The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds
Abstract
:1. Introduction
2. Results
2.1. Polysaccharide Extraction Efficiency
2.1.1. Eucheuma denticulatum (N. L. Burman) Collins and Hervey, 1917
2.1.2. Solieria chordalis (C. Agardh) J. Agardh
2.1.3. Sargassum muticum (Yendo) Fensholt
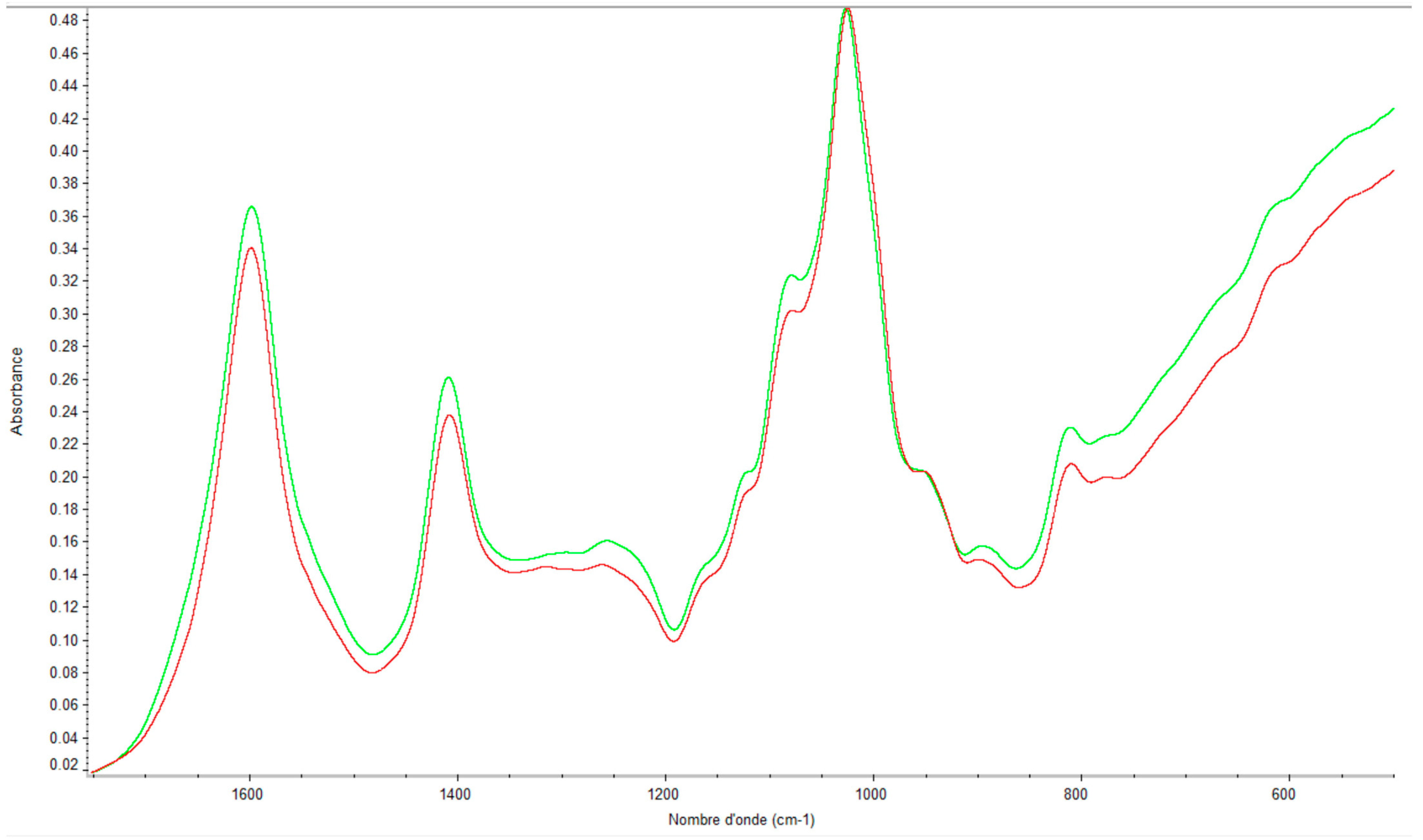
2.2. Quality Control of the Extraction by Using FTIR
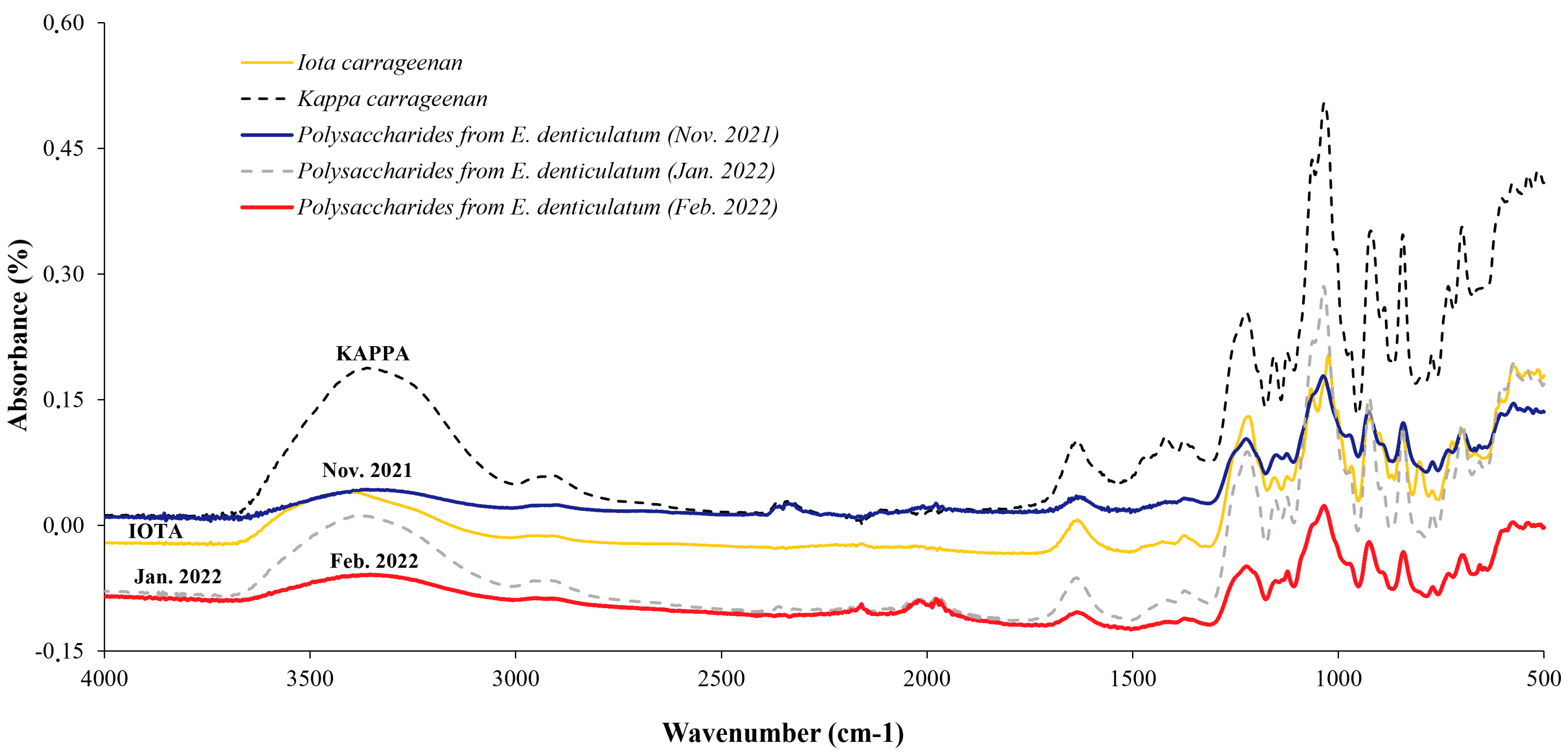
2.2.1. Eucheuma denticulatum
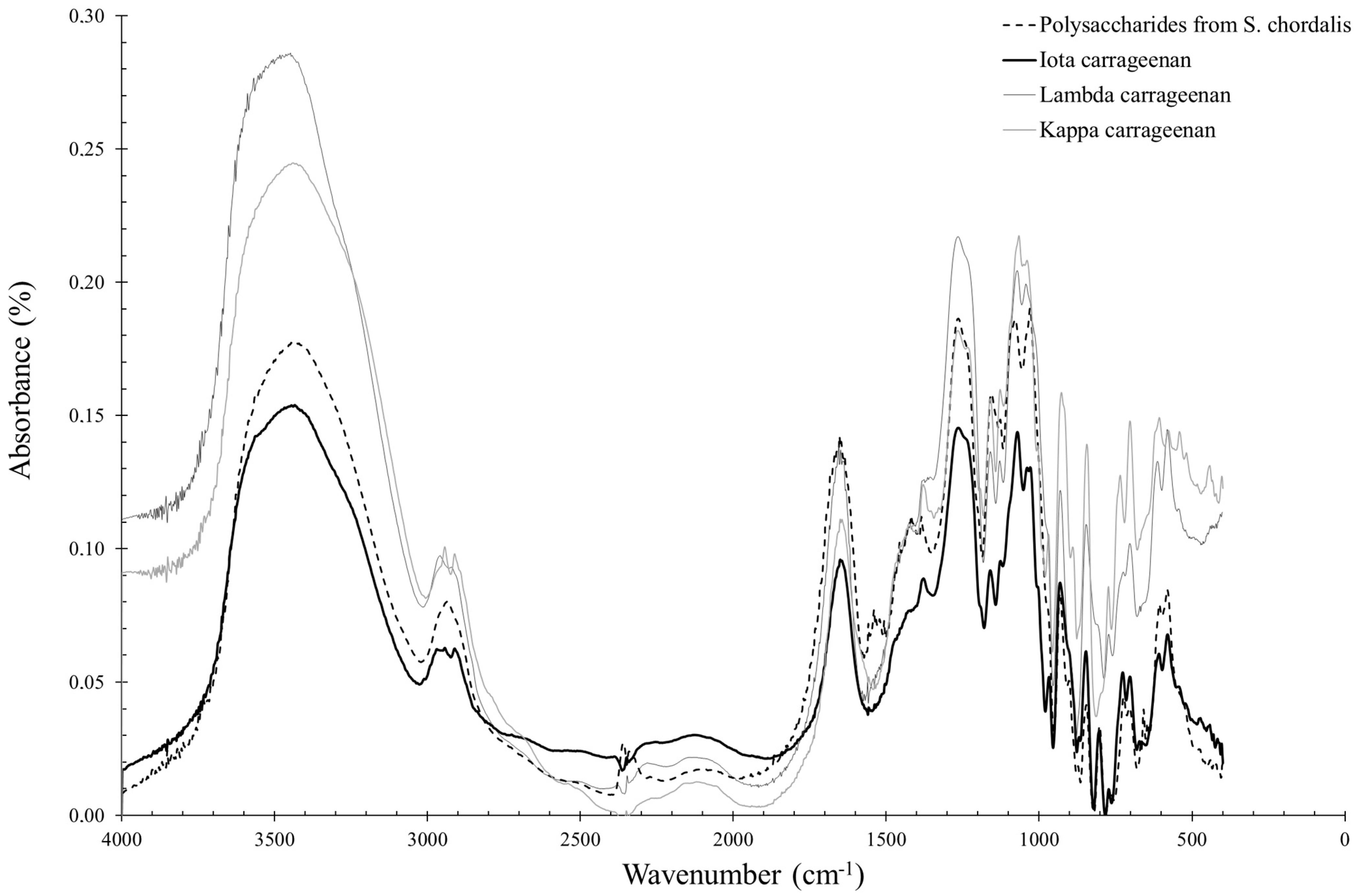
2.2.2. Solieria chordalis
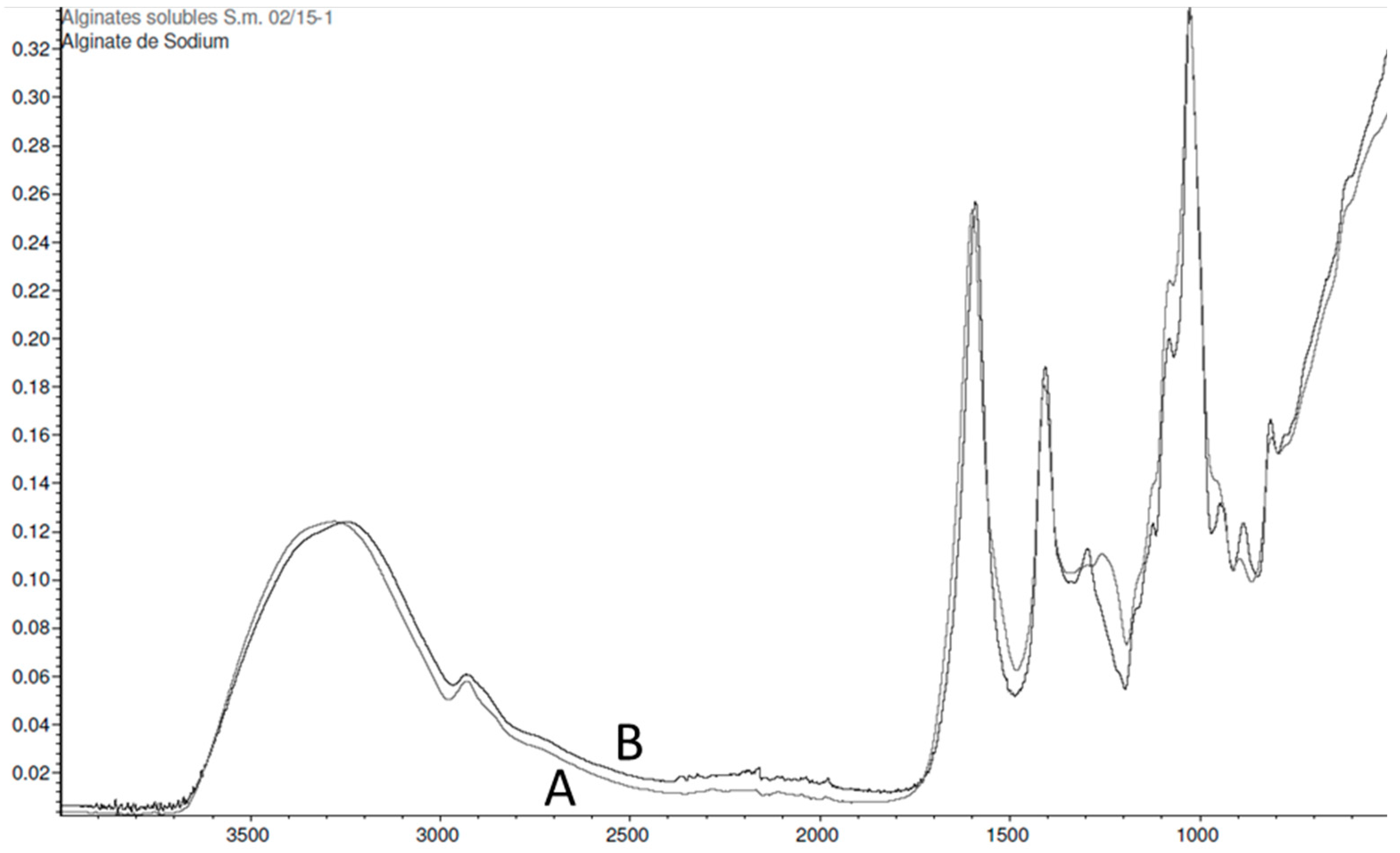
2.2.3. Sargassum muticum
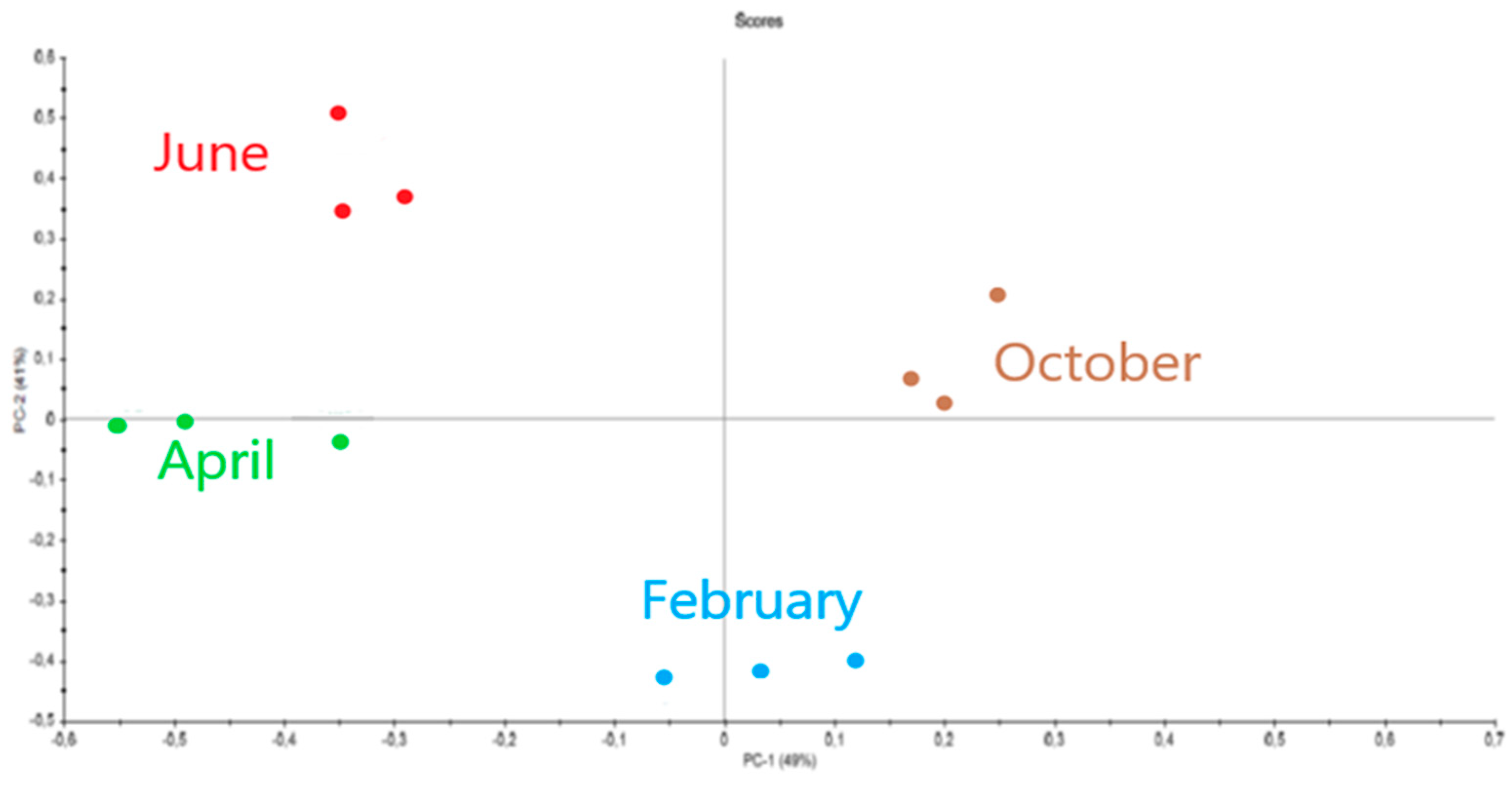
2.3. Influence of Seasonality on Polysaccharides
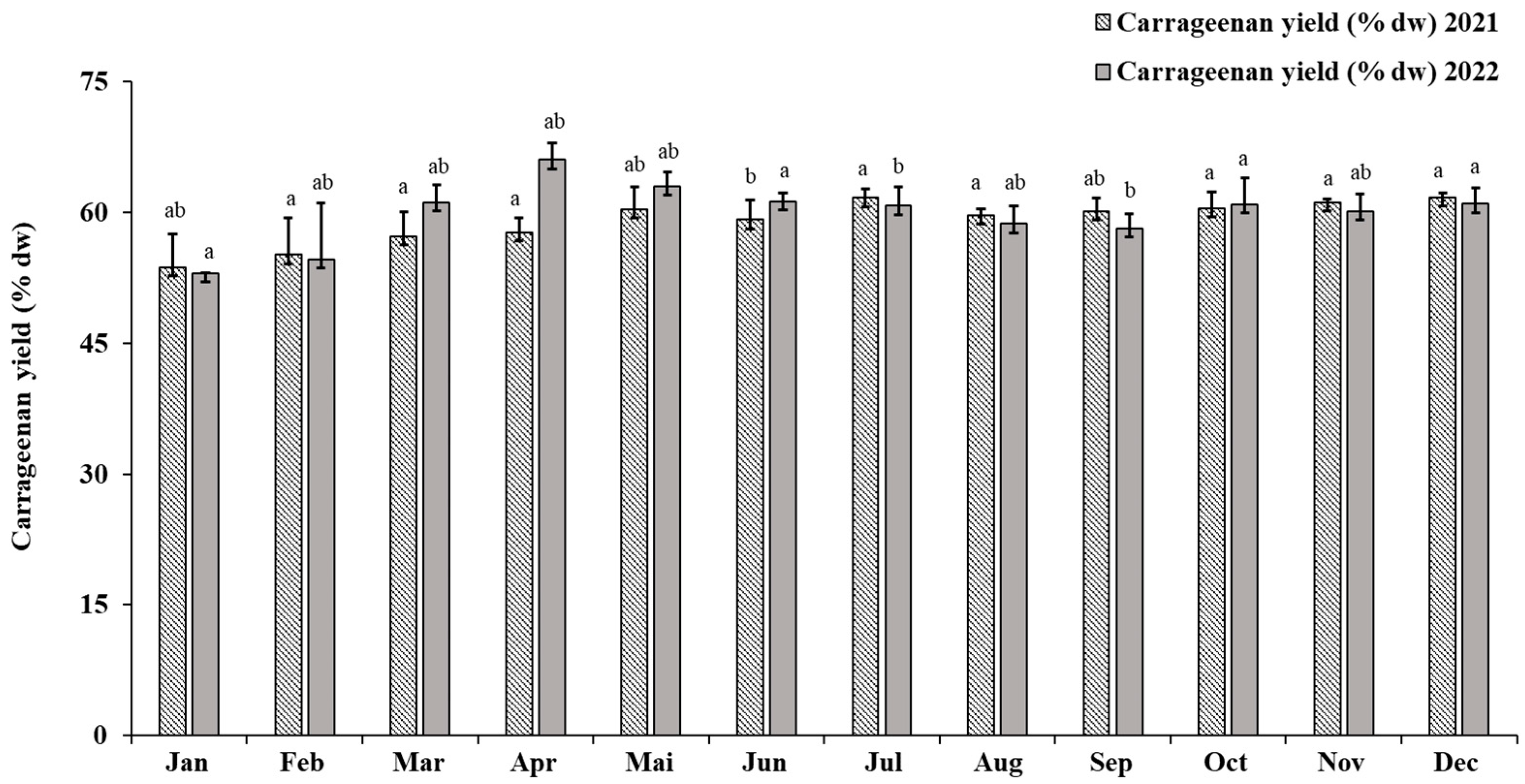
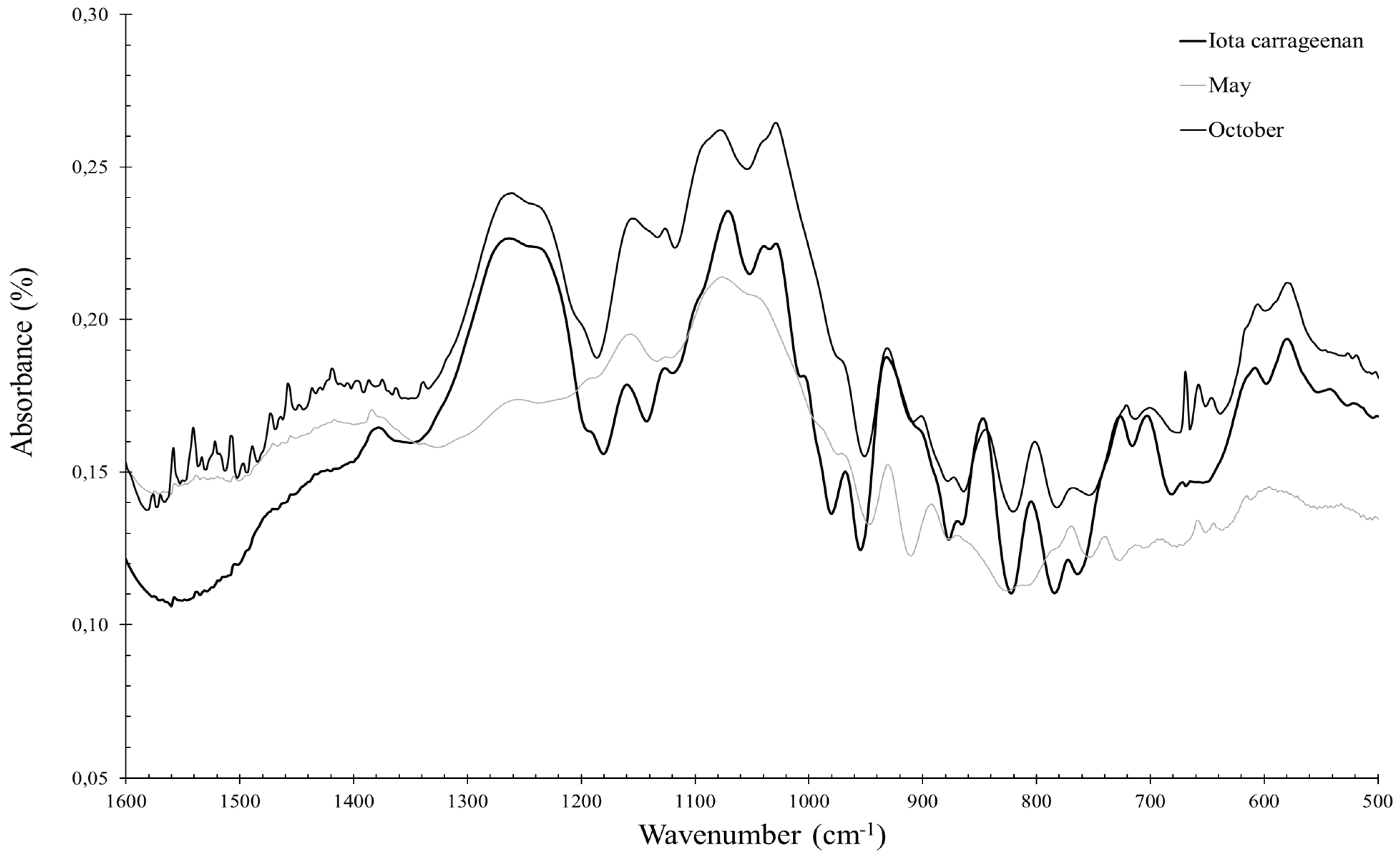
2.3.1. Eucheuma denticulatum
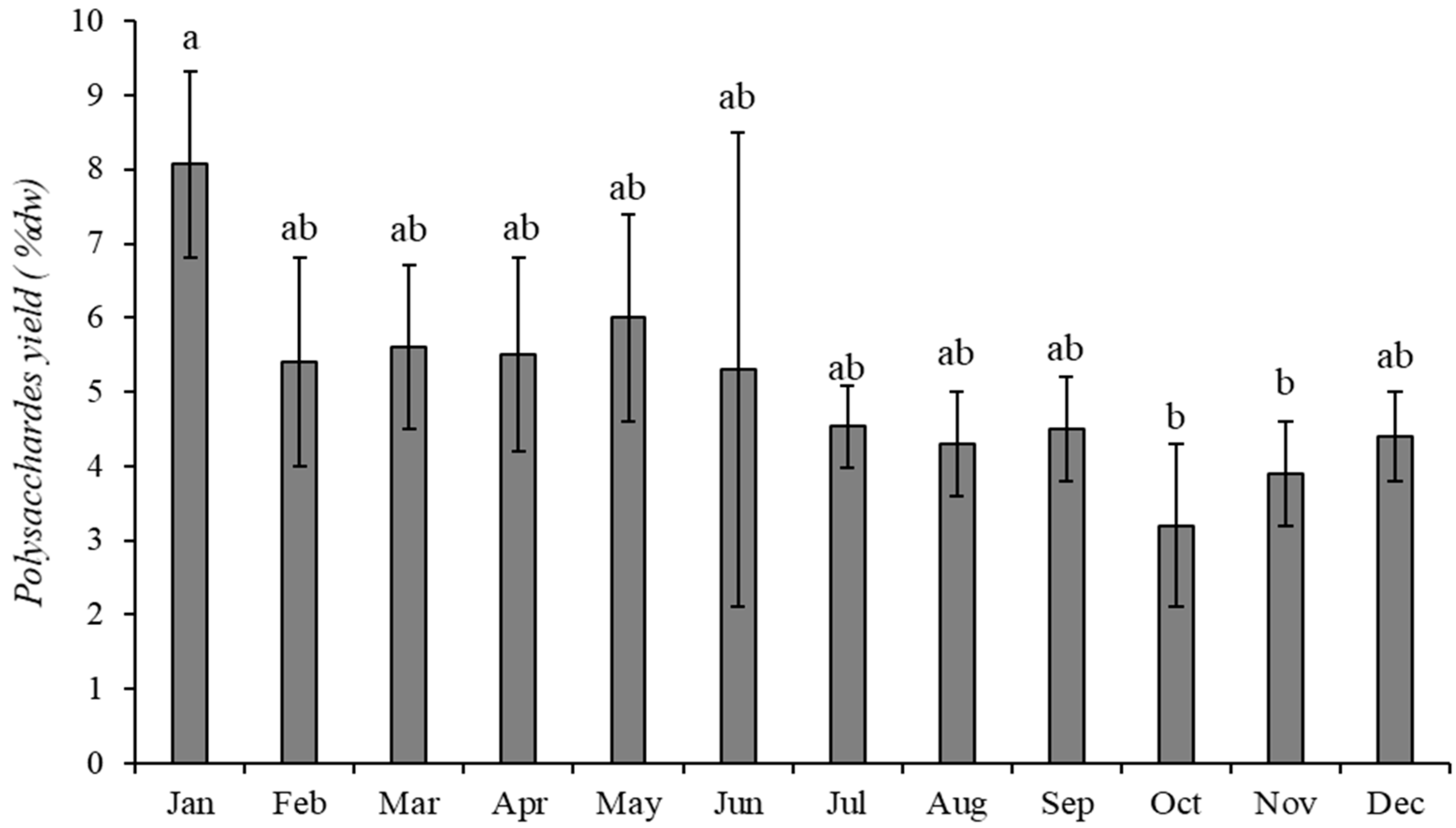
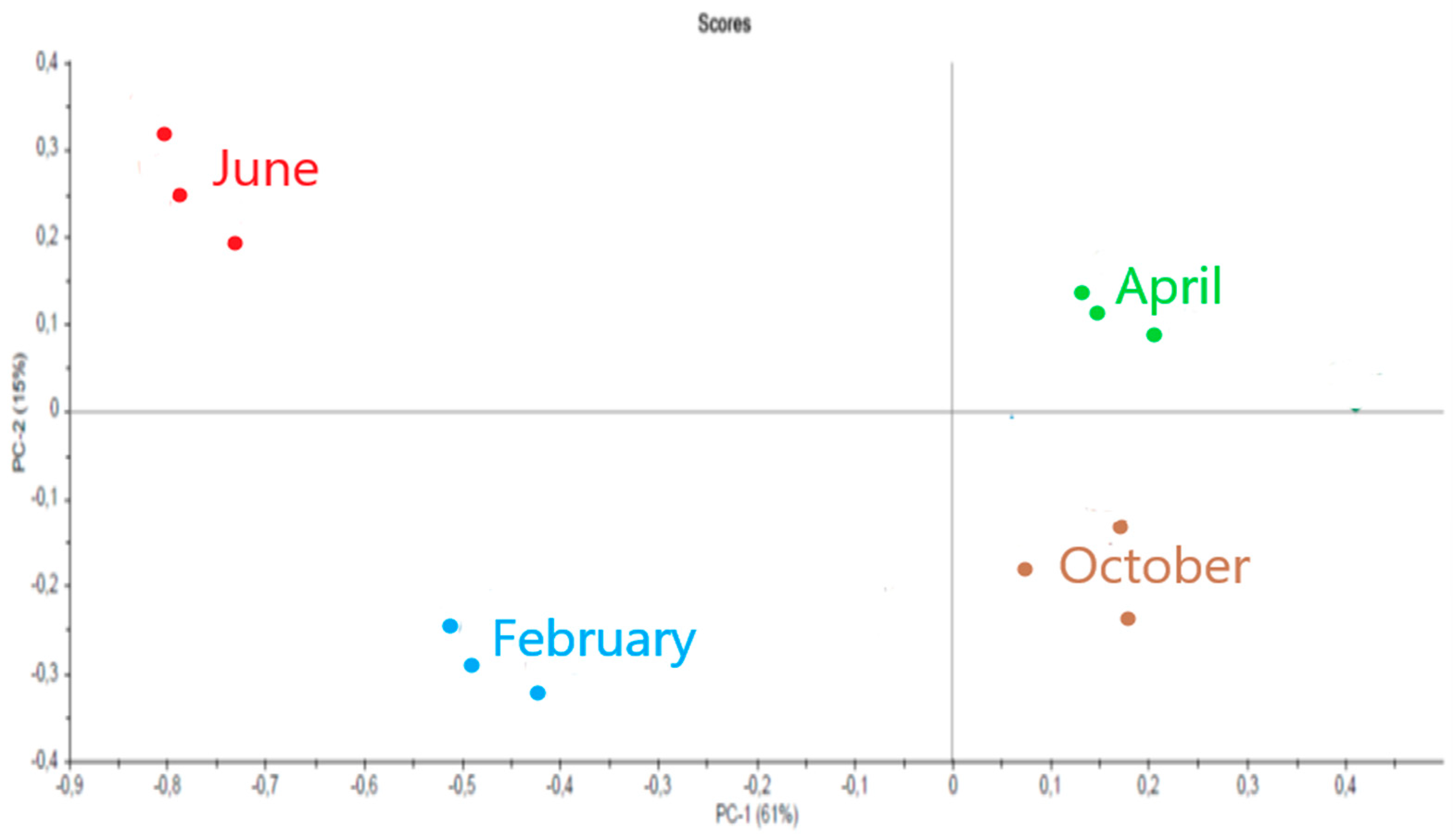
2.3.2. Solieria chordalis
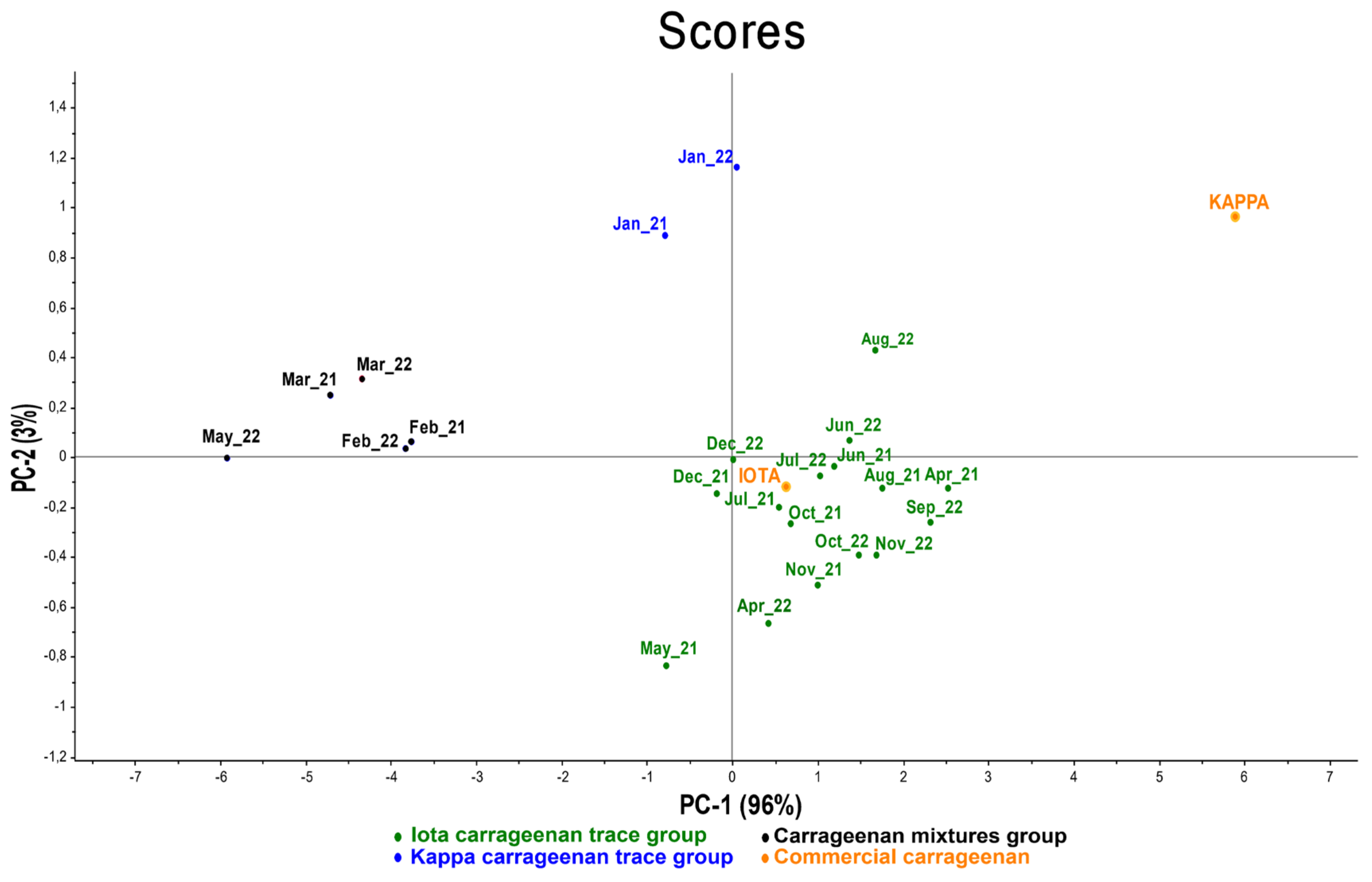
2.3.3. Sargassum muticum
3. Discussion
3.1. Eucheuma denticulatum
3.2. Solieria chordalis
3.3. Sargassum muticum
4. Materials and Methods
4.1. Materials
4.1.1. Eucheuma denticulatum
4.1.2. Solieria chordalis
4.1.3. Sargassum muticum
4.2. Polysaccharide Extraction
4.2.1. Carrageenans
4.2.2. Alginates and Fucoidans
4.3. FTIR Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO; WHO. Report of the Expert Meeting on Food Safety for Seaweed–Current Status and Future Perspectives; Food Safety and Quality Series No. 13; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- FAO. The Global Status of Seaweed Production, Trade and Utilization. Globefish Res. Programme 2018, 124, 120. [Google Scholar]
- Hayashi, L.; Reis, R.P.; dos Santos, A.A.; Castelar, B.; Robledo, D.; de Vega, G.B.; Msuya, F.E.; Eswaran, K.; Yasir, S.M.; Ali, M.K.M.; et al. The Cultivation of Kappaphycus and Eucheuma in Tropical and Sub-Tropical Waters. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 55–90. [Google Scholar] [CrossRef]
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Bedoux, G.; Caamal-Fuentes, E.; Boulho, R.; Marty, C.; Bourgougnon, N.; Freile-Pelegrín, Y.; Robledo, D. Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species. Nat. Prod. Commun. 2017, 12, 807–811. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A Modified System of Nomenclature for Red Algal Galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- Agostinho, D.A.S.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.M.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-Carrageenan Aerogels Prepared by Using Different Dissolution Media and Its Application as Drug Delivery Systems. Mater. Chem. Phys. 2020, 253, 123290. [Google Scholar] [CrossRef]
- Aguilan, J.T.; Broom, J.E.; Hemmingson, J.A.; Dayrit, F.M.; Montaño, M.N.E.; Dancel, M.C.A.; Niñonuevo, M.R.; Furneaux, R.H. Structural Analysis of Carrageenan from Farmed Varieties of Philippine Seaweed. Bot. Mar. 2003, 46, 179–192. [Google Scholar] [CrossRef]
- Bellion, C.; Brigand, G.; Prome, J.-C.; Welti, D.; Bociek, S. Identification et caractérisation des précurseurs biologiques des carraghénanes par spectroscopie de RNM-13C. Carbohydr. Res. 1983, 119, 31–48. [Google Scholar] [CrossRef]
- Distantina, S.; Wiratni; Fahrurrozi, M. Rochmadi Carrageenan Properties Extracted from Eucheuma cottonii, Indonesia. Int. J. Chem. Mol. Eng. 2011, 5, 501–505. [Google Scholar]
- Pacheco-Quito, E.-M.; Roberto, R.-C.; Veiga, M. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Mollion, J.; Braud, J.-P. A Eucheuma (Solieriaceae, Rhodophyta) Cultivation Test on the South-West Coast of Madagascar. Hydrobiologia 1993, 260, 373–378. [Google Scholar] [CrossRef]
- Haug, A.; Myklestad, S.; Larsen, B.; Smidsrød, O.; Eriksson, G.; Blinc, R.; Dumanović, J. Correlation between Chemical Structure and Physical Properties of Alginates. Acta Chem. Scand. 1967, 21, 768–778. [Google Scholar] [CrossRef]
- Rioux, L. Caractéristation Structurale et Evaluation de L’activité Biologique de Polysaccharides Extraits de Saccharina longicruris. Ph.D. Dissertation, Université Laval, Quebec, QC, Canada, 2010. [Google Scholar]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles CB, S.; Dantas-Santos, N.; Camara RB, G.; Cordeiro, S.L.; Rocha, H.A.O. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Colliec, S.; Bretaudiere, J.; Durand, P.; Fischer, A.M.; Jozefonvicz, J.; Kloareg, B.; Vidal, C. Polysaccharides Sulfatés, Agent Anticoagulant et Agent Anti-Complémentaire Obtenus à Partir de Fucanes D’algues Brunes et Leur Procédé D’obtention; Google Patents EP0403377B1 (Ifremer): 1995. Available online: https://patents.google.com/patent/EP0403377A1/fr (accessed on 15 May 2023).
- Han, S.C.; Kang, N.J.; Yoon, W.J.; Kim, S.; Na, M.C.; Koh, Y.S.; Kang, H.K. External Application of Apo-9′-fucoxanthinone, Isolated from Sargassum muticum, Suppresses Inflammatory Responses in a Mouse Model of Atopic Dermatitis. Toxicol. Res. 2016, 32, 109. [Google Scholar] [CrossRef]
- BoissonVidal, C.; Chaubet, F.; Chevolot, L.; Sinquin, C.; Theveniaux, J.; Millet, J.; Fischer, A.M. Relationship between antithrombotic activities of fucans and their structure. Drug Dev. Res. 2000, 51, 216–224. [Google Scholar] [CrossRef]
- Grauffel, V.; Mabeau, S.; Kloareg, B.; Josefonwicz, J. Propriétés Anticoagulantes des Fucanes Sulfatés des Algues Brunes, Rapport Contrat Ifremer. 1985. Available online: https://archimer.ifremer.fr/doc/00101/21217 (accessed on 20 May 2023).
- Balboa, E.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J. Simultaneous Extraction and Depolymerization of Fucoidan from Sargassum muticum in Aqueous Media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef]
- Choi, E.-M.; Kim, A.-J.; Kim, Y.-O.; Hwang, J.-K. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Medica 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Plans, B. Le fucoïdane améliore le système immunitaire et la communication cellulaire, a une puissante activité anticoagulante et antithrombotique, et inhibe certains cancers et virus. Biochem. Biophys. Res. 2006, 350, 501–507. [Google Scholar]
- Sweeney, E.A.; Lortat-Jacob, H.; Priestley, G.V.; Nakamoto, B.; Papayannopoulou, T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bourgougnon, N.; Burlot, A.S.; Jacquin, A.G. Algae for Global Sustainability? Adv. Bot. Res. 2021, 100, 145–212. [Google Scholar] [CrossRef]
- Rueness, J. Sargassum muticum and Other Introduced Japanese Macroalgae: Biological Pollution of European Coasts. Mar. Pollut. Bull. 1989, 20, 173–176. [Google Scholar] [CrossRef]
- Hinds, C.; Oxenford, H.; Cumberbatch, J.; Fardin, F.; Doyle, E.; Cashman, A. Golden Tides: Management Best Practices for Influxes of Sargassum in the Caribbean with a Focus on Clean-up; CERMES, The University of the West Indies, Cave Hill Campus: Wanstead, Barbados, 2016; 17pp. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total Phenolic Content and Biological Activities of Enzymatic Extracts from Sargassum Muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Gorham, J.; Lewey, S.A. Seasonal changes in the chemical composition of Sargassum muticum. Mar. Biol. 1984, 80, 103–107. [Google Scholar] [CrossRef]
- Tadmor Shalev, N.; Ghermandi, A.; Tchernov, D.; Shemesh, E.; Israel, A.; Brook, A. NIR spectroscopy and artificial neural network for seaweed protein content assessment in-situ. Comput. Electron. Agric. 2022, 201, 107304. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuno, J.; Koidis, A.; Theodoridou, K. The use of near-infrared and mid-infrared spectroscopy to rapidly measure the nutrient composition and the in vitro rumen dry matter digestibility of brown seaweeds. Anim. Feed. Sci. Technol. 2022, 285, 115239. [Google Scholar] [CrossRef]
- Pliego-Cortes, H.; Hardouin, K.; Bedoux, G.; Marty, C.; Cerantola, S.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N. Sulfated polysaccharides from seaweed strandings as renewable source for potential antivirals. Mar. Drugs 2022, 20, 116. [Google Scholar] [CrossRef]
- Duarte ME, R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Niemi, C.; Mortensen, A.M.; Rautenberger, R.; Matsson, S.; Gorzsás, A.; Gentili, F.G. Rapid and accurate determination of protein content in North Atlantic seaweed by NIR and FTIR spectroscopies. Food Chem. 2023, 404, 134700. [Google Scholar] [CrossRef]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An Analysis of the Nutritional and Health Values of Caulerpa Racemosa (Forsskål) and Ulva Fasciata (Delile)-Two Chlorophyta Collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef]
- Chopin, T.; Kerin, B.F.; Mazerolle, R. Phycocolloid Chemistry as a Taxonomic Indicator of Phylogeny in the Gigartinales, Rhodophyceae: A Review and Current Developments Using Fourier Transform Infrared Diffuse Reflectance Spectroscopy. Phycol. Res. 1999, 47, 167–188. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T. Structural studies on ι-carrageenan derived oligosaccharides and its application. Adv. Chem. Eng. Sci. 2014, 4, 41925. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M.; Yaphe, W. Sulfate Content of Carrageenan and Agar Determined by Infrared Spectroscopy. Bot. Mar. 1986, 29, 335–340. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR Spectroscopy as a Tool for Polysaccharide Identification in Edible Brown and Red Seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Guerrien, M. L’intérêt de l’analyse en composantes principales (ACP) pour la recherche en sciences sociales. Cah. Des Amériques Lat. 2003, 43, 181–192. [Google Scholar] [CrossRef]
- Azanza, R. Eucheuma; CABI Compendium (Digital Library): 2023; p. 102037. Available online: https://doi.org/10.1079/cabicompendium.102037 (accessed on 30 January 2023).
- Anderson, N.S.; Dolan, T.C.S.; Penman, A.; Rees, D.A.; Mueller, G.P.; Stancioff, D.J.; Stanley, N.F. Carrageenans. Part IV. Variations in the Structure and Gel Properties of κ-Carrageenan, and the Characterisation of Sulphate Esters by Infrared Spectroscopy. J. Chem. Soc. C Organic 1968, 602–606. [Google Scholar] [CrossRef]
- Laserna, E.C.; Veroy, R.L.; Luistro, A.H.; Cajipe, G.J.B. Extracts from some red and brown seaweeds of the Philippines. In Extracts from Some Red and Brown Seaweeds of the Philippines; De Gruyter: Berlin, Germany, 1981; pp. 443–448. ISBN 978-3-11-086527-1. [Google Scholar]
- Matsuhiro, B. Vibrational Spectroscopy of Seaweed Galactans. Hydrobiologia 1996, 326, 481–489. [Google Scholar] [CrossRef]
- Deslandes, E.; Floc’h, J.Y.; Bodeau, B.C.; Brault, D.; Braud, J.P. Short Communication: Evidence for ι-Carrageenans in Solieria Chordalis (Solieriaceae) and Calliblepharis Jubata, Calliblepharis Ciliata, Cystoclonium Purpureum (Rhodophyllidaceae). Bot. Mar. 1985, 28, 317–318. [Google Scholar] [CrossRef]
- Bondu, S.; Deslandes, E.; Fabre Marie, S.; Christian, B.; Guangli, Y. Carrageenan from Solieria Chordalis (Gigartinales): Structural Analysis and Immunological Activities of the Low Molecular Weight Fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus Coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella Thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Bourgougnon, N.; Lahaye, M.; Chermann, J.C.; Kornprobst, J.M. Composition and Antiviral Activities of a Sulfated Polysaccharide from Schizymenia Dubyi (Rhodophyta, Gigartinales). Bioorganic Med. Chem. Lett. 1993, 3, 1141–1146. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Carrageenan of Eucheuma Isiforme (Solieriaceae, Rhodophyta) from Yucatán, Mexico. II. Seasonal Variations in Carrageenan and Biochemical Characteristics. Bot. Mar. 2006, 49, 72–78. [Google Scholar] [CrossRef]
- Burlot, A.-S. Étude de La Macroalgue Rouge Solieria Chordalis: Aspects Écophysiologiques, Production d’extraits et Perspectives d’applications. Ph.D. Dissertation, Université Bretagne Loire, Rennes, France, 2016. [Google Scholar]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic Activity and Mode of Action of Natural Carrageenans of Diverse Structural Types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-Herpes Simplex Virus Activity of Sulfated Galactans from the Red Seaweeds Gymnogongrus Griffithsiae and Cryptonemia Crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zú, E.A. Structural Analysis and Antiviral Activity of a Sulfated Galactan from the Red Seaweed Schizymenia Binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of Local Plant Immunity against Tobacco Mosaic Virus Infection after Treatment with Sulphated-Carrageenan from Red Alga (Hypnea Musciformis). Physiol. Mol. Plant Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Wong, K.F.; Craigie, J.S. Sulfohydrolase Activity and Carrageenan Biosynthesis in Chondrus Crispus (Rhodophyceae). Plant Physiol. 1978, 61, 663–666. [Google Scholar] [CrossRef]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An Overview of Their Most Important Sourcing and Applications as Natural Polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and Its Applications. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: New York, NY, USA, 2015; pp. 753–794. ISBN 9783319162980. [Google Scholar]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Marine Drugs Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic Recovery of Metabolites from Seaweeds: Potential Applications. Adv. Bot. Res. 2014, 71, 279–320. [Google Scholar]
- Penuela, A.; Robledo, D.; Bourgougnon, N.; Bedoux, G.; Hernandez-Nunez, E.; Freile-Pelegrin, Y. Environmentally friendly valorization of Solieria filiformis from IMTA using a biorefinery concept. Mar. Drugs 2018, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V. A Seasonal Study of the Red Seaweeds Solieria Tenera and Three Species of Gracilaria from Jamaica. Hydrobiologia 1986, 140, 167–171. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Mattio, L.; De Ramon N’Yeurt, A.; Uwai, S.; Dominguez, H.; Flórez-Fernández, N.; Connan, S.; Critchley, A.T. A Concise Review of the Highly Diverse Genus Sargassum C. Agardh with Wide Industrial Potential. J. Appl. Phycol. 2023, 35, 1453–1483. [Google Scholar] [CrossRef]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of Alginate from Sargassum Muticum: Process Optimization and Study of Its Functional Activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A Green Approach for Alginate Extraction from Sargassum Muticum Brown Seaweed Using Ultrasound-Assisted Technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Belattmania, Z.; El Atouani, S.; Bentiss, F.; Jama, C.; Falace, A.; Chaouti, A.; Reani, A.; Sabour, B. Seasonal Patterns of Growth, Alginate Content and Block Structure of the Alien Invader Sargassum Muticum (Fucales, Ochrophyta) from the Atlantic Coast of Morocco. Bot. Mar. 2022, 65, 69–78. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H.; Rodríguez-Argüelles, M.C. Synthesis, Process Optimization and Characterization of Gold Nanoparticles Using Crude Fucoidan from the Invasive Brown Seaweed Sargassum Muticum. Algal. Res. 2021, 58, 102377. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-Assisted Extraction of Fucoidan from Sargassum Muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Vandanjon, L.; Deniel, M.; Puspita, M.; Douzenel, P.; Stiger, V.; Bedoux, G.; Bourgougnon, N. Seasonal variation of the biochemical composition of the proliferative macroalgae Sargassum muticum estimated by Fourier Transform Infra-Red (FTIR) spectroscopy. J. Anal. Bioanal. Sep. Tech. 2017, 2, 1–10. [Google Scholar]
- Jaques, L.B.; Balueux, R.E.; Dietrich, C.P.; Kavanagh, L.W. A microelectrophoresis method for heparin. Can. J. Physiol. Pharmacol. 1968, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]



| Sulfate Compounds | Polysaccharides | Uronic Acids | Proteins | Mineral Matter | Total Nitrogen | Phenolic Compounds |
|---|---|---|---|---|---|---|
| 6.9–9.6% | 8.3–19.9% | 1.5–4.1% | 18.2–39.1% | 18.8–28.9% | 1.1–2.1% | 2.3–5.1% |
| Wavenumber (cm−1) | S=O (ι-,κ-,ν-) | DA* (ι-, κ-) | G/D (ι-,κ-,ν-) | DA (ι-, κ-) | DA2S*(ι-) | G/D6S* (ν-) | G4S (ι-,κ-,ν-) | G/D2S (ν-) | G/D6S (ν-) | DA2S (ι-) |
|---|---|---|---|---|---|---|---|---|---|---|
| IOTA Stand. | 1218.05 | 1066.37 | 967.89 | 923.74 | 901.51 | 870.45 | 844.7 | - | - | 802.5 |
| KAPA Stand. | 1224.62 | 1063.61 | 970 | 921.27 | - | 874.26 | 843.27 | - | - | - |
| Jan 21 | 1224.2 | + | 972.99 | 925.48 | + | 871.19 | 843.05 | - | - | + |
| Jan 22 | 1221.5 | 1061.82 | 973.16 | 925.06 | + | 872.43 | 843 | - | - | 805.64 |
| Feb 21 | 1224.38 | + | 973.79 | 925.47 | + | + | 841.56 | - | - | + |
| Feb 22 | 1224.18 | + | 973.65 | 925.35 | + | + | 841.89 | - | - | + |
| Mar 21 | 1223.62 | + | 972.63 | 926.13 | + | + | 841.39 | - | - | + |
| Mar 22 | 1221.17 | + | 972.82 | 925.42 | + | + | 841.96 | - | - | 804.44 |
| Apr 21 | 1224.21 | + | 973.61 | 926.02 | 894.52 | 871.33 | 842.61 | - | - | + |
| Apr 22 | 1223.73 | + | 973.94 | 925.84 | + | + | 842.32 | - | - | + |
| May 21 | 1218,36 | 1061 | 976.18 | 924.64 | 891.69 | + | 841.77 | - | - | 804.49 |
| May 22 | 1224.86 | + | 974.8 | 925.67 | 894.01 | + | 842.21 | - | - | 803.39 |
| Jun 21 | 1224.9 | + | 973.93 | 925.74 | 894.93 | + | 842.84 | - | - | + |
| Jun 22 | 1224.13 | 1062.04 | 972.98 | 924.82 | 892.62 | + | 843.37 | - | - | 805.7 |
| Jul 21 | 1220.79 | 1059.01 | 973.29 | 925.09 | 894.19 | + | 842.52 | - | - | 803.31 |
| Jul 22 | 1224.34 | + | 973.86 | 925.92 | 894.76 | + | 842.74 | - | - | + |
| Aug 21 | 1222.91 | 1061.39 | 975.8 | 925.24 | 892.8 | 870.02 | 843.02 | - | - | 804.79 |
| Aug 22 | 1224.05 | 1061.96 | 975.13 | 925.24 | 893.66 | 872.58 | 843.16 | - | - | + |
| Sep 21 | 1224.39 | 1066.37 | 974.38 | 925.98 | + | + | 842.61 | - | - | + |
| Sep 22 | 1221.76 | + | 974.28 | 925.68 | 895.14 | + | 842.63 | - | - | + |
| Oct 21 | 1221.15 | + | 974.53 | 925.24 | 894.36 | + | 842.28 | - | - | 805.41 |
| Oct 22 | 1222.94 | 1062.19 | 973.39 | 925.14 | 893.76 | 871.16 | 842.89 | - | - | 805.87 |
| Nov 21 | 1224.07 | + | 973.29 | 926.15 | + | 871.7 | 842.23 | - | - | + |
| Nov 22 | 1223.45 | + | 973.57 | 926.13 | 895.96 | + | 842.1 | - | - | + |
| Dec 21 | 1224.26 | + | 974.01 | 926.15 | + | 873.21 | 843.06 | - | - | + |
| Dec 22 | 1224.48 | + | 973.22 | 925.62 | 894.71 | 870.26 | 843.14 | - | - | + |
| February | April | June | October |
|---|---|---|---|
| 72.74% | 72.35% | 64.41% | 75.60% |
| 72.66% | 73.13% | 68.48% | 75.70% |
| 71.54% | 73.21% | 67.06% | 75.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandanjon, L.; Burlot, A.-S.; Zamanileha, E.F.; Douzenel, P.; Ravelonandro, P.H.; Bourgougnon, N.; Bedoux, G. The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Mar. Drugs 2023, 21, 482. https://doi.org/10.3390/md21090482
Vandanjon L, Burlot A-S, Zamanileha EF, Douzenel P, Ravelonandro PH, Bourgougnon N, Bedoux G. The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Marine Drugs. 2023; 21(9):482. https://doi.org/10.3390/md21090482
Chicago/Turabian StyleVandanjon, Laurent, Anne-Sophie Burlot, Elando Fréda Zamanileha, Philippe Douzenel, Pierre Hervé Ravelonandro, Nathalie Bourgougnon, and Gilles Bedoux. 2023. "The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds" Marine Drugs 21, no. 9: 482. https://doi.org/10.3390/md21090482
APA StyleVandanjon, L., Burlot, A.-S., Zamanileha, E. F., Douzenel, P., Ravelonandro, P. H., Bourgougnon, N., & Bedoux, G. (2023). The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Marine Drugs, 21(9), 482. https://doi.org/10.3390/md21090482







