluations and Lipid-Lowering Activity of an Arthrospira Platensis Enriched Diet: A 1-Month Study in Rats. Food Res. Int. 2017, 102, 380–386. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How Much Is Too Much? A Safety Review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira Platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Gómez-Zorita, S.; Aguirre, L.; Portillo, M.P. Effect of Microalgae and Macroalgae Extracts on Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]
- Kagan, M.L.; Matulka, R.A. Safety Assessment of the Microalgae Nannochloropsis Oculata. Toxicol. Rep. 2015, 2, 617–623. [Google Scholar] [CrossRef]
- Kagan, M.L.; Sullivan, D.W.; Gad, S.C.; Ballou, C.M. Safety Assessment of EPA-Rich Polar Lipid Oil Produced from the Microalgae Nannochloropsis Oculata. Int. J. Toxicol. 2014, 33, 459–474. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Salama, A.A.A. Toxicity Assessment of the Green Dunaliella Salina Microalgae. Toxicol. Rep. 2019, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]

| Bioactive Compounds | Microalgae Species | Activity on DM |
|---|---|---|
| Vitamins | ||
| Vitamin E (tocopherols) | A. platensis [23,24] |
|
| N. oculata [26] | ||
| N. gaditana [27] | ||
| Fatty acids | ||
| Linolenic acid | A. platensis [23,28] H. pluvialis [29,30] C. vulgaris [31] D. salina [31] | - |
| Linoleic acid | A. platensis [23,28] | |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| D. salina [36] | ||
| H. pluvialis [30] | ||
| P. cruentum [37,38] | ||
| C. vulgaris [39] | ||
| Arachidonic acid (AA) | A. platensis [23,28] | |
| N. gaditana [35] | ||
| H. pluvialis [30] | ||
| P. cruentum [37,38] | ||
| C. vulgaris [39] | ||
| Eicosapentaenoic acid (EPA) | A. platensis [23,28] | |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| C. pyrenoidosa [43] | ||
| H. pluvialis [30] | ||
| P. cruentum [37,38] | ||
| C. vulgaris [39] | ||
| Docosahexaenoic acid (DHA) | A. platensis [23,28] |
|
| C. pyrenoidosa [43] | ||
| Pigments | ||
| Phycocyanin | A. platensis [45] | |
| P. purpureum [45] | ||
| P. cruentum [49] | ||
| Allophycocyanin | A. platensis [45] | - |
| P. purpureum [45] | ||
| P. cruentum [49] | ||
| Phycoerythrin | A. platensis [45] |
|
| P. purpureum [45] | ||
| P. cruentum [49] | ||
| Astaxanthin | A. platensis [51,52,53] | |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| C. vulgaris [56] | ||
| H. pluvialis [30,57,58] | ||
| Fucoxanthin | N. oculata (Total Lipids Content, Lipid Class, and Fatty Acid Composition of Ten Species of Microalgae) [59] | |
| Antheraxanthin | A. platensis [53] | - |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| C. vulgaris [56] | ||
| Zeaxanthin | A. platensis [53] | |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| P. purpureum [62] | ||
| P. cruentum [63] | ||
| C. pyrenoidosa [64] | ||
| D. salina [65] | ||
| Auraxanthin | N. oculata [34] | - |
| C. pyrenoidosa [64] | ||
| Canthaxanthin | A. platensis [53] | - |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| C. vulgaris [65] | ||
| H. pluvialis [65] | ||
| β-cryptoxanthin | A. platensis [53] | - |
| P. purpureum | ||
| C. pyrenoidosa | ||
| H. pluvialis | ||
| Neoxanthin | N. oculata [34] | - |
| N. gaditana [35] | ||
| C. pyrenoidosa [64] | ||
| C. vulgaris [39] | ||
| Violaxanthin | N. oculata [34] | - |
| N. gaditana [35] | ||
| C. pyrenoidosa [64] | ||
| C. vulgaris [39] | ||
| H. pluvialis [58] | ||
| β-carotene | A. platensis [28,53] |
|
| N. oculata [34] | ||
| N. gaditana [35] | ||
| P. purpureum [62] | ||
| P. cruentum [67] | ||
| C. pyrenoidosa [64] | ||
| C. vulgaris [64] | ||
| D. salina [68] | ||
| H. pluvialis [58,69] | ||
| Chlorophyll | A. platensis [52] | |
| N. oculata [34] | ||
| N. gaditana [35] | ||
| P. purpureum [62] | ||
| P. cruentum [67] | ||
| C. pyrenoidosa [43] | ||
| C. vulgaris [56] | ||
| H. pluvialis [58] | ||
| Pheophytins | N. oculata [34] |
|
| P. purpureum [62] | ||
| C. vulgaris [39] | ||
| Pheophorbide | P. purpureum [62] | |
| C. pyrenoidosa [73] | ||
| C. vulgaris [39] | ||
| Lycopene | H. pluvialis [58] | |
| Lutein | A. platensis [53] | |
| N. gaditana [78] | ||
| C. vulgaris [79] | ||
| C. pyrenoidosa [64] | ||
| D. salina [65] | ||
| H. pluvialis [30,58] | ||
| Carbohydrates | ||
| Polysaccharides | A. platensis [80] | |
| P. cruentum [84] | ||
| C. pyrenoidosa [85] | ||
| C. vulgaris [65] | ||
| β-glucan | N. gaditana [86] | |
| P. purpureum [86] | ||
| Polyphenols | ||
| Gallic acid | A. platensis [51] | |
| Quercetin | A. platensis [51] | |
| N. gaditana [78] | ||
| Apigenin | A. platensis [92] | |
| Catechin | A. platensis [51] | |
| Caffeic acid | A. platensis [51] | |
| N. gaditana [78] | ||
| C. vulgaris [79] | ||
| p-Coumaric acid | A. platensis [51] | |
| C. vulgaris [79] | ||
| Ferulic acid | A. platensis [51] | |
| C. vulgaris [79] | ||
| Kaempferol | A. platensis [51] | |
| Tormentic acid | A. platensis [79] | |
| N. oculata [79] | ||
| C. vulgaris [39,79] | ||
| Neophytadiene | A. platensis [65] | - |
| Alphitolic acid | A. platensis [79] | - |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Maslinic acid | A. platensis [79] | |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Pomolic acid | A. platensis [79] | - |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Corosolic acid | A. platensis [79] | |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Betulinic acid | A. platensis [79] | |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Oleanolic acid | A. platensis [79] | |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Ursolic acid | A. platensis [79] | |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Erythrodiol | A. platensis [79] | - |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| α-Boswellic acid | A. platensis [79] | - |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Uvaol | A. platensis [79] | - |
| N. oculata [79] | ||
| P. purpureum [79] | ||
| C. vulgaris [79] | ||
| Bioactive peptides | A. platensis [126] | |
| C. vulgaris [127] | ||
| C. pyrenoidosa [128] | ||
| H. pluvialis [129] | ||
| Phytol | A. platensis [65] C. vulgaris [65] | |
| Oxohexadecenoic acids | C. karianus [131,132] | |
| Experiments | Cell Model | Experimental Groups | Finding | Mechanisms | Reference |
|---|---|---|---|---|---|
| α-amylase inhibition | - | Arthrospira platensis Nannochloropsis oculata Porphyridium purpureum Chlorella vulgaris | All species: Exhibited α-amylase inhibition (Highest in P. purpureum and N. oculata) | - | [79] |
| α- and β-glucosidase inhibition | - | Synechococcus sp. GFB01 Conduritol β-epoxide (positive control) | Exhibited high α- and β-glucosidase inhibition | - | [134] |
| DPP-IV inhibition, α-amylase, and α-glucosidase inhibition | - | Pepsin hydrolysate Ficin hydrolysate Papain hydrolysate Alcalase hydrolysate Aliskiren (positive control) | All hydrolysates: Exhibited DPP-IV inhibition (Highest in alcalase hydrolysates) Limited and α-glucosidase inhibitory capacities | - | [126] |
| PPARα/γ agonist activity, endogenous PPAR target genes activation analysis, adipocyte differentiation analysis, and adipocyte transcriptomics | COS-1 cells Huh7 cells SGBS pre-adipocyte cells | (7E)-9-OHE or (10E)-9-OHE Rosiglitazone or pirinixic acid (positive controls) Palmitic acid or DMSO (negative controls) | Exhibits PPARα/γ agonist activities Activation of fatty acid catabolism Improvement in adipocyte insulin sensitivity | ↑ CPT1A, ACSL3, PLIN1, and ANGPTL4 gene expressions ↑ Adiponectin and leptin gene expressions ↓ IL-6, TNF-α, CXCL1, CXCL5, and IL-1B gene expressions ↑ IRS1 and SLC2A4 gene expressions | [132] |
| Animal Model | Diabetes Induction | Microalgae and Doses | Experimental Design | Effects on Diabetes Mellitus | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Male Wistar rats | Drug: 55 mg/kg of BW of STZ | Nannochloropsis oculata (NOM) powder (0, 10, 20 mg/kg BW/day) for 21 days | C–H: Non-diabetic + 0 mg/kg BW/day of NOM (normal control) H-10: Non-diabetic + 10 mg/kg BW/day of NOM H-20: Non-diabetic + 20 mg/kg BW/day of NOM C–D: Diabetic + 0 mg/kg BW/day of NOM (diabetic control) D-10: Diabetic + 10 mg/kg BW/day of NOM D-20: Diabetic + 20 mg/kg BW/day of NOM | C–D, D-10, and D-20: ↑ BW ↓ Serum glucose level ↑ Serum insulin level | C–D, D-10, and D-20: ↑ Serum concentrations of GSH-Px, SOD, and FRAP ↓ MDA ↓ Tissue IL-6, NF-κB, IL-1B, and TNF-α | [153] |
| Male Kunming mice (6 weeks old) | Diet: High-glucose high-fat Drug: 45 mg/kg BW/ day of D-gal for 1 month followed by 50 mg/kg BW of STZ | Chlorella pyrenoidosa polysaccharide powder (150 and 300 mg/kg BW/day) for 4 weeks | Normal rats Diabetic rats Diabetic rats + 90 mg/kg BW/day of metformin Diabetic rats + 150 mg/kg BW/day of CPP (CPPL) Diabetic rats + 300 mg/kg BW/day of CPP (CPPH) | CPPL and CPPH: ↑ BW ↑ Insulin level CPPH: ↑ Glucose uptake | CPPL: ↓ FOXO-1 mRNA and protein expressions ↑ GLP-1R mRNA and protein expressions CPPH: ↑ Pancreas weight ↑ Glucose uptake ↑ SOD in liver ↓ MDA in liver CPPL and CPPH: ↑ CAT and GSH-Px in liver Improved pancreatic architecture ↓ IL-6R mRNA and protein expressions Co-modulation of IL-6R/FOXO-1 and GLP-1R/FOXO-1 ↓ Phenylpyruvic acid | [154] |
| Male Wistar rats (2 months old) | Drug: 45 mg/kg of BW STZ | 10% Nannochloropsis gaditana powder/day for 2 months, administered orally | C: normal rats CM: normal rats + 10% N. gaditana/day D: diabetic rats (diabetic control) DM: diabetic rats + 10% N. gaditana/day | DM: ↑ BW ↓ Serum glucose, HbA1c, TG, cholesterol | DM: ↓ IL-6 and TNF-α ↓ MDA and carbonyl proteins in liver mitochondria, and liver and pancreatic tissues ↑ CAT, GSH, and SOD in liver mitochondria and pancreatic tissues ↑ CAT, GSH, SOD, and GST in liver tissue | [2] |
| Male db/db mice (8 weeks old) | Genetically diabetic | LY: 1 mg/g lyophilized EPA+DHA/day from Chlorophyceae and Eustigamatophyceae families for 8 weeks Or MD: 2.0% microalgae EPA+DHA-enriched diet for 8 weeks (ad libitum) | RC: Normal/ diabetic strain + rodent chow LY: Normal/ diabetic strain + LY CO: Normal/ diabetic strain + coconut oil MD: Normal/ diabetic strain + MD | Normal/ diabetic strain LY and MD: No significant changes in blood glucose level | Normal LY: ↑ Total antioxidant capacity in plasma Diabetic LY: ↑ Total antioxidant capacity in adipose tissue and plasma Diabetic MD: ↓ Food intake | [155] |
| Male Sprague–Dawley rats | Drug: 40 mg/kg of BW STZ | Porphyridium cruentum powder (600, 1200, and 1800 mg/kg BW/day) for 14 days Or Porphyridium cruentum extracellular polysaccharide (150, 300, and 450 mg/kg BW/day) for 14 days | Group 1: Normal rats Group 2: Diabetic rats Group 3: Diabetic rats + 0.6 mg/kg BW/day of glibenclamide (positive control 1) Group 4: Diabetic rats + 1 mg/kg BW/day of acarbose (positive control 2) Group 5: Diabetic rats + 600 mg/kg BW/day of P. cruentum powder Group 6: Diabetic rats + 1200 mg/kg BW/day of P. cruentum powder Group 7: Diabetic rats + 1800 mg/kg BW/day of P. cruentum powder Group 8: Diabetic rats + 150 mg/kg BW/day of extracellular polysaccharide Group 9: 300 mg/kg BW/day of extracellular polysaccharide Group 10: Diabetic rats + 450 mg/kg BW/day of extracellular polysaccharide | Group 9–10: ↓ Blood glucose level | Group 5–10: ↑ Food intake ↑ Pancreatic β-cell number and granulation Group 6, 7, 9, 10: ↑ Pancreatic islets area | [82] |
| Male rats | Diet: High-fat high-sucrose chow for 8 weeks | Chlorella pyrenoidosa or Arthrospira platensis ethanol or water extracts (150 mg/kg BW/day) for 8 weeks | NFD: Control group fed normal fat diet HFHS: Control group fed high-fat high-sucrose chow CP55: HFHS + 150 mg/kg BW/day of C. pyrenoidosa ethanol extract CPWE: HFHS + 150 mg/kg BW/day of C. pyrenoidosa water extract SP55: HFHS + 150 mg/kg BW/day of A. platensis ethanol extract SPWE: HFHS + 150 mg/kg BW/day of A. platensis water extract | SPWE, CP55, and CPWE: ↓ FBG SP55, SPWE, CP55, and CPWE: Improvement in glucose tolerance | SP55, SPWE, CP55, and CPWE: Improvement in gut microbiota composition | [43] |
| Male Wistar rats | Drug: 55 mg/kg of BW STZ | Nannochloropsis oculata (0, 10, 20 mg/kg BW/day) for 3 weeks | Healthy NOM-0: Healthy rats + 0 mg/kg BW/day of N. oculata Healthy NOM-10: Healthy rats + 10 mg/kg BW/day of N. oculata Healthy NOM-20: Healthy rats + 20 mg/kg BW/day of N. oculata Diabetic NOM-0: Diabetic rats + 0 mg/kg BW/day of N. oculata Diabetic NOM-10: Diabetic rats + 10 mg/kg BW/day of N. oculata Diabetic NOM-20: Diabetic rats + 20 mg/kg BW/day of N. oculata | Diabetic NOM-10 and Diabetic NOM-20: ↑ BW ↓ Serum glucose, TG, cholesterol, and LDL-C ↑ Serum insulin and HDL-C | - | [156] |
| Male Wistar rats (2.5 months old) | Drug: 55 mg/kg of BW STZ | Arthrospira plantensis extract (10, 20, 30 mg/kg BW/day) for 3 weeks | HC: Healthy control rats DC: Diabetic control rats SH10: Healthy rats + 10 mg/kg BW/day of A. plantensis SH20: Healthy rats + 20 mg/kg/day BW of A. plantensis SH30: Healthy rats + 30 mg/kg/day BW of A. plantensis SD10: Diabetic rats + 10 mg/kg BW/day of A. plantensis SD20: Diabetic rats + 20 mg/kg BW/day of A. plantensis SD30: Diabetic rats + 30 mg/kg BW/day of S. plantensis | SD20 and SD30: ↓ Plasma glucose, TG, cholesterol, LDL-C | SD20 and SD30: ↑ Zinc, iron, selenium, and copper ↓ TNF-α and 1L-6 ↑ SOD, GSH-Px, and CAT ↓ MDA | [157] |
| Male db/db mice (8 weeks old) | Genetically diabetic | LY: 1 mg/g lyophilized EPA+DHA/day from Chlorophyceae and Eustigamatophyceae families for 8 weeks Or MD: 2.0% microalgae EPA+DHA-enriched diet for 8 weeks (ad libitum) | BL: Normal/ diabetic strain baseline (control) RC: Normal/ diabetic strain + rodent chow LY: Normal/ diabetic strain + LY SAT: Normal/ diabetic strain + coconut oil MD: Normal/ diabetic strain + MD | Normal/ diabetic strain LY and MD: No significant changes in blood glucose level | Diabetic MD: ↓ % CD3+ ↓ % CD4+ ↓ % CD8+ ↓ IFN-γ, TNF-α ↑ IL-12 ↑ IL-17A ↓ IL-5 ↑ IL-4, IL-10 ↑ IL-6, TGF-β Diabetic LY: ↓ % CD3+ ↓ % CD8+ ↓ IFN-y, TNF-α ↑ IL-12 ↑ IL-17A ↓ IL-5 ↓ IL-4 ↑ IL-6, IL-10, TGF-β | [158] |
| Male Albino rats | Drug: 45 mg/kg of BW STZ | Arthrospira platensis powder (500 mg/kg BW) twice weekly for 2 months | Healthy rats STZ: Diabetic rats SP: Healthy rats + 500 mg/kg BW, twice weekly of A. platensis STZ-SP: Diabetic rats + 500 mg/kg BW, twice weekly of A. platensis | STZ-SP: ↓ Serum glucose ↓ HbA1C ↑ Serum insulin | STZ-SP: ↓ MDA and TBARS ↑ GSH ↑ GST activity ↑ SOD and catalase activities ↑ SOD, CAT, and GST mRNA expression ↓ PC mRNA expression ↓ Bax, CASP-3, and TNF-α mRNA expressions ↑ Bcl-2 mRNA expression Prevention of MAPK pathways activation ↓ Vacuolation of β-cells of langerhan’s islets | [159] |
| Male Wistar rats or Swiss mice | Drug: 40 mg/kg of alloxan | Arthrospira platensis (Spi, 25, 50, and 100 mg/kg BW/day) for 5 and 10 days | Diabetic control: Diabetic + distilled water Spi 25: Diabetic + 25 mg/kg BW/day of A. platensis Spi 50: Diabetic + 50 mg/kg BW/day of A. platensis Spi 100: Diabetic + 100 mg/kg BW/day of A. platensis Gli: Diabetic + 5 mg/kg BW of glibenclamide | Spi 50 and Spi 100: ↓ Serum TG and cholesterol Spi 25, Spi 50, and Spi 100: ↓ Serum glucose | Spi 50 and Spi 100: ↓ Improved paw tissue architecture ↓ Neutrophil infiltration in paw tissue Spi 100: ↑ Pancreas islet area ↓ Paw TNF-α in paw tissue | [136] |
| Male Sprague– Dawley rats (12 ± 2 weeks old) | Drug: 50 mg/kg of BW STZ | Dunaliella salina (150 mg/kg BW/day) at 72, 64, 48, 40, and 24 h before sacrifice for 3 days | C: Normal rats D: Diabetic rats CD: Normal rats + 150 mg/kg BW/day of D. salina DD: Diabetic rats + 150 mg/kg BW/day of D. salina | DD: ↑ BW ↓ TG | DD: ↓ TBARS ↑ GSH + GSSG in liver ↓ GSSG/GSH in liver ↓ α-tocopherol liver ↑ α-tocopherol/lipids in plasma | [160] |
| Female db/db mice | Genetically diabetic | Astaxanthin from marine microalgae (1.0 mg/mouse/day) for 12 weeks | Normal rats Untreated diabetic rats Treated diabetic rats: Diabetic rats + 1.0 mg/mouse/day of astaxanthin | Treated diabetic rats: ↓ Non-FBG ↑ Serum insulin Improvement in glucose tolerance | - | [55] |
| Human Subject | Microalgae and Doses | Experimental Design | Effects on Diabetes Mellitus | Reference |
|---|---|---|---|---|
| 60 male patients with T2DM (40–60 years old) | Arthrospira platensis capsule (1 g and 2 g/day) for 2 months | E1: Diabetic patients + 1 g/day of A. platensis E2: Diabetic patients + 2 g/day of A. platensis C: Diabetic control | ↓ FBG ↓ Postprandial blood glucose ↓ TG, TC, LDL-C, and VLDL-C | [237] |
| 160 male patients with T2DM (45–60 years old) | Arthrospira platensis capsule (1 g/day) for 12 weeks | Group-I: Diabetic control + placebo Group-II: Diabetic patients + diet regimen + 1 g/day of A. platensis Group-III: Diabetic patients + diet and drug regimen + 1 g/day of A. platensis Group-IV: Diabetic patients + diet, drug, and insulin regimen + 1 g/day of A. platensis | ↓ FBG ↓ HbA1C ↓ TG, TC, LDL-C ↑ HDL-C | [238] |
| 11 male and 11 female patients with T2DM | Arthrospira platensis tablet (2 g/day) for 2 months | Control group: Diabetic patients Treatment group: Diabetic patients + 2 g/day of A. platensis | ↓FBG ↓ Glycated serum protein↓TG, FFA, TC, LDL-C, and VLDL-C | [239] |
| 40 male and female with T2DM (30–60 years old) | Arthrospira platensis powder (14 g/day) for 45 days | Positive control group: Diabetic patients + 500 mg of metformin Treatment group: Diabetic patients + 7 g of A. platensis powder | ↓ FBG ↓ Postprandial blood glucose level | [240] |
| 50 male and female patients with T2DM (30–60 years old) | Arthrospira platensis powder (8 g/day) for 3 months | Control group: Diabetic patients Treatment group: Diabetic patients + 8 g/day of A. platensis | ↓ FBG | [241] |
| 60 male and female patients with T2DM (25–50 years old) | Arthrospira platensis powder (2 g/day) for 3 months | Control group: Diabetic patients + metformin + placebo Treatment group: Diabetic patients + metformin + 2 g/day of A. platensis | ↓ FBG ↓ HbA1C ↓ TG, TC, LDL-C ↑ HDL-C | [242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Therapeutic Potentials of Microalgae and Their Bioactive Compounds on Diabetes Mellitus. Mar. Drugs 2023, 21, 462. https://doi.org/10.3390/md21090462
Tamel Selvan K, Goon JA, Makpol S, Tan JK. Therapeutic Potentials of Microalgae and Their Bioactive Compounds on Diabetes Mellitus. Marine Drugs. 2023; 21(9):462. https://doi.org/10.3390/md21090462
Chicago/Turabian StyleTamel Selvan, Kartthigeen, Jo Aan Goon, Suzana Makpol, and Jen Kit Tan. 2023. "Therapeutic Potentials of Microalgae and Their Bioactive Compounds on Diabetes Mellitus" Marine Drugs 21, no. 9: 462. https://doi.org/10.3390/md21090462
APA StyleTamel Selvan, K., Goon, J. A., Makpol, S., & Tan, J. K. (2023). Therapeutic Potentials of Microalgae and Their Bioactive Compounds on Diabetes Mellitus. Marine Drugs, 21(9), 462. https://doi.org/10.3390/md21090462