Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Structural and Biological Activity Studies
2.1. Terpenoids
2.1.1. Monoterpenoids
2.1.2. Sesquiterpenes
2.1.3. Diterpenoids
2.1.4. Meroterpenoids
2.2. Polyketides
2.2.1. Lactones
2.2.2. Azaphilones
2.2.3. Xanthones
2.2.4. Other Polyketides
2.3. Nitrogen-Containing Compounds
2.3.1. Alkaloids
2.3.2. Peptides
2.4. Steroids
2.5. Other Classes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Inflammation and its role in regeneration and repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An inflammation-centric view of neurological disease: Beyond the neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A review of anti-inflammatory compounds from marine fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution pharmacology: Opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Zhuo, Y.; Li, D.; Cui, L.; Li, C.; Zhang, S.; Zhang, Q.; Zhang, L.; Wang, X.; Yang, L. Treatment with 3,4-dihydroxyphenylethyl alcohol glycoside ameliorates sepsis-induced ALI in mice by reducing inflammation and regulating M1 polarization. Biomed. Pharmacother. 2019, 116, 109012. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Vo, T.; Ngo, D.; Kim, S. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy-Drug Targets 2012, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.11. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Nah, J.; Jeon, Y. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Wu, N.N.; Wu, Y.W.; Qi, Y.X.; Wei, M.Y.; Pineda, L.M.; Ng, M.G.; Spadafora, C.; Zheng, J.Y.; Lu, L.; et al. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022, 4, 88–97. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.T.; Wei, J.E.; Huang, Z.N. Marine Natural Products in Clinical Use. Marine Drugs. 2022, 20, 528. [Google Scholar] [CrossRef]
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A.; et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br. J. Cancer 2017, 117, 628–638. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine polyhydroxynaphthoquinone, echinochrome a: Prevention of atherosclerotic inflammation and probable molecular targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Zhang, Q.; Xu, W.F.; Hai, Y.; Chao, R.; Wang, C.F.; Hou, X.M.; Wei, M.Y.; Gu, Y.C.; Wang, C.Y.; et al. Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide A, using LC-MS/MS-based molecular networking. Mar. Life Sci. Technol. 2023, 5, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Niu, S.W.; Yang, L.H.; Chen, T.T.; Hong, B.H.; Pei, S.X.; Shao, Z.Z.; Zhang, G.Y. New monoterpenoids and polyketides from the deep-sea sediment-derived fungus Aspergillus sydowii MCCC 3A00324. Mar. Drugs 2020, 18, 561. [Google Scholar] [CrossRef]
- Sun, B.; Wang, D.; Ren, J.; Wang, C.; Yan, P.; Gustafson, K.R.; Jiang, W. Paraconulones A–G: Eremophilane sesquiterpenoids from the marine-derived fungus Paraconi othyrium sporulosum DL-16. J. Nat. Prod. 2023, 86, 1360–1369. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.J.; Zou, G.; Yang, W.C.; Zhang, L.S.; Yan, Z.Y.; Long, Y.H.; She, Z.G. Bioactive sesquiterpene derivatives from mangrove endophytic fungus Phomopsis sp. SYSU-QYP- Structures and nitric oxide inhibitory activities. Bioorg. Chem. 2021, 107, 104530. [Google Scholar] [CrossRef]
- Hu, Z.B.; Chen, J.J.; Liu, Q.Q.; Wu, Q.L.; Chen, S.H.; Wang, J.J.; Li, J.; Liu, L.; Gao, Z.Z. Cyclohexenone derivative and drimane sesquiterpenes from the seagrass-derived fungus Aspergillus insuetus. Chem. Biodivers. 2023, 20, e202300424. [Google Scholar] [CrossRef]
- Ning, Y.D.; Gu, Q.W.F.; Zheng, T.; Xu, Y.; Li, S.; Zhu, Y.P.; Hu, B.; Yu, H.B.; Liu, X.Y.; Zhang, Y.; et al. Genome mining leads to diverse sesquiterpenes with anti-inflammatory activity from an arctic-derived fungus. J. Nat. Prod. 2024, 87, 1426–1440. [Google Scholar] [CrossRef]
- Wang, G.S.; Yuan, Y.L.; Li, Z.K.; Liu, X.G.; Chu, Y.H.; She, Z.G.; Kang, W.Y.; Chen, Y. Pleosmaranes A–R, isopimarane and 20-nor isopimarane diterpenoids with anti-inflammatory activities from the mangrove endophytic fungus Pleosporales sp. HNQQJ. J. Nat. Prod. 2024, 87, 304–314. [Google Scholar] [CrossRef]
- He, J.X.; Zou, Q.H.; Deng, H.M.; He, S.T.; Yan, D.; Pan, K.H.; Zhou, Y.W.; Zhao, Z.X.; Cui, H.; Liu, Y.N. Novel 6/7/6 ring system diterpenoids and cytochalasins from the fungus Eutypella scoparia GZU-4-19Y and their anti-inflammatory activity. Fitoterapia 2024, 173, 105804. [Google Scholar] [CrossRef]
- Wang, G.S.; Wu, J.Y.; Li, Z.K.; Chen, T.; Liu, Y.F.; Wang, B.; Chen, Y.; She, Z.G. Talaroacids A–D and talaromarane A, diterpenoids with anti-Inflammatory activities from mangrove endophytic fungus Talaromyces sp. JNQQJ-4. Int. J. Mol. Sci. 2024, 25, 6691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X.C.; Pan, W.C.; Liu, X.; Tan, S.L.; Cui, H.; Zhao, Z.X. Meroterpenoids from the fungus penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Phytochemistry 2022, 202, 113307. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liu, Y.N.; Ruan, Q.F.; Zhao, M.; Zhao, Z.X.; Cui, H. Aspermeroterpenes A–C: Three meroterpenoids from the marine-derived fungus Aspergillus terreus GZU-31-1. Org. Lett. 2020, 22, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Cui, X.; Xiong, B.; Yang, M.S.; Zhang, Y.X.; Liu, X.M. Terretonin D1, a new meroterpenoid from marine-derived Aspergillus terreus ML-44. Nat. Prod. Res. 2019, 33, 2262–2265. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Luo, L.; Liu, Y.C.; Bo, X.L.; Wu, F.R.; Wang, F.F.; Tan, M.J.; Wei, Y.Q.; Dou, X.B.; Wang, C.Y.; et al. Diisoprenyl-cyclohexene-type meroterpenoids from a mangrove endophytic fungus Aspergillus sp. GXNU-Y65 and their anti-nonalcoholic steatohepatitis activity in AML12 cells. Phytochemistry 2024, 218, 113955. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.M.; Ye, Y.X.; Xu, Z.Y.; Zhao, Q.L.; Luo, X.W.; Liu, Y.H.; Guo, P. Anti-inflammatory compounds from the mangrove endophytic fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 2022, 13, 976399. [Google Scholar] [CrossRef]
- Yuan, S.W.; Chen, L.T.; Wu, Q.L.; Jiang, M.H.; Guo, H.; Hu, Z.B.; Chen, S.H.; Liu, L.; Gao, Z.Z. Genome mining of α-pyrone natural products from ascidian-derived fungus Amphichorda felina SYSU-MS7908. Mar. Drugs 2022, 20, 294. [Google Scholar] [CrossRef]
- Zhai, G.; Chen, S.; Shen, H.; Guo, H.; Jiang, M.; Liu, L. Bioactive monoterpenes and polyketides from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 553. [Google Scholar] [CrossRef]
- Ding, W.J.; Wang, F.F.; Li, Q.W.; Xue, Y.X.; Dong, Z.T.; Tian, D.M.; Chen, M.; Zhang, Y.W.; Hong, K.; Tang, J.S. Isolation and characterization of anti-inflammatory sorbicillinoids from the mangrove-derived fungus Penicillium sp. DM815. Chem. Biodivers. 2021, 18, e2100229. [Google Scholar] [CrossRef]
- Chen, C.; Ye, G.T.; Tang, J.; Li, J.L.; Liu, W.B.; Wu, L.; Long, Y.H. New polyketides from mangrove endophytic fungus Penicillium sp. BJR-P2 and their anti-inflammatory activity. Mar. Drugs 2022, 20, 583. [Google Scholar] [CrossRef]
- Guo, H.; Wu, Q.L.; Chen, D.N.; Jiang, M.H.; Chen, B.; Lu, Y.J.; Li, J.; Liu, L.; Chen, S.H. Absolute configuration of polypropionate derivatives: Decempyrones A–J and their MptpA inhibition and anti-inflammatory activities. Bioorg. Chem. 2021, 115, 105156. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ren, X.; Tao, H.M.; Cai, W.T.; Chen, Y.C.; Luo, X.W.; Guo, P.; Liu, Y.H. Anti-inflammatory polyketides from an alga-derived fungus Aspergillus ochraceopetaliformis SCSIO 41020. Mar. Drugs 2022, 20, 295. [Google Scholar] [CrossRef]
- Liu, Z.M.; Qiu, P.; Liu, H.J.; Li, J.; Shao, C.L.; Yan, T.; Cao, W.H.; She, Z.G. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg. Chem. 2019, 88, 102973. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, M.; Fan, H.; Chen, Y.; Dong, S.; Zhou, F.; Wang, B.; Liu, J.; Jin, J.; Luo, Y.; et al. The marine Penicillium sp. GGF16-1-2 metabolite dicitrinone G inhibits pancreatic angiogenesis by regulating the activation of NLRP3 inflammasome. J. Nat. Med. 2024, 78, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Suping Xiao, S.P.; Huiyi Liao, H.Y.; Jiang, Q.J.; Chen, G.; Wen, L. A new chloro-containing γ-butyrolactone from the mangrove endophytic fungus Neofusicoccum parvum Y2NBKZG1016. Chem. Nat. Comp. 2023, 59, 424–427. [Google Scholar] [CrossRef]
- Zou, Z.B.; Wu, T.Z.; Yang, L.H.; He, X.W.; Liu, W.Y.; Zhang, K.; Xie, C.L.; Xie, M.M.; Zhang, Y.; Yang, X.W.; et al. Hepialiamides A–C: Aminated fusaric acid derivatives and related metabolites with anti-inflammatory activity from the deep-sea-derived fungus Samsoniella hepiali W7. Mar. Drugs 2023, 21, 596. [Google Scholar] [CrossRef]
- Dong, L.; Kim, H.J.; Cao, T.Q.; Liu, Z.; Lee, H.; Ko, W.; Kim, Y.C.; Sohn, J.H.; Kim, T.K.; Yim, J.H.; et al. Anti-inflammatory effects of metabolites from antarctic fungal strain Pleosporales sp. SF-7343 in HaCaT human keratinocytes. Int. J. Mol. Sci. 2021, 22, 9674. [Google Scholar] [CrossRef]
- Hsiao, G.; Chi, W.C.; Chang, C.H.; Chiang, Y.R.; Fu, Y.J.; Lee, T.H. Bioactive pulvinones from a marine algicolous fungus Aspergillus terreus NTU243. Phytochemistry 2022, 200, 113229. [Google Scholar] [CrossRef]
- Tilvi, S.; Parvatkar, R.; Singh, K.S.; Devi, P. Chemical investigation of marine-derived fungus Aspergillus flavipes forpotential anti-inflammatory agents. Chem. Biodivers. 2021, 18, e2000956. [Google Scholar] [CrossRef]
- Chen, S.W.; Zhang, Y.; Niu, X.T.; Mohyuddin, S.G.; Wen, J.Y.; Bao, M.L.; Ju, X.H. Coral-derived endophytic fungal product, butyrolactone-I, alleviates LPS induced intestinal epithelial cell inflammatory response through TLR4/NF-κB and MAPK signaling pathways: An in vitro and in vivo studies. Front. Nutr. 2021, 8, 748118. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.Y.; Zhu, H.R.; Sun, Y.T.; Wu, Y.; Liao, H.Z.; Gui, Y.H.; Li, L.; Liu, L.; Sun, F.; et al. Butyrolactone-I, an efficient α-glucosidase inhibitor, improves type 2 diabetes with potent TNF-α–lowering properties through modulating gut microbiota in db/db mice. FASEB J. 2019, 33, 12616. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.S.; Tian, D.M.; Wei, J.H.; Ma, Y.H.; Zhang, Y.X.; Chen, M.; Ding, W.J.; Wu, B.; Tang, J.S. New drimane sesquiterpenes and polyketides from marine-derived fungus Penicillium sp. TW58-16 and their anti-inflammatory and α-glucosidase inhibitory effects. Mar. Drugs 2021, 19, 416. [Google Scholar] [CrossRef]
- Hu, Y.W.; Zhao, X.Y.; Song, Y.; Jiang, J.H.; Long, T.; Cong, M.J.; Miao, Y.H.; Liu, Y.Y.; Yang, Z.Y.; Zhu, Y.G.; et al. Anti-inflammatory and neuroprotective α-Pyrones from a marine-derived strain of the fungus Arthrinium arundinis and their heterologous expression. J. Nat. Prod. 2024, 87, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.B.; Wang, Z.; Chang, W.J.; Zhao, W.B.; Wang, H.; Chen, H.Q.; Dai, H.F.; Lv, F. New azaphilones from the marine-derived fungus Penicillium sclerotiorum E23Y-1A with their anti-inflammatory and antitumor activities. Mar. Drugs 2023, 21, 75. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, Y.B.; Yin, J.J.; Chang, W.J.; Zhao, X.L.; Mao, Y. Two new azaphilones from the marine-derived fungus Penicillium sclerotiorum E23Y-1A. Phytochem. Lett. 2022, 47, 76–80. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Z.X.; Chen, T.; Ye, G.T.; Qiu, L.Y.; Long, Y.H. New azaphilones from mangrove endophytic fungus Penicillium sclerotiorin SCNU-F0040. Nat. Prod. Res. 2023, 37, 296–304. [Google Scholar] [CrossRef]
- Wang, H.C.; Ke, T.Y.; Ko, Y.C.; Lin, J.J.; Chang, J.S.; Cheng, Y.B. Anti-inflammatory azaphilones from the edible alga-derived fungus Penicillium sclerotiorum. Mar. Drugs 2021, 19, 529. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Jiang, M.; Wu, Q.; Li, J.; Shen, H.; Liu, L. Mono-and dimeric xanthones with anti-glioma and anti-inflammatory activities from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 51. [Google Scholar] [CrossRef]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Fu, S.L.; Chen, J.J. Anti-cancer and anti-inflammatory activities of three new chromone derivatives from the marine-derived Penicillium citrinum. Mar. Drugs 2021, 19, 408. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wu, H.C.; Huang, S.J.; Hsiao, G.; Chi, W.C.; Lee, T.H. Anti-inflammatory constituents from a sea anemone-derived fungus Arthrinium arundinis MA30. Phytochemistry 2024, 219, 113998. [Google Scholar] [CrossRef]
- Koopklang, K.; Choodej, S.; Hantanong, S.; Intayot, R.; Jungsuttiwong, S.; Insumran, Y.; Ngamrojanavanich, N.; Pudhom, K. Anti-Inflammatory properties of oxygenated isocoumarins and xanthone from thai mangrove-associated endophytic fungus Setosphaeria rostrata. Molecules 2024, 29, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhang, Y.; Li, G.; Dong, K.; Wang, J.L.; Xiao, S.J.; Lou, H.X.; Peng, X.P. Anti-inflammatory monomeric sorbicillinoids from the marine-fish-derived fungus Trichoderma sp. G13. Fitoterapia 2024, 175, 105963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Deng, Y.L.; Lin, X.J.; Chen, B.; Li, J.; Liu, H.J.; Chen, S.H.; Liu, L. Anti-inflammatory mono-and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Chen, T.; Sun, B.; Tan, Q.; Ouyang, H.; Wang, B.; Yu, H.J.; She, Z.G. Mono-and dimeric sorbicillinoid inhibitors targeting IL-6 and IL-1β from the mangrove-derived fungus Trichoderma reesei BGRg-3. Int. J. Mol. Sci. 2023, 24, 16096. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Meng, Q.Y.; Liu, D.; Fan, A.; Huang, J.; Lin, W.H. Targeted isolation of sorbicilinoids from a deep-sea derived fungus with anti-neuroinflammatory activities. Phytochemistry 2024, 219, 113976. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.X.; Song, X.S.; Pan, W.C.; Cui, H.; Zhao, Z.X. New chromone compounds from the marine derived fungus Diaporthe sp. XW12-1. Fitoterapia 2023, 164, 105384. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, W.; Liao, Q.; She, Z. Pyrone derivatives from a mangrove endophytic fungus Phomopsis asparagi LSLYZ-87. Chem. Biodivers. 2022, 19, e202200491. [Google Scholar] [CrossRef]
- Qin, X.Y.; Huang, J.G.; Zhou, D.X.; Zhang, W.X.; Zhang, Y.J.; Li, J.; Yang, R.Y.; Huang, X.S. Polyketide derivatives, guhypoxylonols A–D from a mangrove endophytic fungus Aspergillus sp. GXNU-Y45 that inhibit nitric oxide production. Mar. Drugs 2021, 20, 5. [Google Scholar] [CrossRef]
- Lei, H.; Bi, X.X.; Lin, X.P.; She, J.L.; Luo, X.W.; Niu, H.; Zhang, D.; Yang, B. Heterocornols from the sponge-derived fungus Pestalotiopsis heterocornis with anti-inflammatory activity. Mar. Drugs 2021, 19, 585. [Google Scholar] [CrossRef]
- Cong, M.J.; Zhang, Y.; Feng, X.Y.; Pang, X.Y.; Liu, Y.H.; Zhang, X.Y.; Yang, Z.Y.; Wang, J.F. Anti-inflammatory alkaloids from the cold-seep-derived fungus Talaromyces helicus SCSIO41311. 3 Biotech. 2022, 12, 161. [Google Scholar] [CrossRef]
- Lu, C.J.; Liang, L.F.; Zhang, G.S.; Li, H.Y.; Fu, C.Q.; Yu, Q.; Zhou, D.M.; Su, Z.W.; Liu, K.; Gao, C.H.; et al. Carneusones A–F, benzophenone derivatives from sponge-derived fungus Aspergillus carneus GXIMD00543. Mar. Drugs 2024, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Quang, T.H.; Tien, N.T.; Kim, K.W.; Kim, Y.C.; Ngan, N.T.T.; Cuong, N.X.; Nam, N.H.; Oh, H. Anti-neuroinflammatory effect of oxaline, isorhodoptilometrin, and 5-hydroxy-7-(2′-hydroxypropyl)-2-methyl-chromone obtained from the marine fungal strain Penicillium oxalicum CLC-MF05. Arch. Pharm. Res. 2022, 45, 90–104. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, Y.C.; Li, S.N.; Zhang, W.Y.; Yan, H.J.; Liu, H.X.; Zhang, W.M. 3-Carboxy-indole derivatives from the deep-sea-derived fungus Phomopsis tersa FS441. Fitoterapia 2024, 172, 105772. [Google Scholar] [CrossRef]
- Song, Y.Y.; She, J.L.; Chen, W.H.; Wang, J.M.; Tan, Y.H.; Pang, X.Y.; Zhou, X.F.; Wang, J.F.; Liu, Y.H. New fusarin derivatives from the marine algicolous fungus Penicillium steckii SCSIO41040. Mar. Drugs 2023, 21, 532. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.Q.; Zheng, Y.Y.; Wang, C.Y.; Liu, Y.; Yao, G.S. Sclerotioloids A–C: Three new alkaloids from the marine-derived fungus Aspergillus sclerotiorum ST0501. Mar. Drugs 2023, 21, 219. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Guo, X.; Wu, J.S.; Liu, D.; Gu, Y.C.; Huang, J.; Fan, A.; Lin, W.H. Prenylated notoamide-type alkaloids isolated from the fungus Aspergillus sclerotiorum and their inhibition of NLRP3 inflammasome activation and antibacterial activities. Phytochemistry 2022, 203, 113424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Wang, J.Z.; Tian, Y.; Li, M.; Xu, S.H.; Zhang, L.J.; Luo, X.W.; Tan, Y.H.; Liang, H.; Chen, M. Equisetin protects from atherosclerosis in vivo by binding to STAT3 and inhibiting its activity. Pharmacol. Res. 2024, 206, 107289. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.T.; Yang, L.; Guo, J.C.; Ma, Q.Y.; Xie, Q.Y.; Jiang, L.; Yu, Z.F.; Dai, H.F.; Zhao, Y.X. Anti-diabetic and anti-inflammatory indole diterpenes from the marine-derived fungus Penicillium sp. ZYX-Z-143. Bioorg. Chem. 2024, 145, 107205. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, L.; Fu, P.; Leao, T.F.; Li, T.; Wang, T.; Poulin, R.X.; Gaspar, L.R.; Naman, C.B.; Thavarool, P.S. Targeted isolation of two new anti-inflammatory and UV-A protective dipyrroloquinones from the sponge-associated fungus Aspergillus tamarii MCCF102. Planta Med. 2022, 88, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Anh, C.V.; Yoon, Y.D.; Kang, J.S.; Lee, H.S.; Heo, C.S.; Shin, H.J. Nitrogen-containing secondary metabolites from a deep-sea fungus Aspergillus unguis and their anti-inflammatory activity. Mar. Drugs 2022, 20, 217. [Google Scholar] [CrossRef]
- Yao, G.S.; Ma, Z.L.; Zheng, Y.Y.; Lv, L.; Mao, J.Q.; Wang, C.Y. Bioactive alkaloids from the marine-derived fungus Metarhizium sp. P2100. J. Fungi 2022, 8, 1218. [Google Scholar] [CrossRef]
- Liu, Z.M.; Chen, Y.C.; Li, S.N.; Hu, C.Y.; Liu, H.X.; Zhang, W.M. Indole diketopiperazine alkaloids from the deep-sea-derived fungus Aspergillus sp. FS445. Nat. Prod. Res. 2022, 36, 5213–5221. [Google Scholar] [CrossRef]
- Li, P.H.; Zhang, M.Q.; Li, H.N.; Wang, R.C.; Hou, H.R.; Li, X.B.; Liu, K.C.; Chen, H. New prenylated indole homodimeric and pteridine alkaloids from the marine-derived fungus Aspergillus austroafricanus Y32-2. Mar. Drugs 2021, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Liu, Z.M.; Tan, H.B.; Chen, Y.C.; Zhu, S.; Liu, H.X.; Zhang, W.M. Photeroids A and B, unique phenol–sesquiterpene meroterpenoids from the deep-sea-derived fungus Phomopsis tersa. Org. Biomol. Chem. 2020, 18, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wu, L.; Liu, R.R.; Li, J.L.; Liu, L.L.; Chen, C.; Li, J.S.; Zhang, K.; Liao, J.J.; Long, Y.H. Penifuranone A: A novel alkaloid from the mangrove endophytic fungus Penicillium crustosum SCNU-F0006. Int. J. Mol. Sci. 2024, 25, 5032. [Google Scholar] [CrossRef]
- Chen, S.H.; Jiang, M.H.; Chen, B.; Salaenoi, J.; Niaz, S.I.; He, J.G.; Liu, L. Penicamide A, a unique N, N′-ketal quinazolinone alkaloid from ascidian-derived fungus Penicillium sp. 4829. Mar. Drugs 2019, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Du, H.F.; Liu, Y.F.; Cao, F.; Luo, D.Q.; Wang, C.Y. Novel anti-inflammatory diketopiperazine alkaloids from the marine-derived fungus Penicillium brasilianum. Appl. Microbiol. Biot. 2024, 108, 194. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhu, Q.; Li, J.; Yang, R.; Zhang, J.; You, M.; Luo, L.; Yang, B. Optimization of Fermentation Process for New Anti-Inflammatory Glycosylceramide Metabolite from Aspergillus sp. Metabolites 2024, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, X.; Xu, L.; El-Shazly, M.; Ma, C.; Yuan, S.; Wang, P.; Luo, L. Two new cerebroside metabolites from the marine fungus Hortaea werneckii. Chem. Biodivers. 2022, 19, e202200008. [Google Scholar] [CrossRef]
- Hsiao, G.; Wang, S.W.; Chiang, Y.R.; Chi, W.C.; Kuo, Y.H.; Phong, D.A.; Chen, C.Y.; Lee, T.H. Anti-inflammatory effects of peptides from a marine algicolous fungus Acremonium sp. NTU492 in BV-2 microglial cells. J. Food. Drug Anal. 2020, 28, 283. [Google Scholar] [CrossRef]
- Ding, W.J.; Tian, D.M.; Chen, M.; Xia, Z.X.; Tang, X.Y.; Zhang, S.H.; Wei, J.H.; Li, X.N.; Yao, X.S.; Wu, B.; et al. Molecular networking-guided isolation of cyclopentapeptides from the hydrothermal vent sediment derived fungus Aspergillus pseudoviridinutans TW58-5 and their anti-inflammatory effects. J. Nat. Prod. 2023, 86, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Chen, Y.H.; Bian, H.H.; Zhang, J.P.; Su, L.; Han, H.; Zhang, W. Anti-inflammatory ergosteroid derivatives from the coral-associated fungi Penicillium oxalicum HL-44. Molecules 2023, 28, 7784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, Z.; Qiu, P.; Wang, Q.; Yan, J.; Lu, Y.; Wasu, P.A.; Hong, K.; She, Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liang, J.; Wang, Y.; Liu, Y.; Zhou, C.; Hong, P.; Zhang, Y.; Qian, Z.J. A new benzaldehyde from the coral-derived fungus Aspergillus terreus C23-3 and its anti-inflammatory effects via suppression of MAPK signaling pathway in RAW264. 7 cells. J. Zhejiang Univ. Sci. B 2022, 23, 230–240. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, X.M.; Yang, X.; Tang, M.M.; Liao, Q.Y.; Meng, B.Z.; Liao, S.; Chen, G.Y. Three new bioactive natural products from the fungus Talaromyces assiutensis JTY2. Bioorg. Chem. 2020, 94, 103362. [Google Scholar] [CrossRef]
- Wen, H.L.; Chen, C.M.; Sun, W.G.; Zang, Y.; Li, Q.; Wang, W.X.; Zeng, F.R.; Liu, J.J.; Zhou, Y.; Zhou, Q.; et al. Phenolic C-glycosides and aglycones from marine-derived Aspergillus sp. and their anti-inflammatory activities. J. Nat. Prod. 2019, 82, 1098–1106. [Google Scholar] [CrossRef]
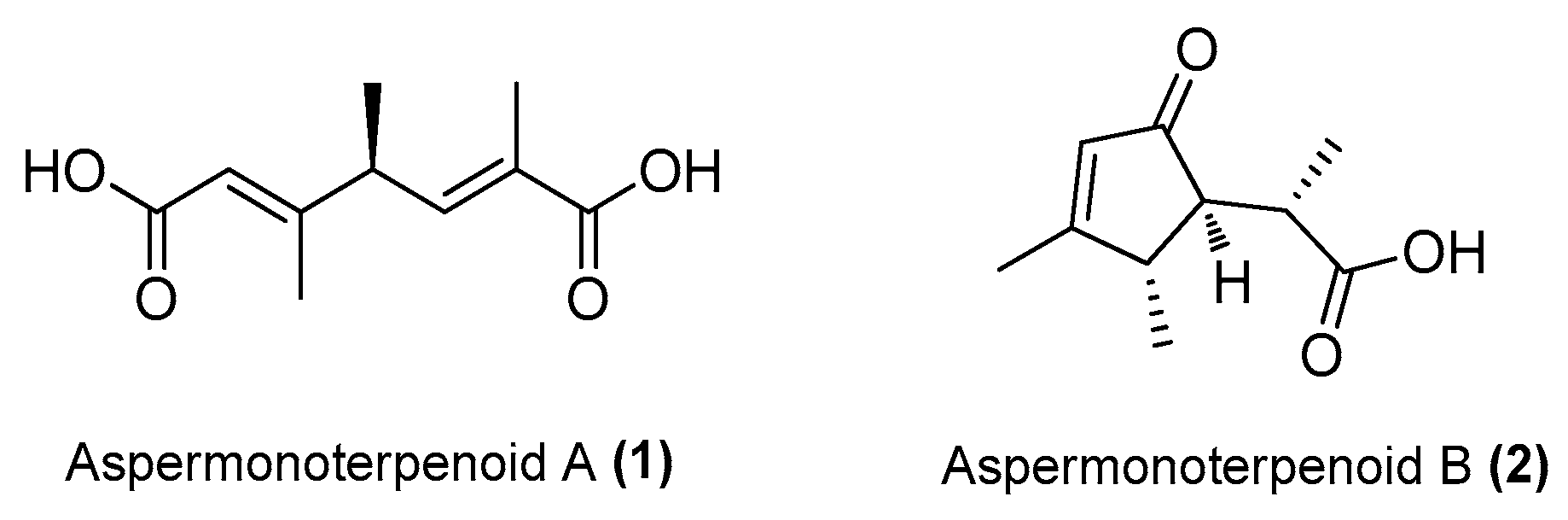
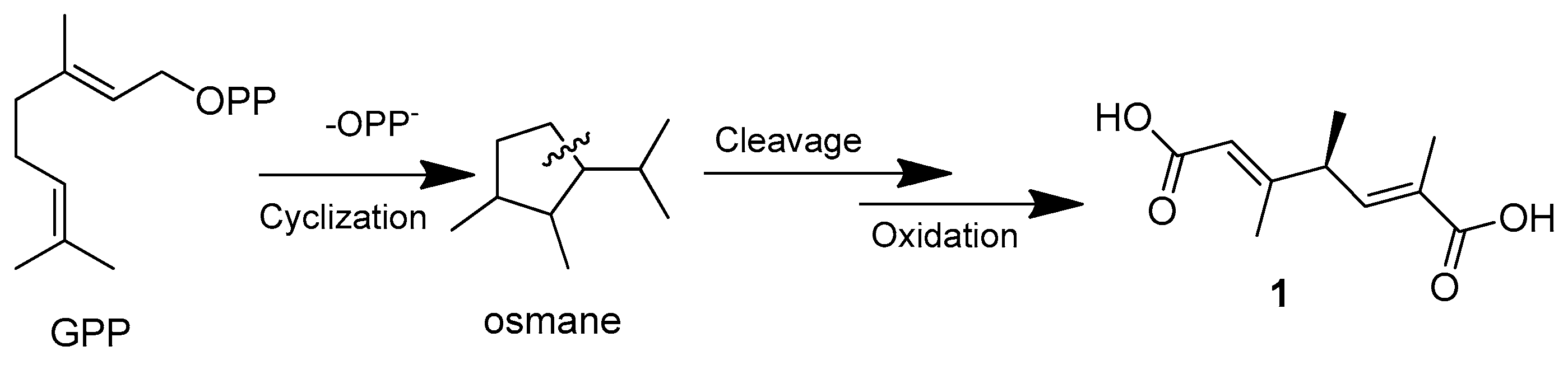
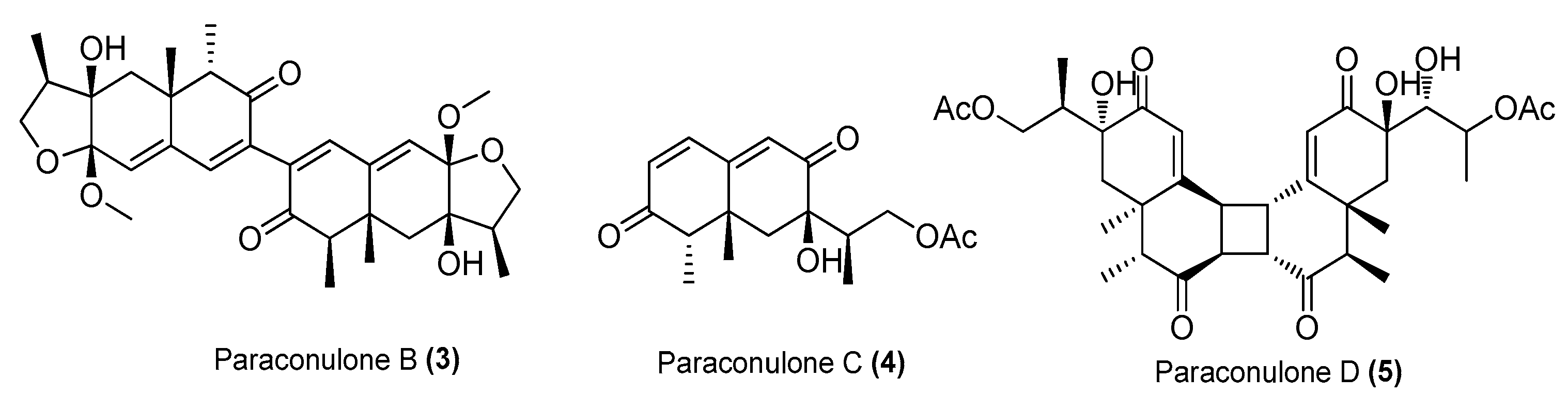
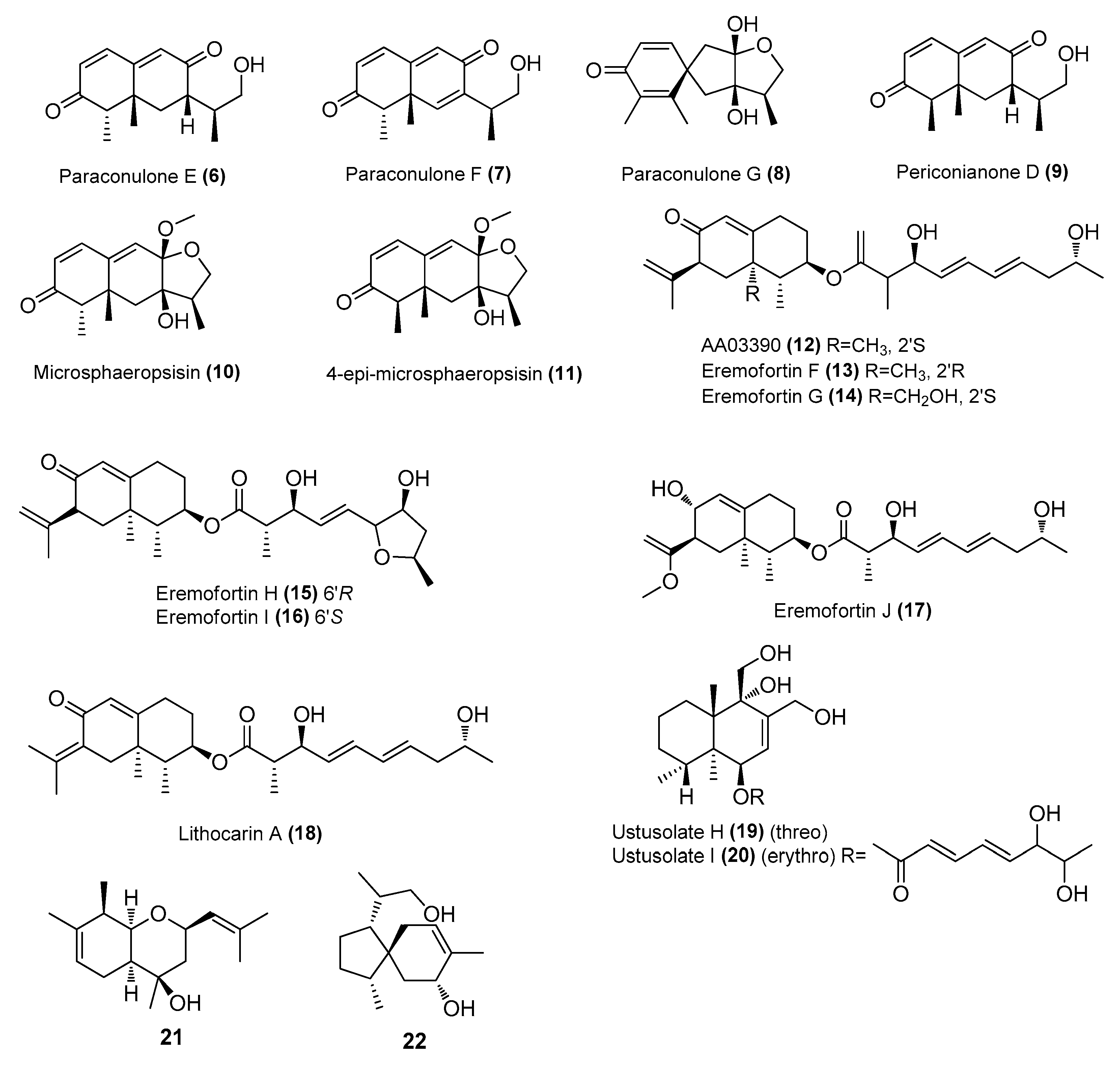

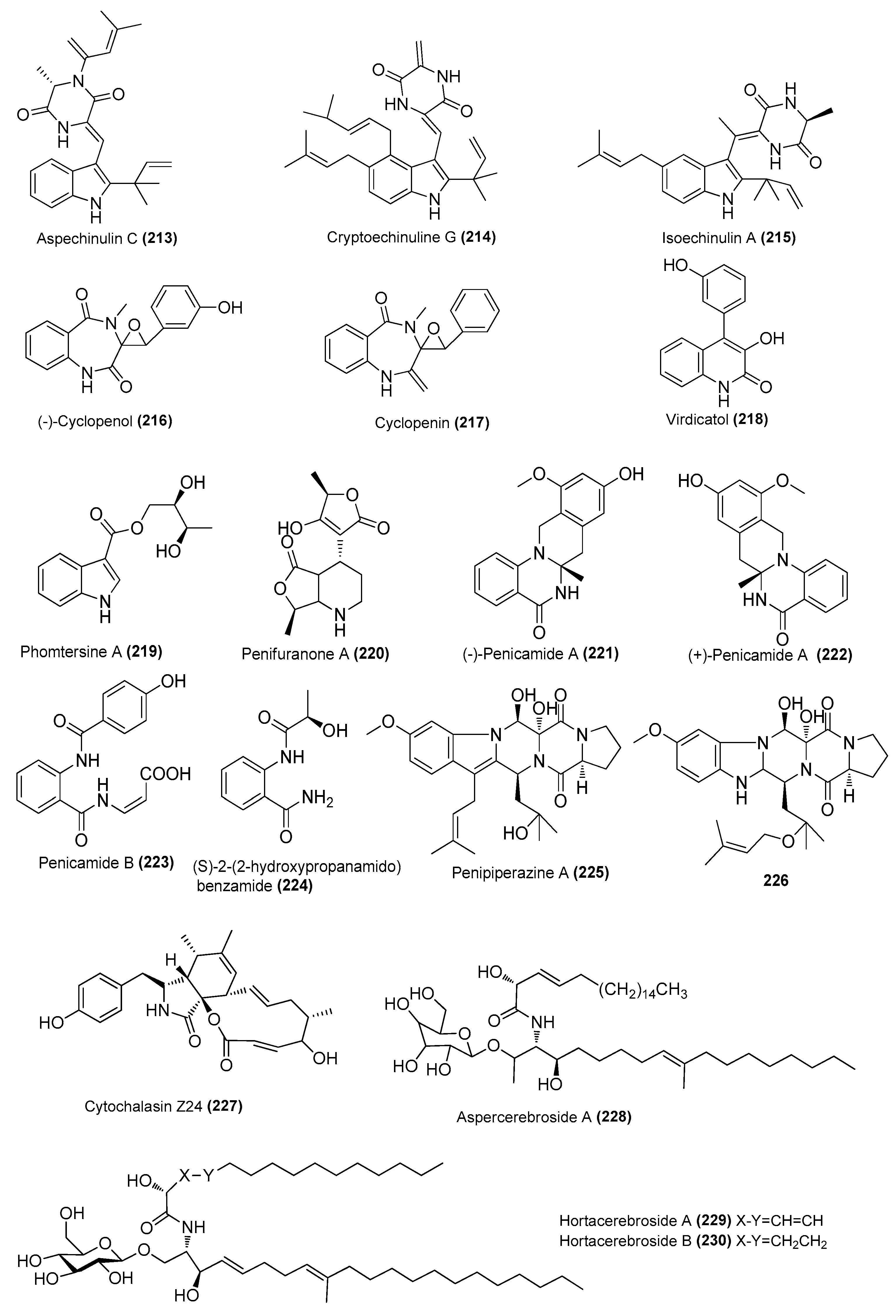
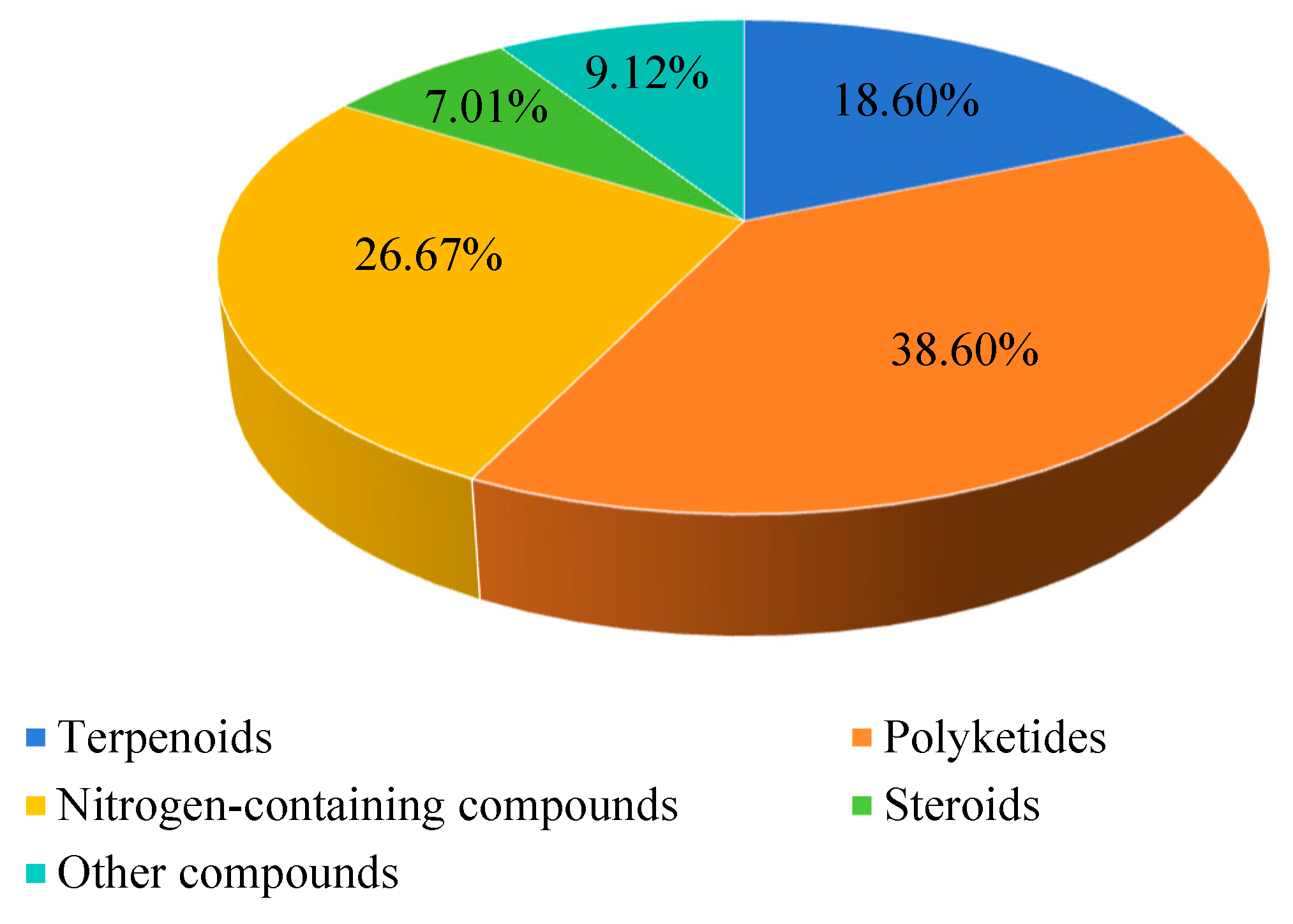
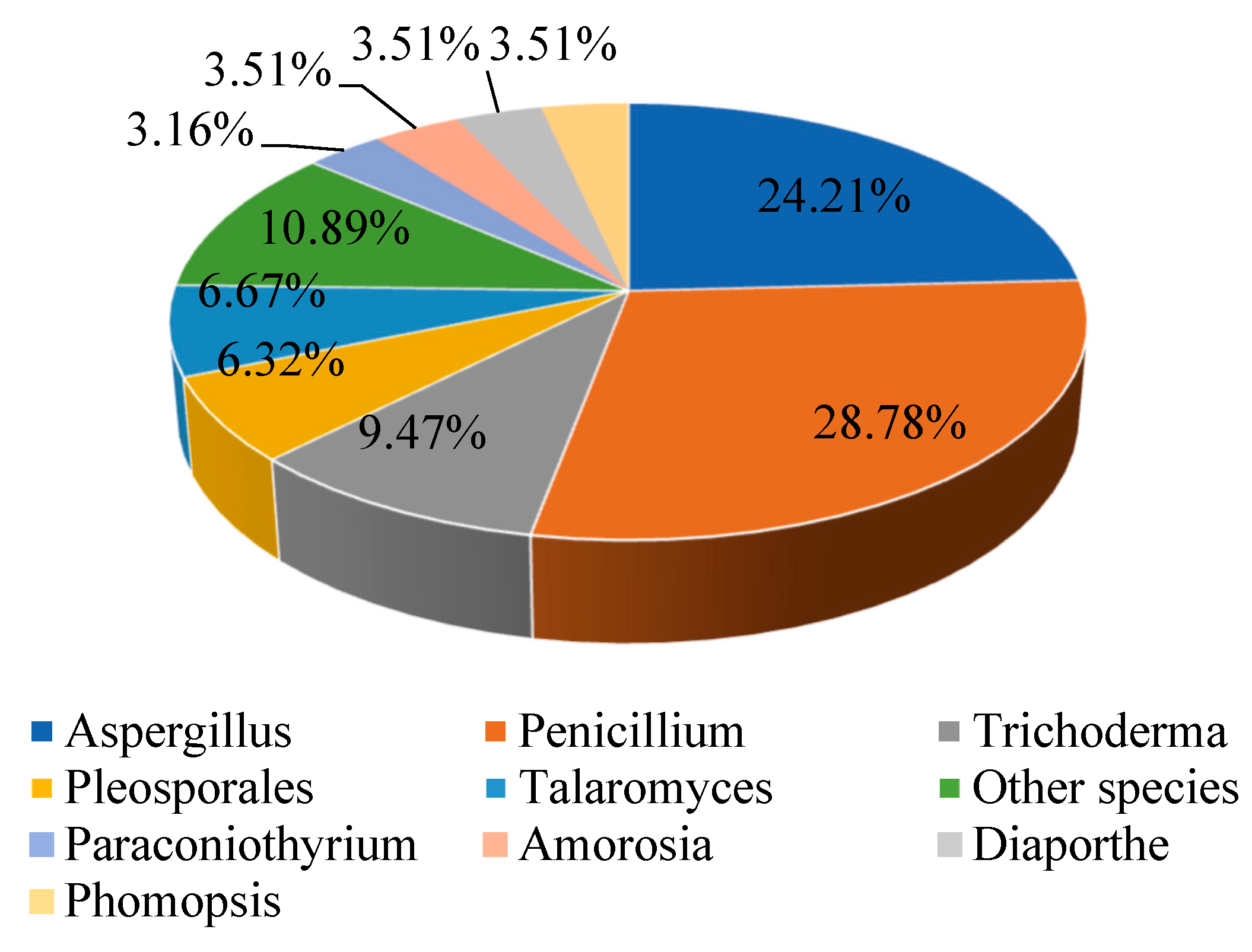
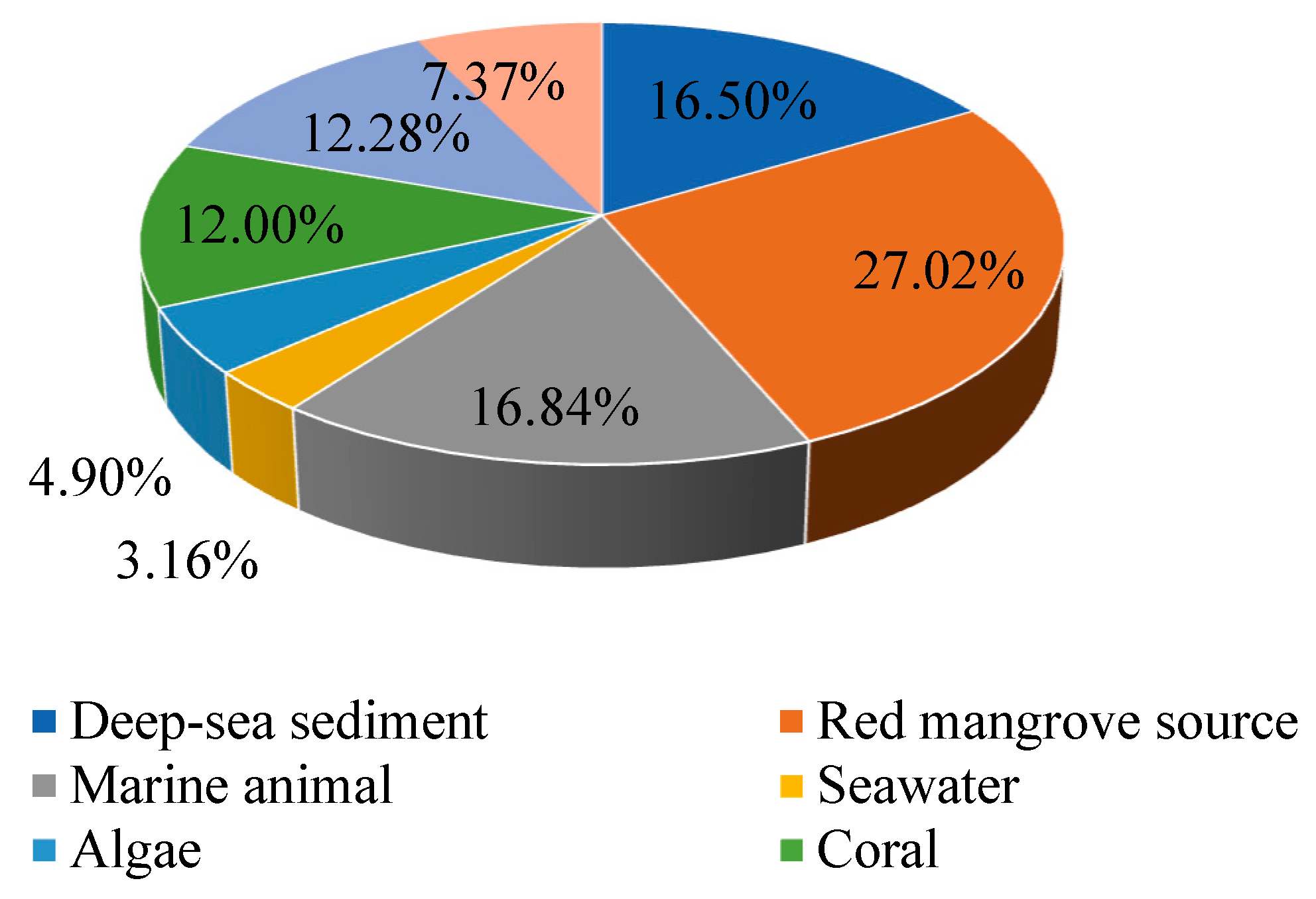
| Compounds | Producing Strains | Habitats | Genbank Accession Number | Bioactivities | References |
|---|---|---|---|---|---|
| Aspermonoterpenoid A (1) | Aspergillus sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 20 µM | [24] |
| Aspermonoterpenoid B (2) | A. sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 10 µM | [24] |
| Paraconulones B−E (3−6) | Paraconiothyrium sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.9 ± 2.6, 7.7 ± 2.0, 2.8 ± 0.5, 8.1 ± 2.9 μΜ, respectively | [25] |
| Paraconulone F (7) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 40 ± 15 μΜ | [25] |
| Paraconulone G (8) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 8.1 ± 3.5 μΜ | [25] |
| Periconianone D (9) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 98 ± 17 μΜ | [25] |
| Microsphaeropsisin (10) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 80 ± 38 μΜ | [25] |
| 4-epi-microsphaeropsisin (11) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 4.6 ± 3.5 μΜ | [25] |
| AA03390 (12) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14.5 μΜ | [26] |
| Eremofortins G-J (13-17) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 8.6−13.5 μΜ | [26] |
| lithocarin A (18) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 10.5 μΜ | [26] |
| Ustusolates H-J (19-20) | Aspergillus insuetus SYSU6925 | Seagrass, Zhuhai, Guangdong, China | MZ411391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.5 and 32.6 μΜ, respectively | [27] |
| 21 and 22 | Eutypella sp. D-1 | London Island, Arctic | FJ430580 | Modulated the MAPK and NLRP3/caspase-1 signaling pathways | [28] |
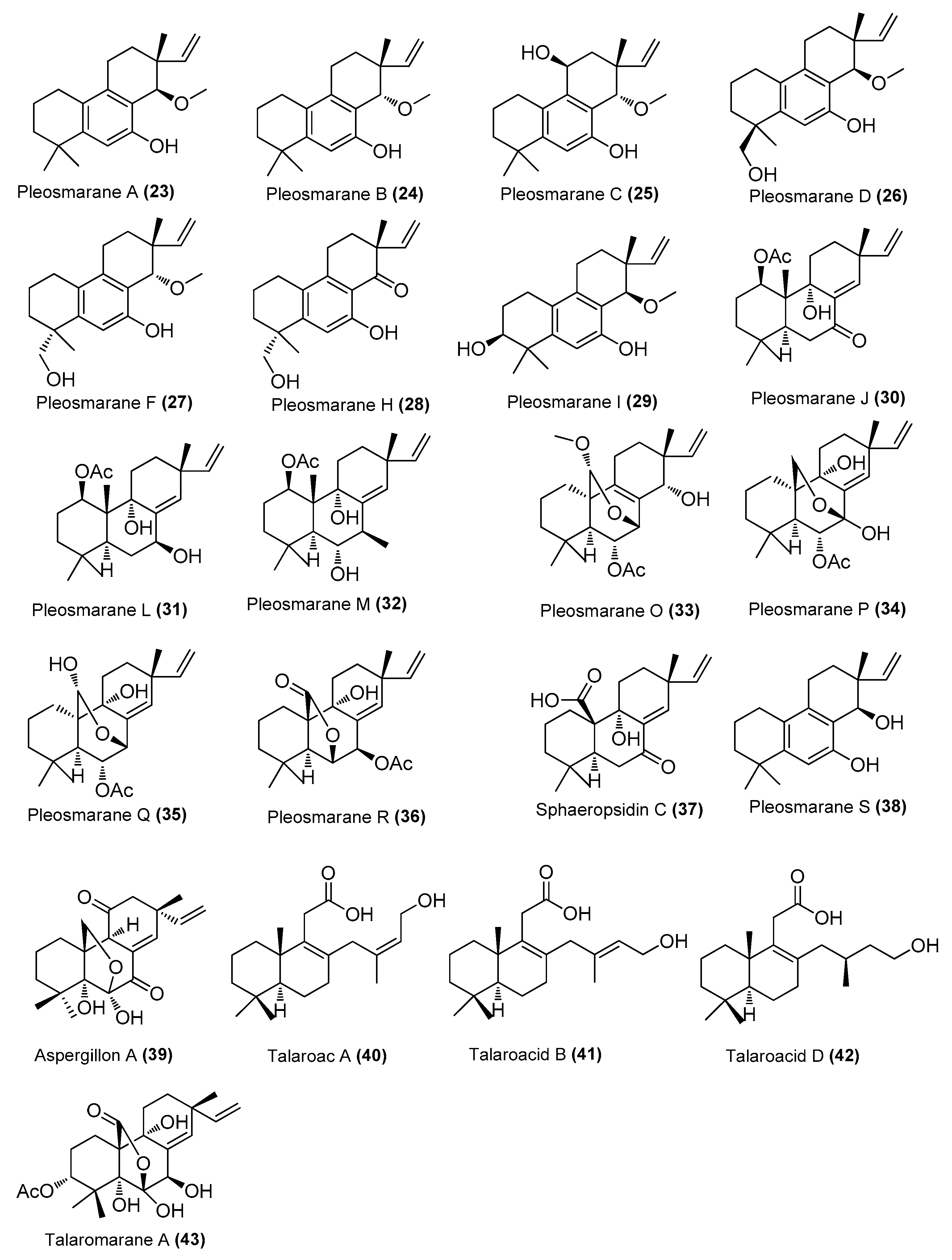
| Pleosmaranes A-D (23-26), F (27), H–J (28–30), L (31), M (32), and O–R (33–36); sphaeropsidin C (37), pleosmarane S (38) | Pleosporales sp. HNQQJ-1 | Mangrove, Dongzhai Harbor Mangrove Nature Reserve, Hainan, China | OR616722 | Inhibited NO production in LP S induced RAW 264.7 cells, IC50 = 30, 37, 38, 42, 42, 19, 35, 33, 25, 35, 37, 30, 33, 35, 31 and 40 μM, compared with the positive control (L-NMMA, 33 μM). | [29] |
| Aspergillon A (39) | Eutypella scoparia GZU-4-19Y | Xuwen, Guangdong, China | OM920979 | Inhibited NO production, IC50 = 2.0 μM, suppressed the protein expression of iNOS and COX-2 at 2.5 μM | [30] |
| Talaroacids A (40) and D (42), Talaromarane A (43) | Talaromyces sp. JNQQJ-4 | Mangrove, Jinniu Island Mangrove Nature Reserve, Guangzhou, China | MK450749.1 | Inhibited NO production, IC50 = 15.78, 21.60, and 13.38 μM, respectively | [31] |
| Talaroacid B (41) | Talaromyces sp. JNQQJ-4 | Jinniu Island Mangrove Nature Reserve, Guangzhou, China | MK450749.1 | Inhibited NO production, IC50 = 21.60 μΜ, positive control quercetin (IC50, 11.33 μM) | [31] |
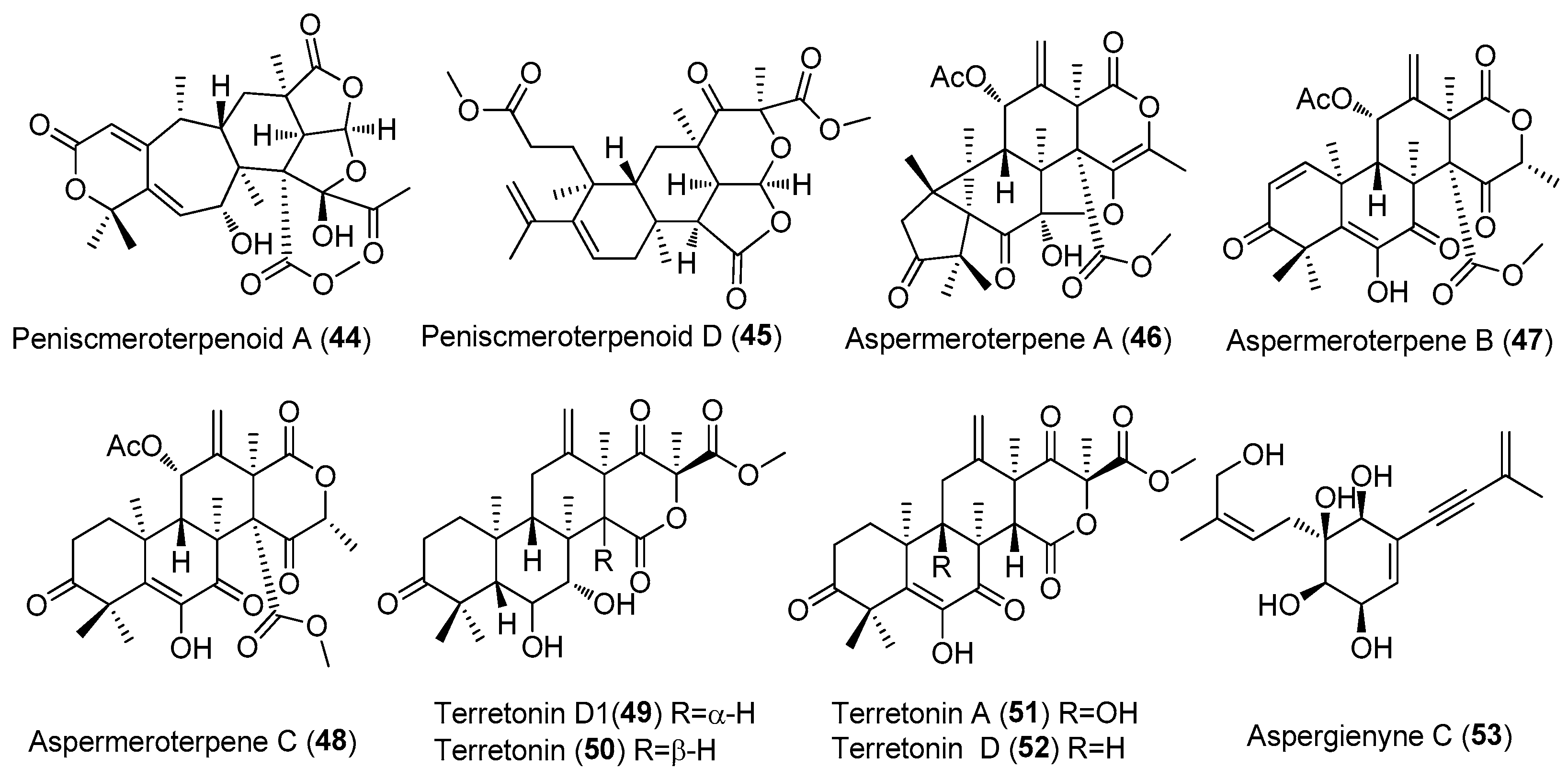
| Peniscmeroterpenoids A and D (44 and 45) | Penicillium sclerotiorum GZU-XW03-2. | Onchidium sp., Guangdong, China. | MT071304) | Inhibited NO production, IC50 = 26.60 and 8.79 μM, respectively | [32] |
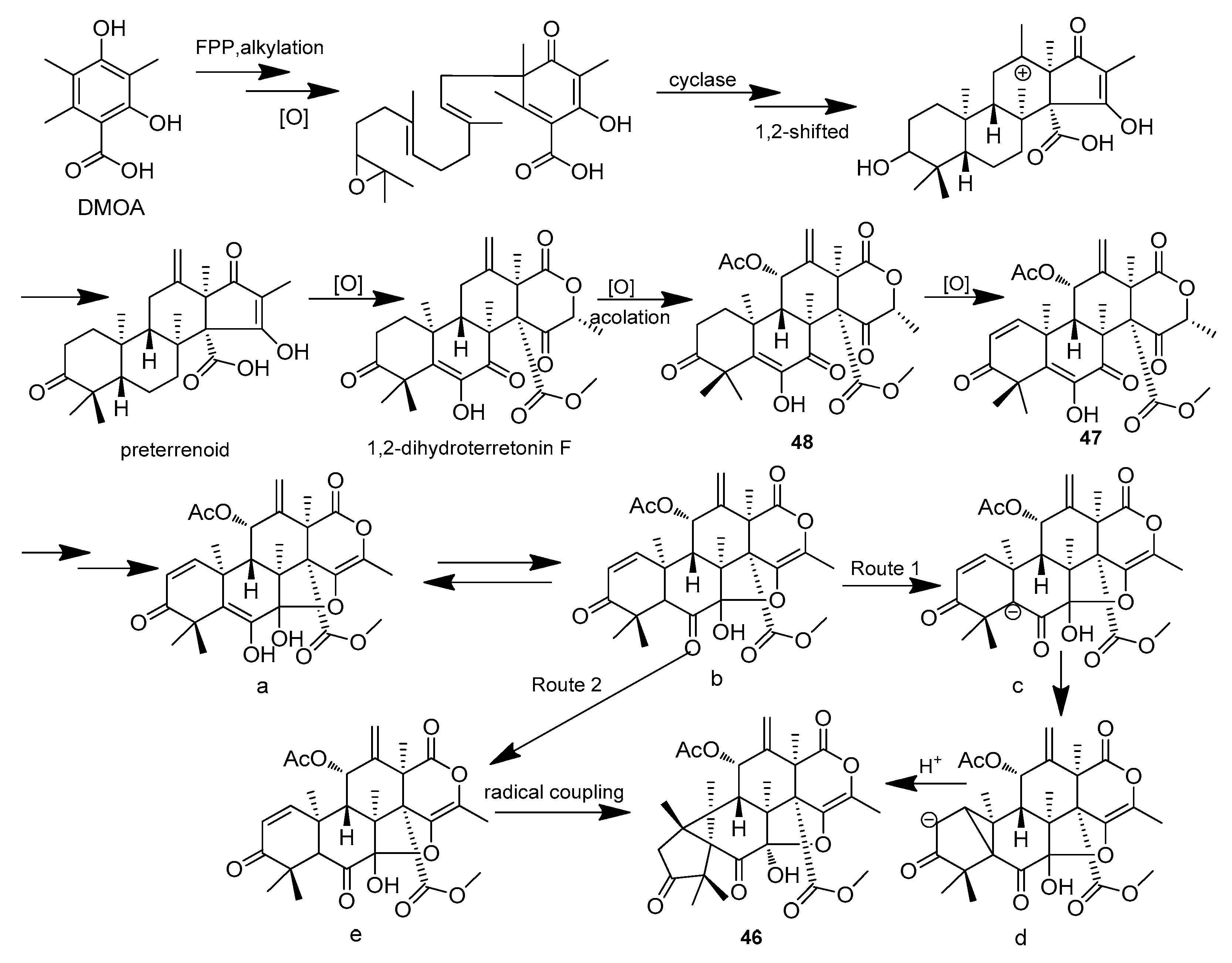
| Aspermeroterpene A-C (46–48) | Aspergillus terreus GZU 31-1 | Guangdong province (Zhanjiang, Xuwen), China | MN860009 | Inhibited NO production, IC50 (anti-inflammatory)17.8,14.1 and 13.4 μM | [33] |
| Terretonin D1(49), Terretonin (50), Terretonins A and D (51 and 52) | Aspergillus terreus ML-44 | Pacific oyster, Yangma Island in Yantai, China | CGMCC 15664 | Inhibited NO production, inhibitory rates of 30.2%, 34.0%, 22.5% and 23.5%, at 50 μg/mL | [34] |
| Aspergienyne C (53) | Aspergillus sp. GXNU-Y65 | Mangrove Kandelia cande, Beihai, China | MT626087 | Aspergienyne C had strong anti-nonalcoholic steatohepatitis activity against AML12 cells treated with PA (palmitic acid) + OA (oleic acid). | [35] |
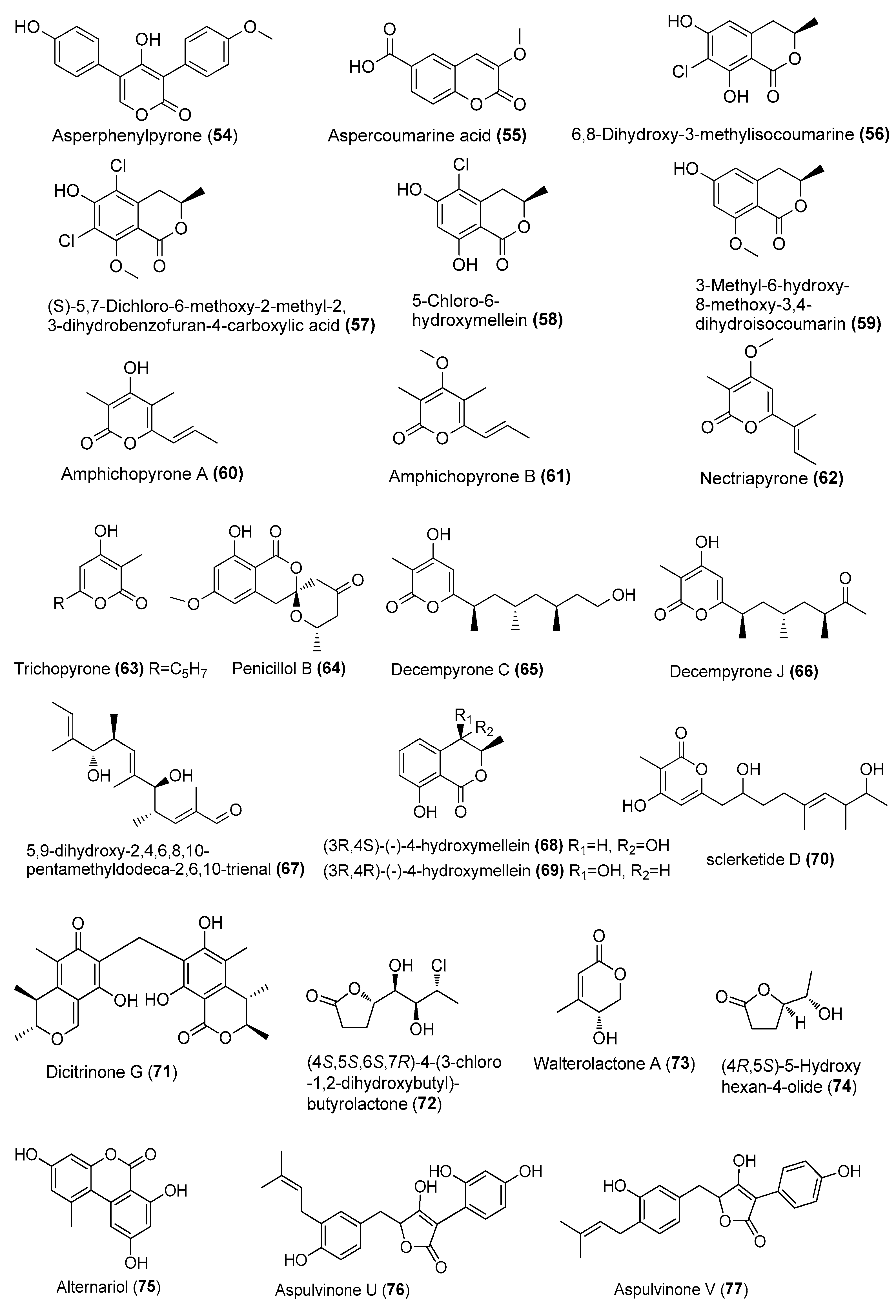
| Asperphenylpyrone (54) | Aspergillus sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 20 µM | [24] |
| Aspercoumarine acid (55) | A. sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 10 µM | [24] |
| 6,8-dihydroxy-3-methylisocoumarine (56) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| (S)-5,7-dichloro-6-methoxy-2-methyl-2,3-dihydrobenzofuran-4-carboxylic acid (57) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| 5-chloro-6-hydroxymellein (58) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| 3-methyl-6-hydroxy-8-methoxy-3,4-dihydroisocoumarin (59) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| Amphichopyrones A (60) and B (61) | Amphichorda felina SYSU-MS7908 | Culturing ascidian, Xisha Islands, South China Sea, China. | MT786206 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 18.09 and 7.18 μΜ, respectively | [37] |
| Nectriapyrone (62) | Diaporthe sp. SYSU-MS4722 | Shenzhen City, Guangdong, Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 35.4 μΜ, positive control indomethacin, IC50 = 35.8 µM | [38] |
| Trichopyrone (63) | Penicillium sp. DM815 | Qinglan, Wenchang, Hainan Province | MW497629 | Weakly inhibited LPS-induced NO release at 10 μM | [39] |
| Penicillol B (64) | Penicillium sp. BJR-P2 | Mangrove Avicennia marinav, Yangjiang Hailing Island Mangrove Wetland Park, China | PRJNA793386 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 12 μΜ | [40] |
| Decempyrones C (65) and J (66) | Fusarium decemcellulare SYSU-MS6716 | Sea grass, Lingshui Xincungang and Li’angang Special Protected Area, Hainan, China | MW851212 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.4 and 21.7 μΜ, respectively | [41] |
| 5,9-dihydroxy-2,4,6,8,10-pentamethyldodeca-2,6,10-trienal (67) | A. ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Blocked the release of pro-inflammatory cytokines (IL-6, MCP-1, and TNF-α) induced by LPS both in vivo and in vitro | [42] |
| (3R,4S)-(−)-4-hydroxymellein (68) | A. ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Inhibited NO production in LPS-induced RAW 264.7 cells | [42] |
| (3R,4R)-(−)-4-hydroxymellein (69) | A.ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Inhibited NO production in LPS-induced RAW 264.7 cells | [42] |
| sclerketide D (70) | Penicillium sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.5 μΜ | [43] |
| Dicitrinone G (71) | Penicillium sp. GGF 16-1-2 | in the South China Sea | _ | Regulated the activation of NLRP3 infammasome | [44] |
| (4S,5S,6S,7R)-4-(3-chloro-1,2-dihydroxybutyl)-butyrolactone (72) | Neofusicoccum parvum Y2NBKZG1016 | Mangrove Sonneratia glauca, Nansha District, Guangzhou, China | _ | Weak anti-inflammatory activity at concentrations ≥ 6.25 μM | [45] |
| Walterolactone A (73) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-activated BV-2 microglia cells, with inhibition rates of 38.6% at 1 µM | [46] |
| (4R,5S)-5-hydroxyhexan-4-olide (74) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-activated BV-2 microglia cells, IC50 = 426.2 nM | [46] |
| Alternariol (75) | Pleosporales sp. SF-7343 | King George Island, Antarctica | MK785420 | Inhibited inflammatory factors | [47] |
| Aspulvinone U (76) | Aspergillus terreus NTU243 | Marine alga Ulva lactuca, northeastern coast, Taiwan, China | PRJNA611016 | Inhibited LPS-induced MMP-9-mediated gelatinolysis, inhibition rate of 56.0% at 10 µM | [48] |
| Aspulvinone V (77) | A. terreus NTU243 | Marine alga Ulva lactuca, northeastern coast, Taiwan, China | PRJNA611016 | Inhibited NO production in LPS-induced RAW 264.7 cells, and LPS-induced MMP-9-mediated gelatinolysis, with inhibition rates of 45.0% and 67.8%, 10 µM | [48] |
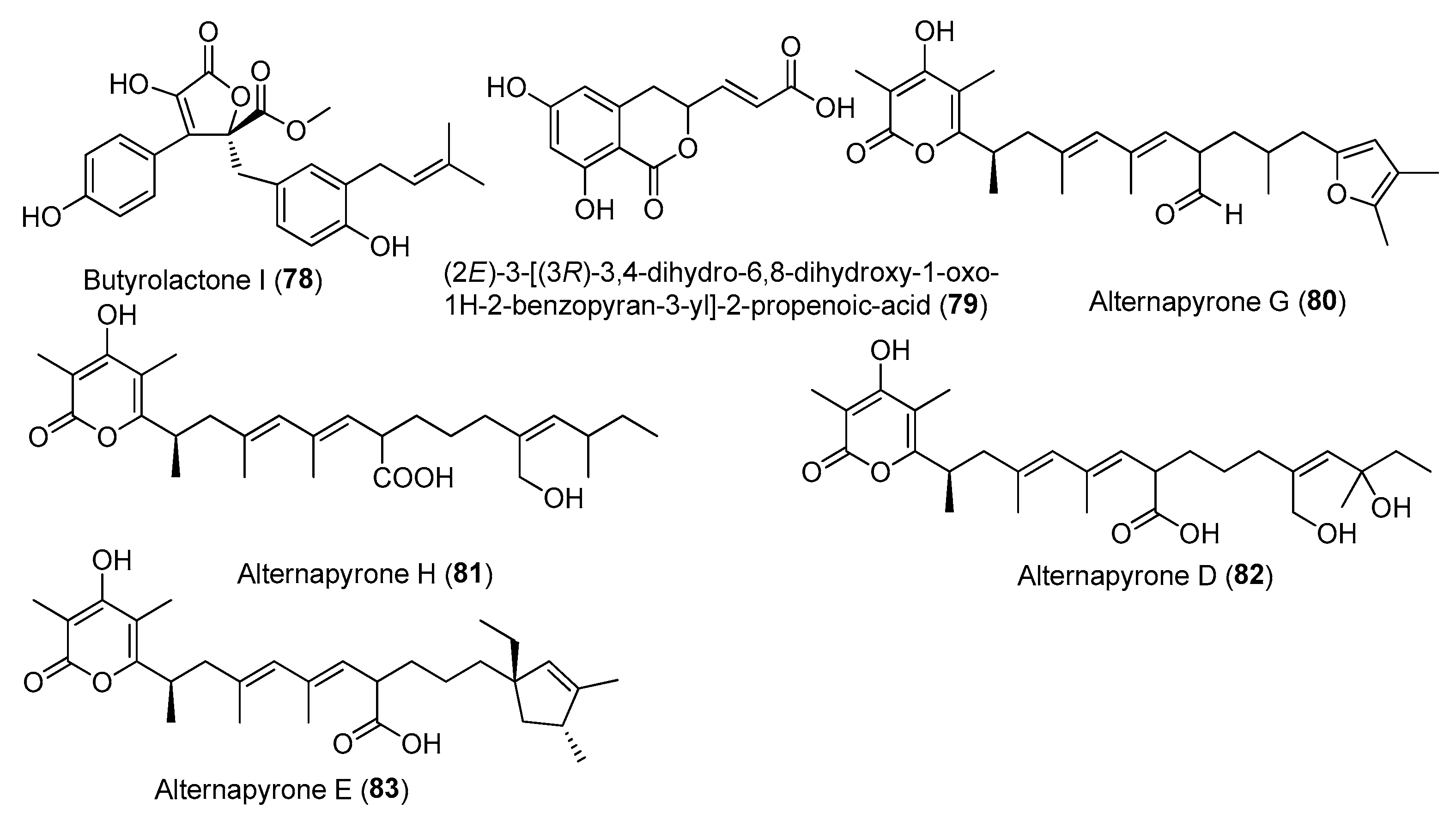
| Butyrolactone I (78) | Aspergillus flavipes MTCC 5220 | Mangrove plant Acanthus illicifolius, Goa, India | PRJNA611016 | IC50 (anti-inflammatory) 12.03 µM (IL-6), 43.29 µM (TNF-α) | [49] |
| Butyrolactone I (78) | Aspergillus terreus XWC21-10 | Coral Porites pukoensis, Zhanjiang seawaters of the South China Sea | PRJNA611016 | Inhibited the expression of iNOS and COX-2 | [50] |
| Butyrolactone I (78) | A. terreus var. africanus IFO 8835 | _ | _ | Regulating inflammation by regulating the gut microbiota | [51] |
| (2E)-3-[(3R)-3,4-dihydro-6,8-dihydroxy-1-oxo-1H-2-benzopyran-3-yl]-2-propenoic-acid (79) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | The regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| Alternapyrones G (80) and H (81) | Arthrinium arundinis ZSDS-F3 | Phakellia fusca, Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
| Alternapyrone D (82) | A. arundinis ZSDS-F3 | Phakellia fusca, Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
| 6-alkenylpyrone polyketides alternapyrones E (83) | A. arundinis ZSDS-F3 | The Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
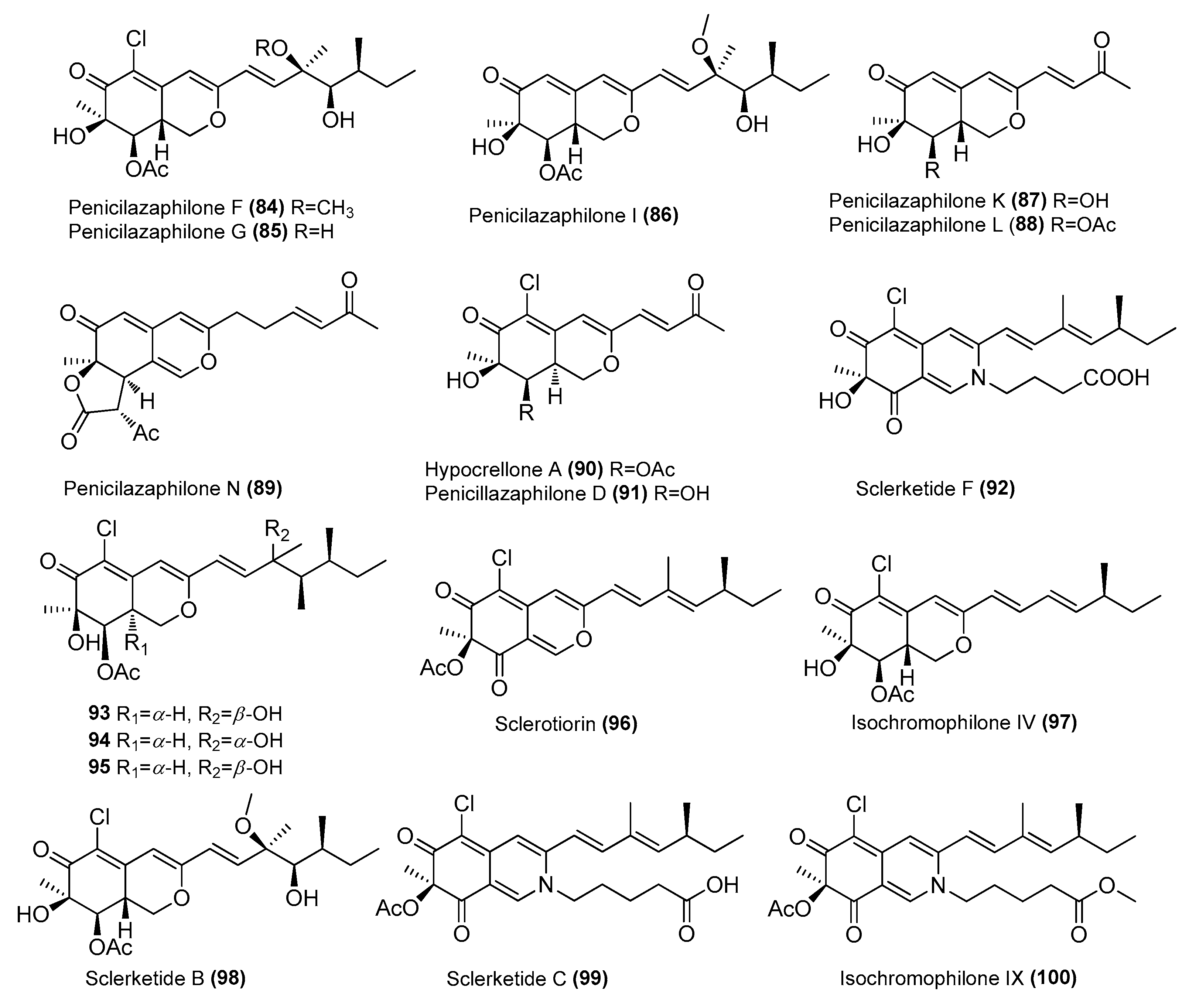
| Penicilazaphilones F (84) and G (85) | Penicillium sclerotiorum E23Y-1A | Sponge Holoxea sp., Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 31.7 ± 1.5 and 34.5 ± 1.4, respectively | [54,55] |
| Penicilazaphilones I, K, L and N (86, 87, 88 and 89) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.63–65.30 µM | [54,55] |
| Hypocrellone A (90) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 25.3 ± 2.2 μΜ | [55] |
| Penicillazaphilone D (91) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 34.8 ± 1.9 μΜ | [55] |
| Sclerketide F (92) | Penicillium sclerotiorin SCNU-F0040 | Mangrove Bruguiera gymnorhiza, Zhanjiang Mangrove Nature Reserve, Guangdong, China | MW-541637 | COX-2 inhibitory activity, IC50 = 47.8 μΜ | [56] |
| 8a-epi-hypocrellone A (93) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| 8a-epi-eupenicilazaphilone C (94) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Promote both TGF-β/Smad signaling and transcriptional function | [57] |
| Hypocrellone A (95) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| Sclerotiorin (96) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited both TGF-β/Smad-mediated signaling and transcriptional function | [57] |
| Isochromophilone IV (97) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| Sclerketide B (98) and Sclerketide C (99) | P. sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.4 and 17.6 μΜ, respectively | [43] |
| Isochromophilone IX (100) | P. sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.7 μΜ, respectively | [43] |
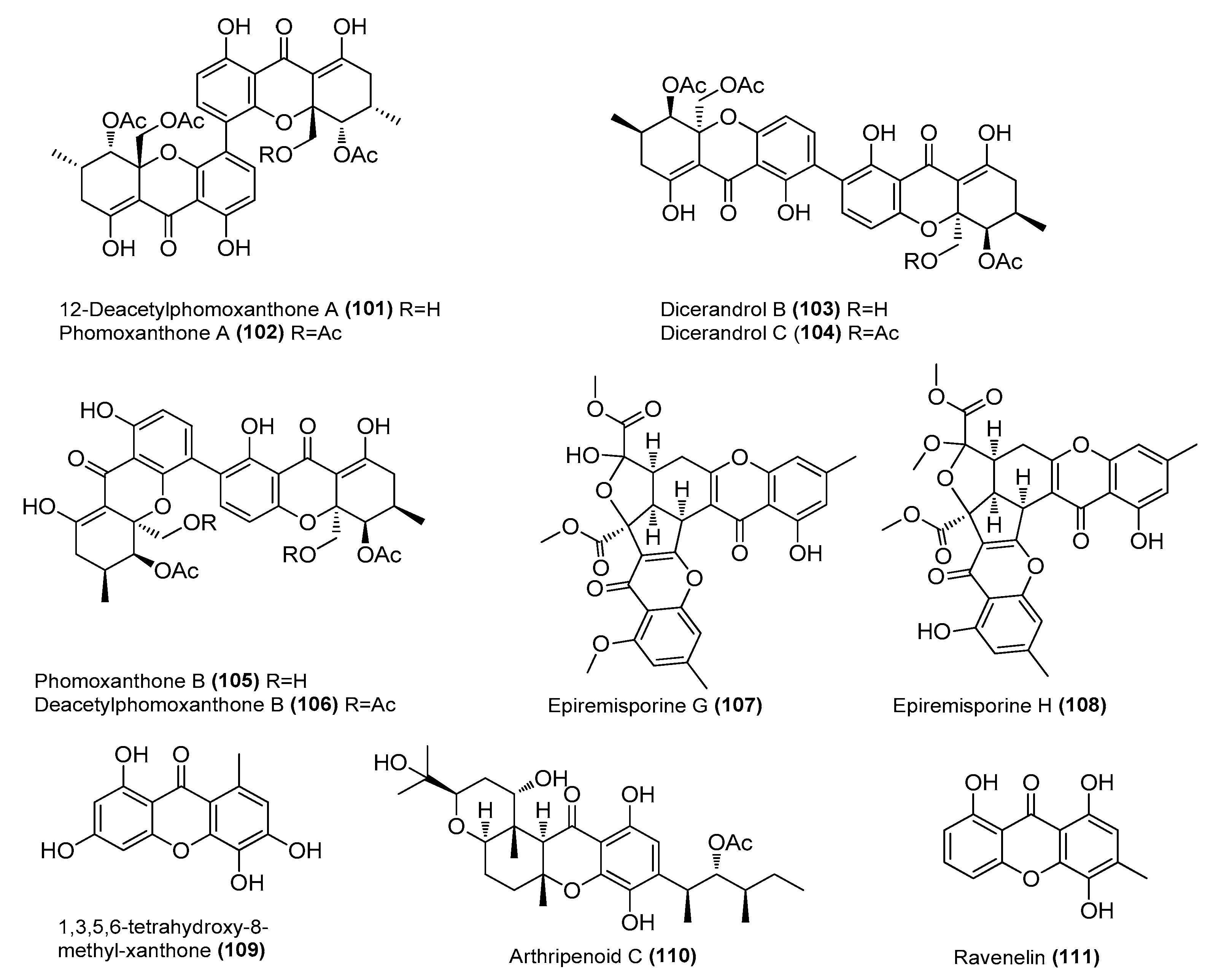
| 12-Deacetylphomoxanthone A (101) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.3 μΜ | [58] |
| Phomoxanthones A (102) and B (105) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.5 and 8.0 μΜ, respectively | [58] |
| Dicerandrols B (103) and C (104) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.3 and 7.6 μΜ, respectively | [58] |
| Deacetylphomoxanthone B (106) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.8 μΜ | [58] |
| Epiremisporines G (107) and H (108) | Penicillium citrinum BCRC 09F458 | Hazailiao, Dongshi, Chiayi, Taiwan, China | _ | Significantly inhibited the production of superoxide ions by fMLP, IC50 = 31.68 ± 2.53, and 33.52 ± 0.42 µM, respectively. Positive control ibuprofen, IC50 = 28.56 µM | [59] |
| 1,3,5,6-tetrahydroxy-8-methyl-xanthone (109) | Arthrinium arundinis MA30 | Sea anemone, Badouzi | OM761170 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 5.3 μΜ | [60] |
| Arthripenoid C (110) | A. arundinis MA30 | Sea anemone, Badouzi | OM761170 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 5.3 μΜ | [60] |
| Ravenelin (111) | Setosphaeria rostrata | Mangrove, Prachuap Kiri Khan Province, Thailand | OK047731 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.27 μΜ Suppressed iNOS and COX-2 expression | [61] |
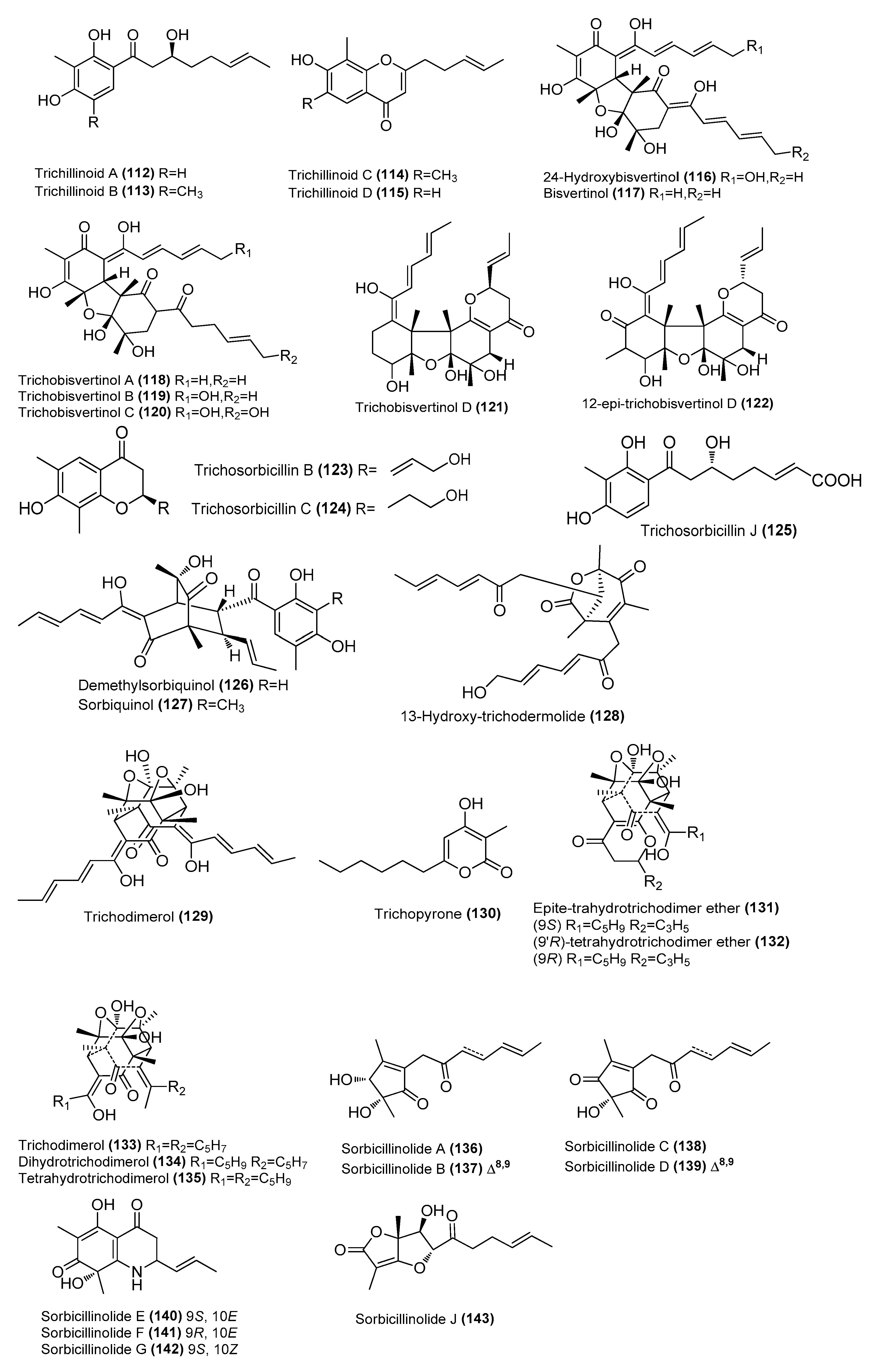
| Trichillinoids A-D (112–115) | Trichoderma sp. G13 | Marine fish Sebastes schlegelii, Yangma Island, Yantai, China | OQ781262 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14, 14, 16, and 20 µM, respectively | [62] |
| 24-Hydroxybisvertinol (116) | Trichoderma reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.1 μΜ | [63] |
| Bisvertinol (117) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.9 μΜ | [63] |
| Trichobisvertinols A-D (118-121) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.9, 22 and 24 μΜ, respectively | [63] |
| 12-epi-trichobisvertinol D (122) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32 μΜ | [63] |
| Trichosorbicillins B (123) and C (124) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 8.5 and 38 μΜ, respectively | [63] |
| Trichosorbicillin J (125) | Trichoderma reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 45%, and IL-1β 21%, respectively, at 25 µM | [64] |
| Demethylsorbiquinol (126) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 27%, and IL-1β 75%, respectively, at 25 µM | [64] |
| Sorbiquinol (127) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 35%, and IL-1β, 58%, respectively, at 25 µM | [64] |
| 13-hydroxy-trichodermolide (128) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | 47% inhibition of IL-6, 85% inhibition of IL-1β at 25 µM | [64] |
| Trichodimerol (129) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | 67% inhibition of IL-6, 87% inhibition of IL-1β at 25 µM | [64] |
| Trichopyrone (130) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [36] |
| Epite-trahydrotrichodimer ether (131) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| (9′R)-tetrahydrotrichodimer ether (132) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Trichodimerol (133) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Dihydrotrichodimerol (134) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Tetrahydrotrichodimerol (135) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Sorbicillinolides A–G (136–142) | Penicillium rubens F54 | Deep-sea sediment, Pacific Ocean | OR016127 | Inhibitory effects on the production of NO and PGE2, inhibition rates of 68.6%, 36.6%, 64.7%, 44.5%, 54.9%, 41.9%, and 44.5%, respectively, at 10 μM | [65] |
| Sorbicillinolide J (143) | Penicillium rubens F54 | Deep-sea sediment, Pacific Ocean | OR016127 | Inhibitory effects on the production of NO and PGE2, inhibition rate of 33.4%, at 10 μM | [65] |
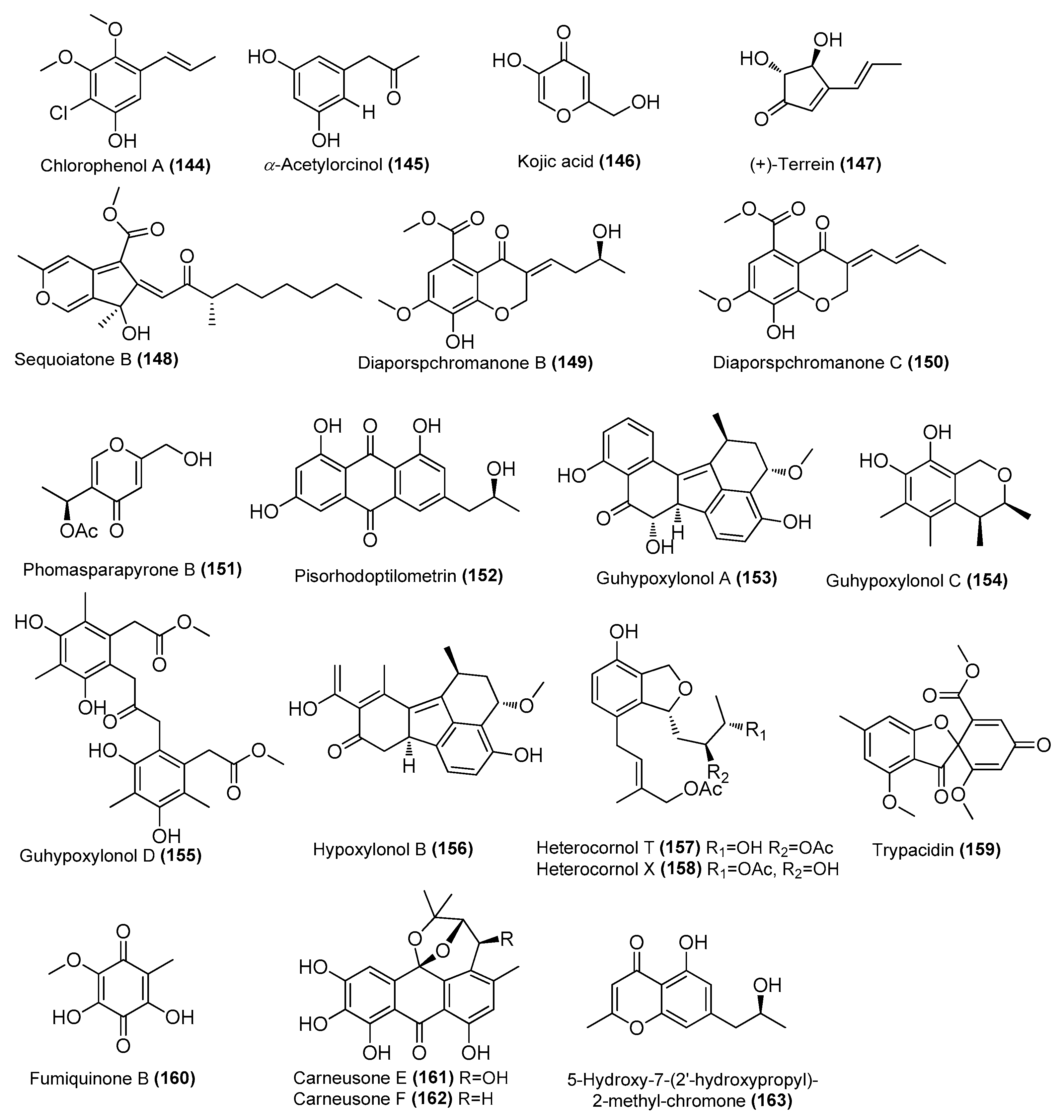
| Chlorophenol A (144) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| α-acetylorcinol (145) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Kojic acid (146) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| (+)-Terrein (147) | Aspergillus flavipes MTCC 5220 | Alga Ulva lactuca, Goa, India | PRJNA611016 | Inhibitory activity against IL-6 and TNF-α, IC50 = 8.5 ± 0.68 and 15.76 ± 0.18 µM, respectively | [48] |
| Sequoiatone B (148) | Penicillium sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.2 μΜ | [43] |
| Diaporspchromanones B–C (149–150) | Diaporthe sp. XW12-1 | Mangrove, Xuwen, Guangdong, China | MW566595.1 | IC50 (anti-inflammatory) = 19.06 ± 3.60 and 9.56 ± 0.18 μM, respectively, positive control (indomethacin, IC50 = 70.33 ± 0.95 μM) | [66] |
| Phomasparapyrone B (151) | Phomopsis asparagi LSLYZ-87 | Mangrove Acanthus ilicifolius, Huizhou Mangrove National Nature Reserve, Guangdong, China | ON341023 | Inhibition of LPS-induced NO accumulation on BV-2 cells in a dose-dependent manner | [67] |
| Pisorhodoptilometrin (152) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | MT597864.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 15.2 µM | [58] |
| Guhypoxylonols A (153), C (154), D (155) | Aspergillus sp. GXNU-Y45 | Mangrove Acanthus ilicifolius, Beihai City, China | MT626059 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14.42 ± 0.11, 18.03 ± 0.14, 16.66 ± 0.21, and 21.05 ± 0.13 µM, respectively | [68] |
| Hypoxylonol B (156) | Aspergillus sp. GXNU-Y45 | Mangrove Acanthus ilicifolius, Beihai City, China | MT626059 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.05 ± 0.13 µM | [68] |
| Heterocornols T (157) and X (158) | Pestalotiopsis heterocornis XWS03F09 | Xisha Islands, China | JN943628.1 | Inhibited NOS protein expression in a concentration-dependent manner | [69] |
| Trypacidin (159) | Talaromyces helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.65 μΜ | [70] |
| Fumiquinone B (160) | T. helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 15.54 μΜ | [70] |
| Carneusones E-F (161–162) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, EC50 = 34.6 and 20.2 μΜ, respectively | [71] |
| 5-Hydroxy-7-(2′-hydroxypropyl)-2-methyl-chromone (163) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | NR 121232.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 75.5 μΜ | [72] |
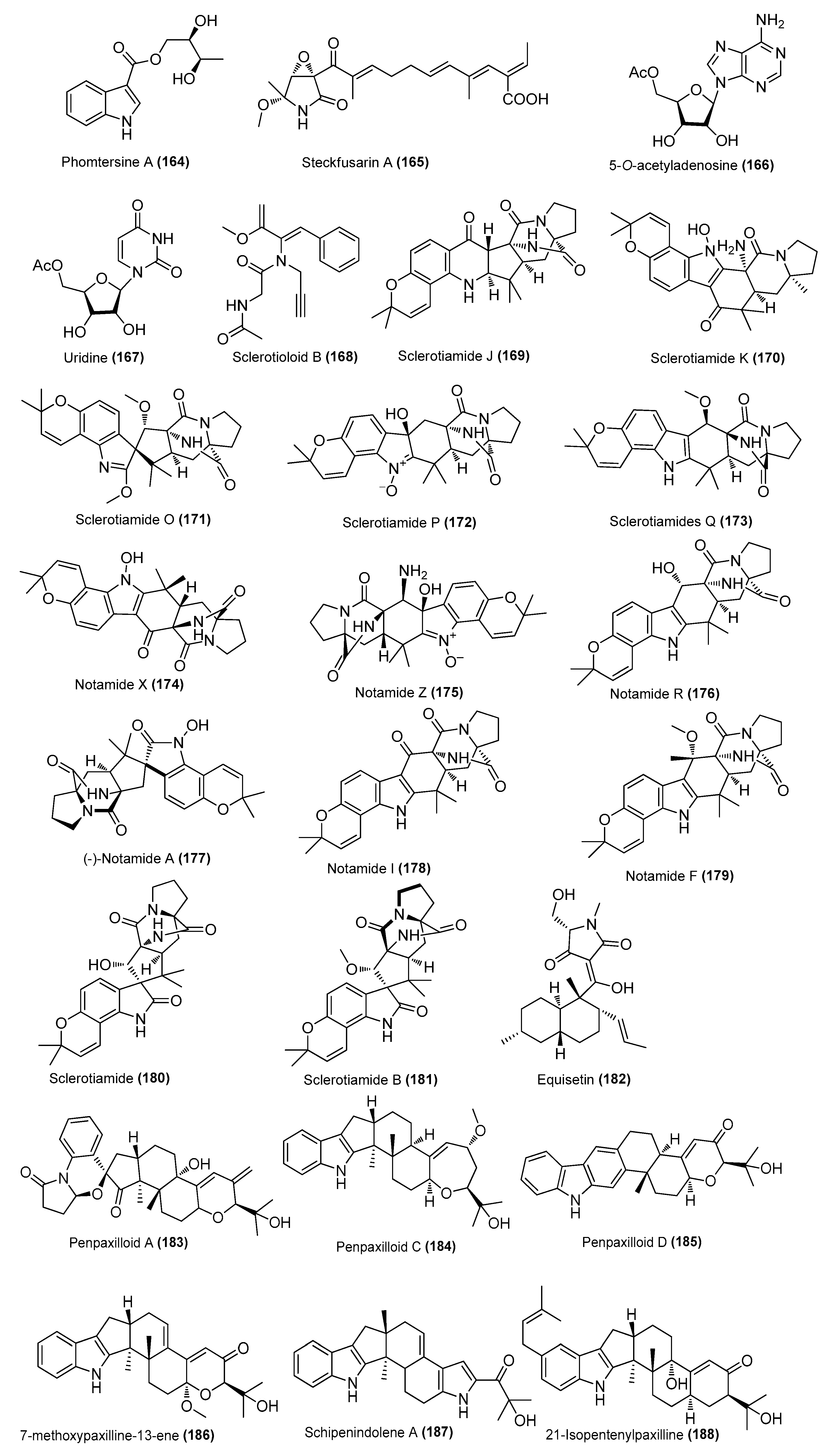
| Phomtersine A (164) | Phomopsis tersa FS441 | Deep sea in the Indian Ocean | MK592793 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 83.57 μΜ | [73] |
| Steckfusarin A (165) | Penicillium steckii SCSIO 41040 | Green algae Botryocladia sp., South China Sea | OP349656 | Anti-inflammatory activity at 20 µM | [74] |
| 5-O-acetyladenosine (166) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | OR398925 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rates of 34.2%, at 1 µM | [46] |
| Uridine (167) | S. hepiali W7 | Deep-sea sulfide sample, South Atlantic | OR398925 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rates of 30.7%, at 1 µM | [46] |
| Sclerotioloid B (168) | Aspergillus sclerotiorum ST0501 | Guangdong, China | MT534582 | Inhibited NO production in LPS-induced RAW 264.7 cells, inhibition rate of 28.92%, postive control dexamethasone (25.87%) | [75] |
| Sclerotiamide J (169) | Aspergillus sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamides K (170) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamides O-Q (171–173) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide X (174) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide Z (175) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide R (176) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| (−)-notamide A (177) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide I (178) | |||||
| Notamide F (179) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamide (180) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamide B (181) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Equisetin (182) | Fusarium equiseti | Sponge, Xuwen County, Zhanjiang, China | SCSIO 41019 | EQST inhibits macrophage inflammatory response in vitro | [77] |
| Penpaxilloids A (183), C (184), D (185) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.11, 27.25 and 33.09 μΜ, respectively | [78] |
| 7-methoxypaxilline-13-ene (186) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 38.79 μΜ | [78] |
| Schipenindolene A (187) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 11.87 μΜ | [78] |
| 21-isopentenylpaxilline (188) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.95 μΜ | [78] |
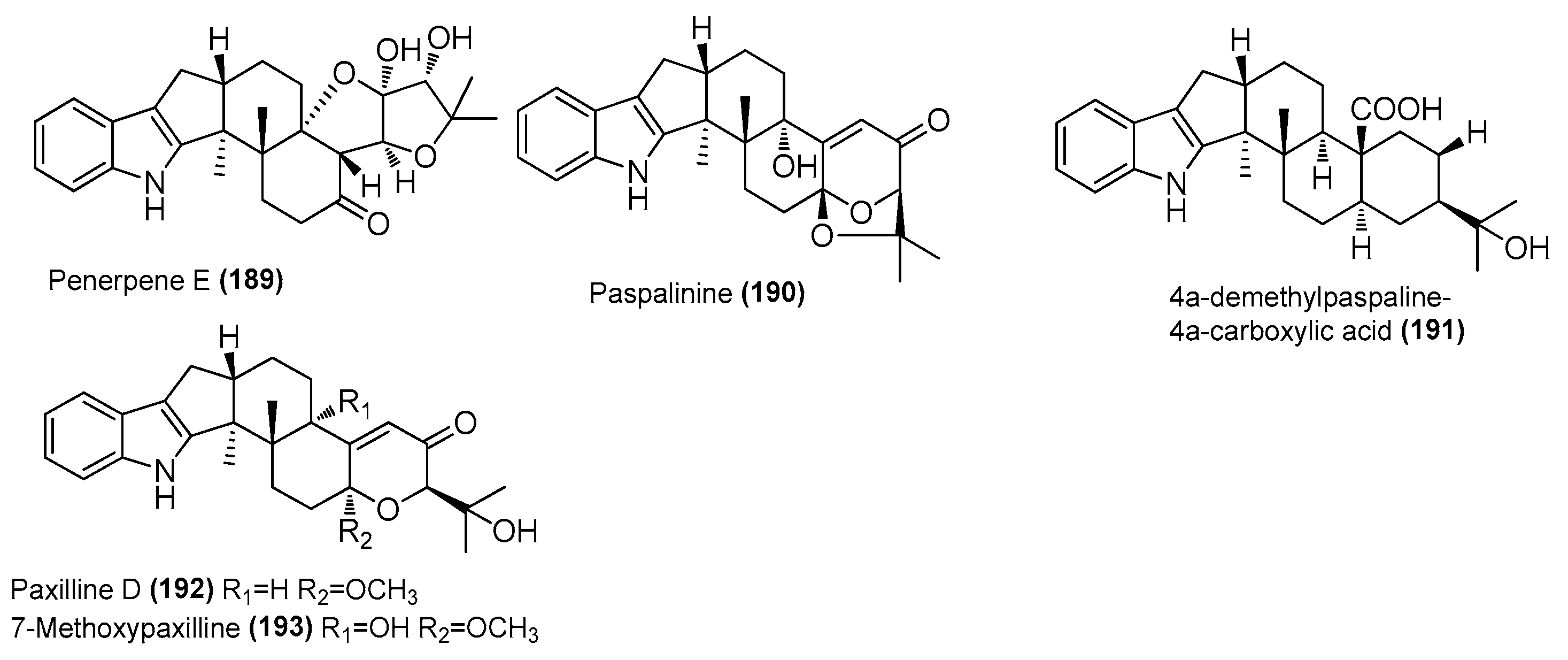
| Penerpene E (189) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 23.89 μΜ | [78] |
| Paspalinine (190) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 19.34 μΜ | [78] |
| 4a-demethylpaspaline-4a-carboxylic acid (191) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 28.22 μΜ | [78] |
| Paxilline D (192) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 4.46 μΜ | [78] |
| Arthropod Dardanus scutellatus, Yinyu Island in South China’s Hainan province | |||||
| 7-methoxypaxilline (193) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.88 μΜ | [78] |
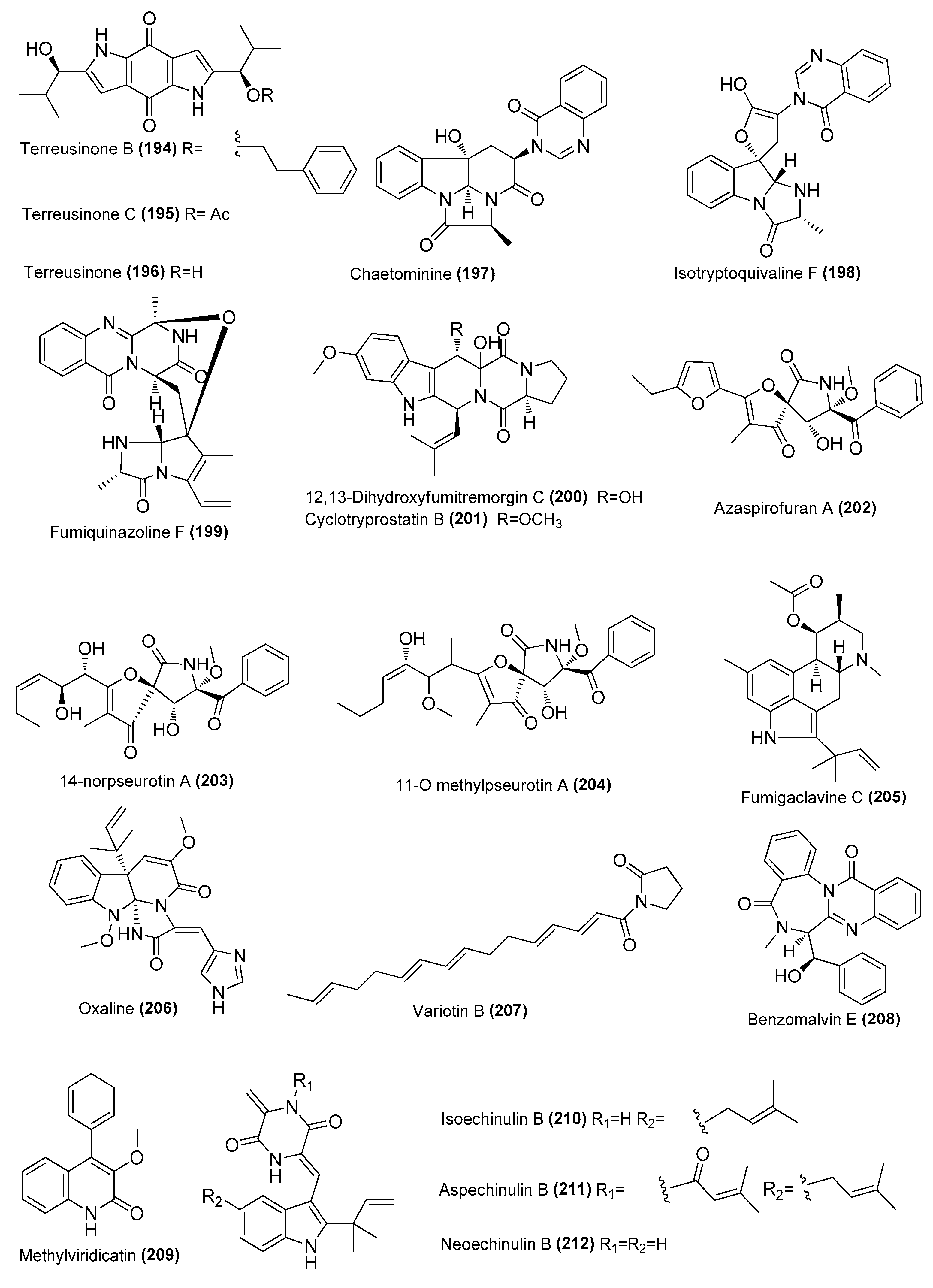
| Terreusinones B (194) and C (195) | Aspergillus tamarii MCCF102 | Sponge, Vizhinjam, Southwest coast of India | JAGJCD000000000 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.032, 0.046 and 0.096μΜ. respectively | [79] |
| Terreusinone (196) | A. tamarii MCCF102 | Sponge, Vizhinjam, Southwest coast of India | JAGJCD000000000 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.032 μΜ | [79] |
| Chaetominine (197) | Talaromyces helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 103.2 μΜ | [70] |
| Isotryptoquivaline F (198) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 26.51 μΜ | [70] |
| Fumiquinazoline F (199) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.35 μΜ | [70] |
| 12,13-dihydroxyfumitremorgin C (200) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 24.95 μΜ | [70] |
| Cyclotryprostatin B (201) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 29.58 μΜ | [70] |
| Azaspirofurans A (202) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.65 μΜ | [70] |
| 14-norpseurotin A (203) | T. Helicus SCSIO41311 | Cold seep, South China Sea Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.37 μΜ | [70] |
| 11-O methylpseurotin A (204) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.22 μΜ | [70] |
| Fumigaclavine C (205) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 23.46 μΜ | [70] |
| Oxaline (206) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | MT597864.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 9.2 μΜ | [72] |
| Variotin B (207) | Aspergillus unguis IV17-109 | Deep sea, Indian Ocean | OL700797 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 20.0 μΜ | [80] |
| Benzomalvin E (208) | Metarhizium sp. P2100 | Seawater, Qingdao Huiquan Bay, Yellow Sea | OP028052 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37.08 μΜ | [81] |
| Methylviridicatin (209) | Metarhizium sp. P2100 | Seawater, Qingdao Huiquan Bay, Yellow Sea | OP028052 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37.48 μΜ | [81] |
| Isoechinulin B (210) | Aspergillus sp. nFS445 | Deep sea, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 16 ± 1.3 µM, positive control aminoguanidine (IC50, 23.7 µM) | [82] |
| Aspechinulins B (211) and C (213) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 20 ± 0.28 and 25 ± 1.7 µM, respectively | [82] |
| Neoechinulin B (212) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 89 ± 2.0 µM | [82] |
| Cryptoechinuline G (214) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37 ± 0.75 µM | [82] |
| Isoechinulin A (215) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 57 ± 2.3 µM | [82] |
| (−)-cyclopenol (216) | Aspergillus austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 70 µg/mL | [83] |
| Cyclopenin (217) | A. austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 130 µg/mL | [83] |
| Virdicatol (218) | austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 30 µg/mL | [83] |
| Phomtersine A (219) | Phomopsis tersa FS441 | Deep sea, Indian Ocean | MK592793 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 83.57 μΜ | [84] |
| Penifuranone A (220) | Penicillium crustosum SCNU-F0006 | Mangrove, Yangjiang Hailing Island Mangrove Wetland Park, China | MH345907 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 42.2 μΜ | [85] |
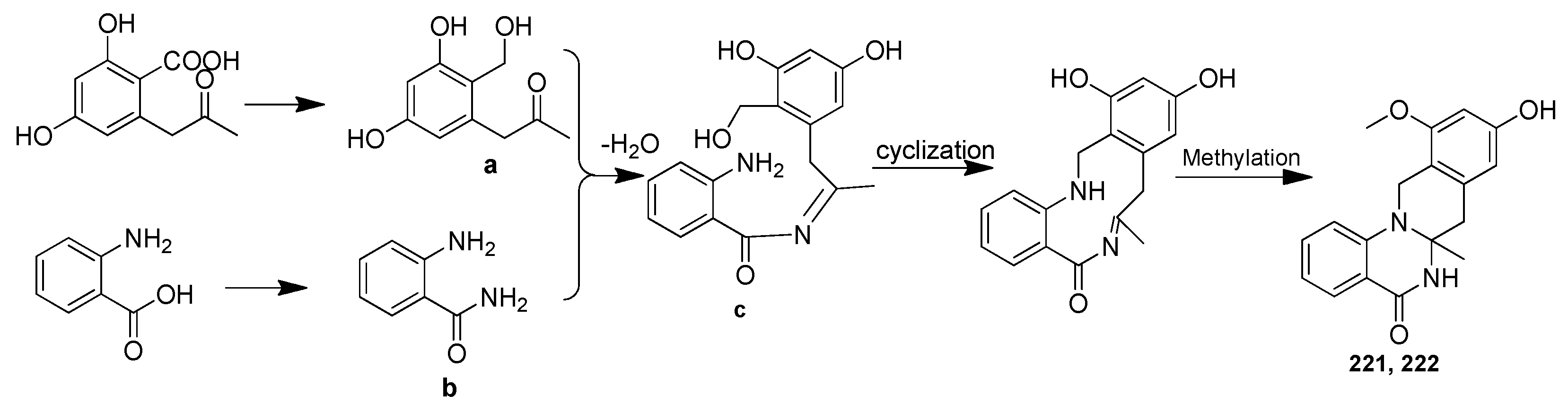
| (±)-penicamide A [(−)-221 and (+)-222] | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 27.2 and 47.5 μΜ, respectively | [86] |
| Penicamide B (223) | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 45.9 ± 2.0 μΜ | [86] |
| (S)-2-(2-hydroxypropanamido) benzamide (224) | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.8 ± 1.3 µM, respectively | [86] |
| Penipiperazine A (225) | Penicillium brasilianum HBU-136 | Bohai Sea, China | MH377073 | Inhibited the expression of inflammatory factors at 25.0 µM | [87] |
| Metabolite (226) | P. brasilianum HBU-136 | Bohai Sea, China | MH377073 | Inhibited the expression of inflammatory factors at 25.0 µM | [87] |
| Cytochalasins Z24 (227) | Eutypella scoparia GZU-4-19Y | Xuwen, Guangdong province, China | OM920979 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 17.1 μΜ | [30] |
| Aspercerebroside A (228) | Aspergillus sp. | Dongshan Island, Fujian Province | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, at 30 and 40 μg/mL | [88] |
| Hortacerebrosides A (229) and B (230) | Hortaea werneckii | Sponge, Danzhou, Hainan, China | HN-YPG-2-5 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7 and 5 μΜ, respectively | [89] |
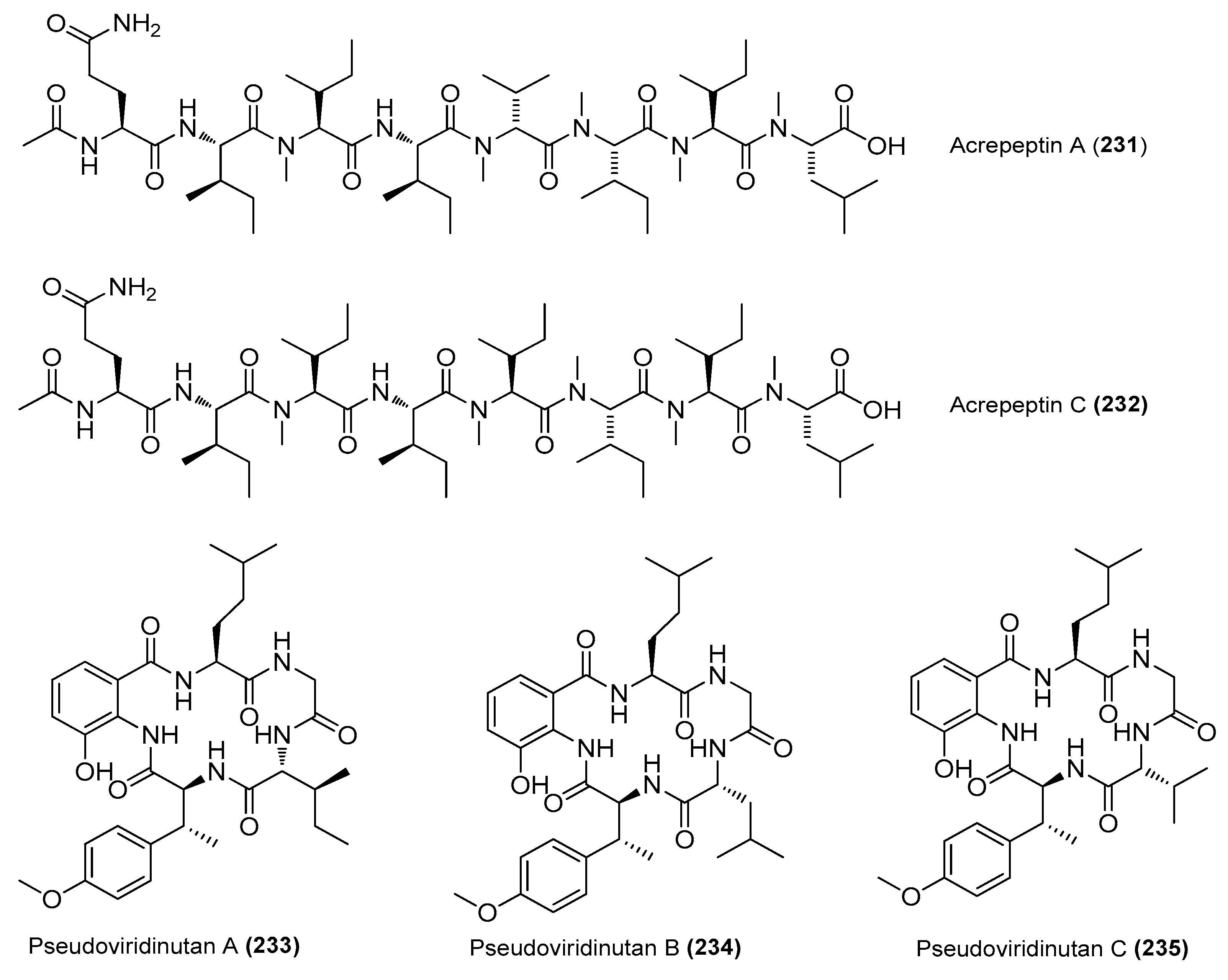
| Acrepeptins A (231) and C (232) | Acremonium sp. NTU492 | Red alga Mastophora rosea, Taiwan, China | KY753131 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 12.0 and 10.6 μΜ, respectively | [90] |
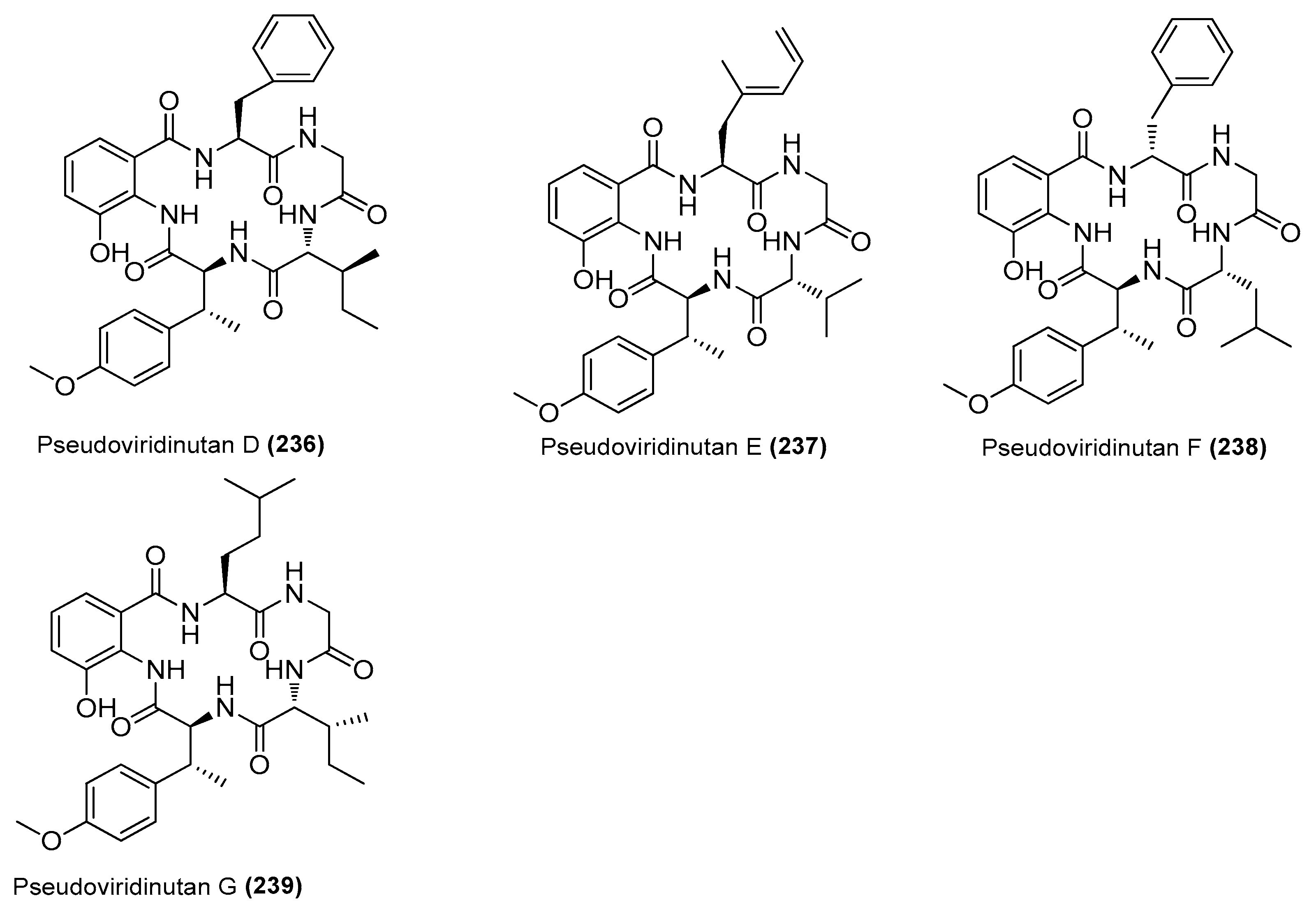
| Pseudoviridinutans A−G (233−239) | Aspergillus pseudoviridinutans TW58-5 | Ahydrothermal vent sediment, Kueishantao, Taiwan, China | OQ405296 | Inhibited NO production in LPS-induced RAW 264.7 cells | [91] |
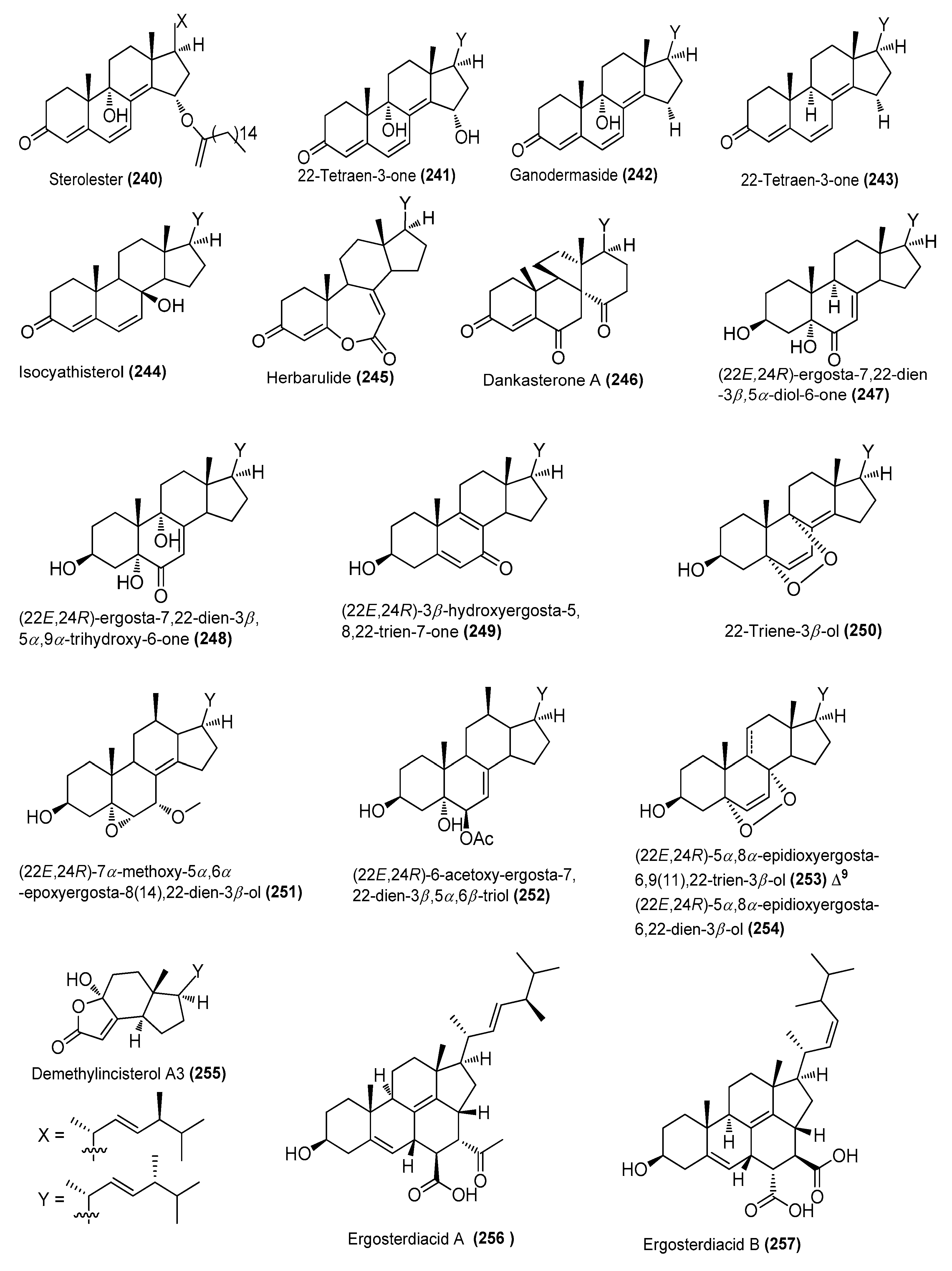
| Sterolester (240) | Penicillium oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-Tetraen-3-one (241) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Ganodermaside (242) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-Tetraen-3-one (243) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Isocyathisterol (244) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Herbarulide (245) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Dankasterone A (246) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-ergosta-7,22-dien-3β,5α-diol-6-one (247) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-ergosta-7,22-dien-3β,5α,9α-trihydroxy-6-one (248) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-3β-hydroxyergosta-5,8,22-trien-7-one (249) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-triene-3β-ol (250) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-7α-methoxy-5α,6α-epoxyergosta-8(14),22-dien-3β-ol (251) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-6-acetoxy-ergosta-7,22-dien-3β,5α,6β-triol (252) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-5α,8α-epidioxyergosta-6,9(11),22-trien-3β-ol (253) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-5α,8α-epidioxyergosta-6,22-dien-3β-ol (254) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Demethylincisterol A3 (255) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Ergosterdiacids A and B (256 and 257) | Aspergillus sp. | Mangrove Aegiceras corniculatum, Thailand | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 4.5 and 3.6 μΜ, respectively | [93] |
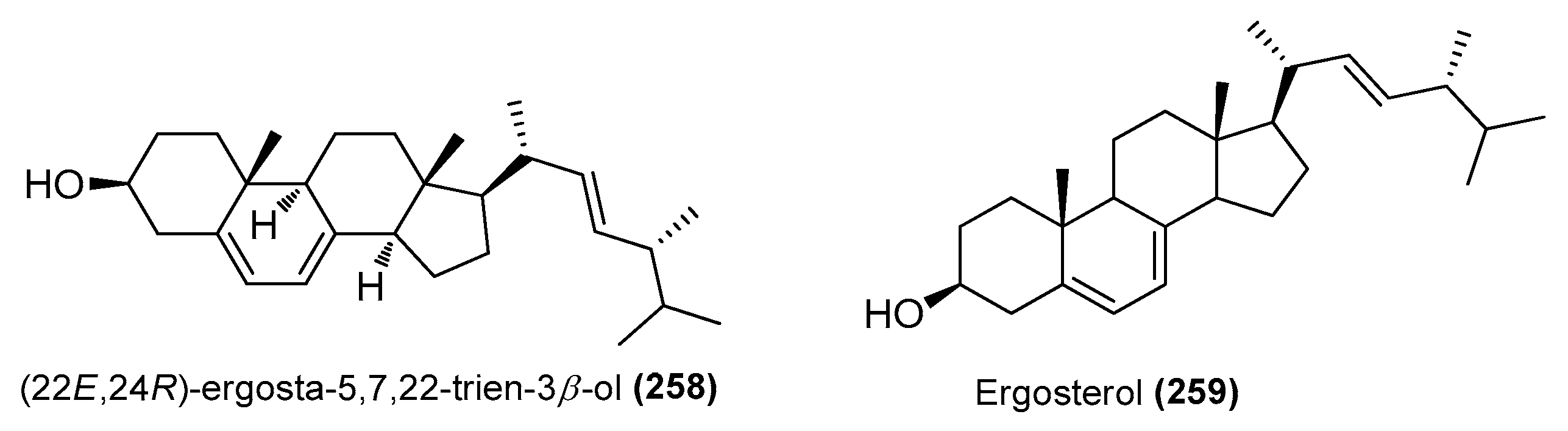
| (22E,24R)-ergosta-5,7,22-trien-3β-ol (258) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Ergosterol (259) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rate of 32.9% (1 μΜ) | [46] |
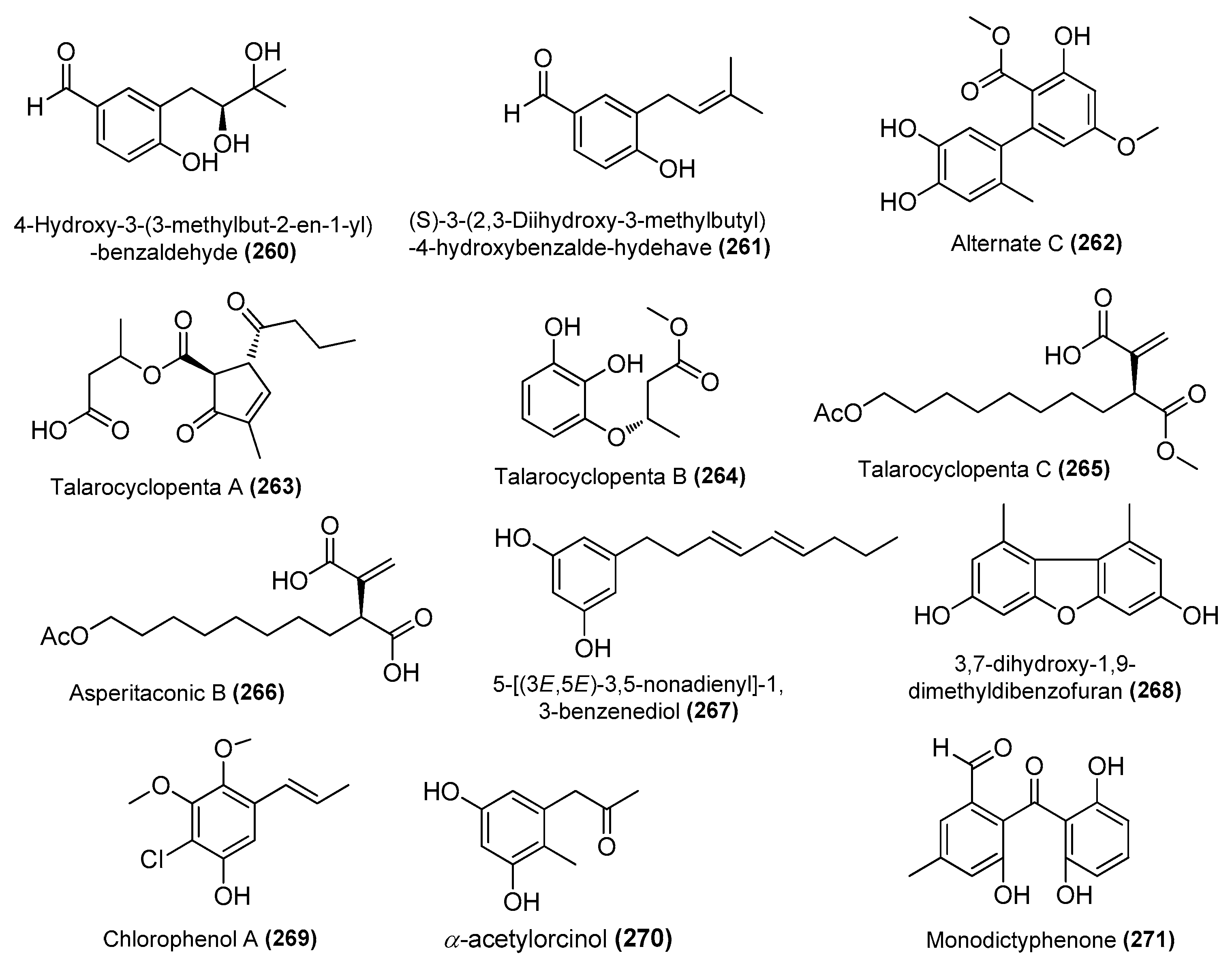
| 4-hydroxy-3-(3-methylbut-2-en-1-yl)-benzaldehyde (260) | Aspergillus terreus C23-3 | Coral, Xuwen natural reserve located, South China Sea | _ | Inhibited the MAPK signaling pathway in RAW264.7 cells | [94] |
| (S)-3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzalde-hydehave (261) | Aspergillus terreus C23-3 | Coral, Xuwen natural reserve located, South China Sea | _ | Inhibited the MAPK signaling pathway in RAW264.7 cells | [94] |
| Alternate C (262) | Pleosporales sp. SF-7343 | King George Island, Antarctica | MK785420 | Inhibition of IL-6 and IL-8 | [47] |
| Talarocyclopentas A (263), B (264) and C (265) | Talaromyces assiutensis JTY2 | Mangrove Ceriops tagal, South China Sea | JN899320.1 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.38, 6.26, and 12.56 μΜ, respectively | [95] |
| Asperitaconic B (266) | T. assiutensis JTY2 | Mangrove Ceriops tagal, South China Sea | JN899320.1 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 15.63 μΜ | [95] |
| 5-[(3E,5E)-3,5-nonadienyl]-1,3-benzenediol (267) | Aspergillus sp. | Brown alga Saccharina cichorioides, South China Sea | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.0 μΜ | [96] |
| 3,7-dihydroxy-1,9-dimethyldibenzofuran (268) | Aspergillus sydowii MCCC 3A00324 | Deep sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced BV-2 cells, 94.4% (10 μΜ) | [24] |
| Chlorophenol A (269) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| α-acetylorcinol (270) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Monod-ictyphenone (271) | Diaporthe sp. SYSU-MS4722 | Shenzhen City, Guangdong, Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 40.8 μΜ | [38] |
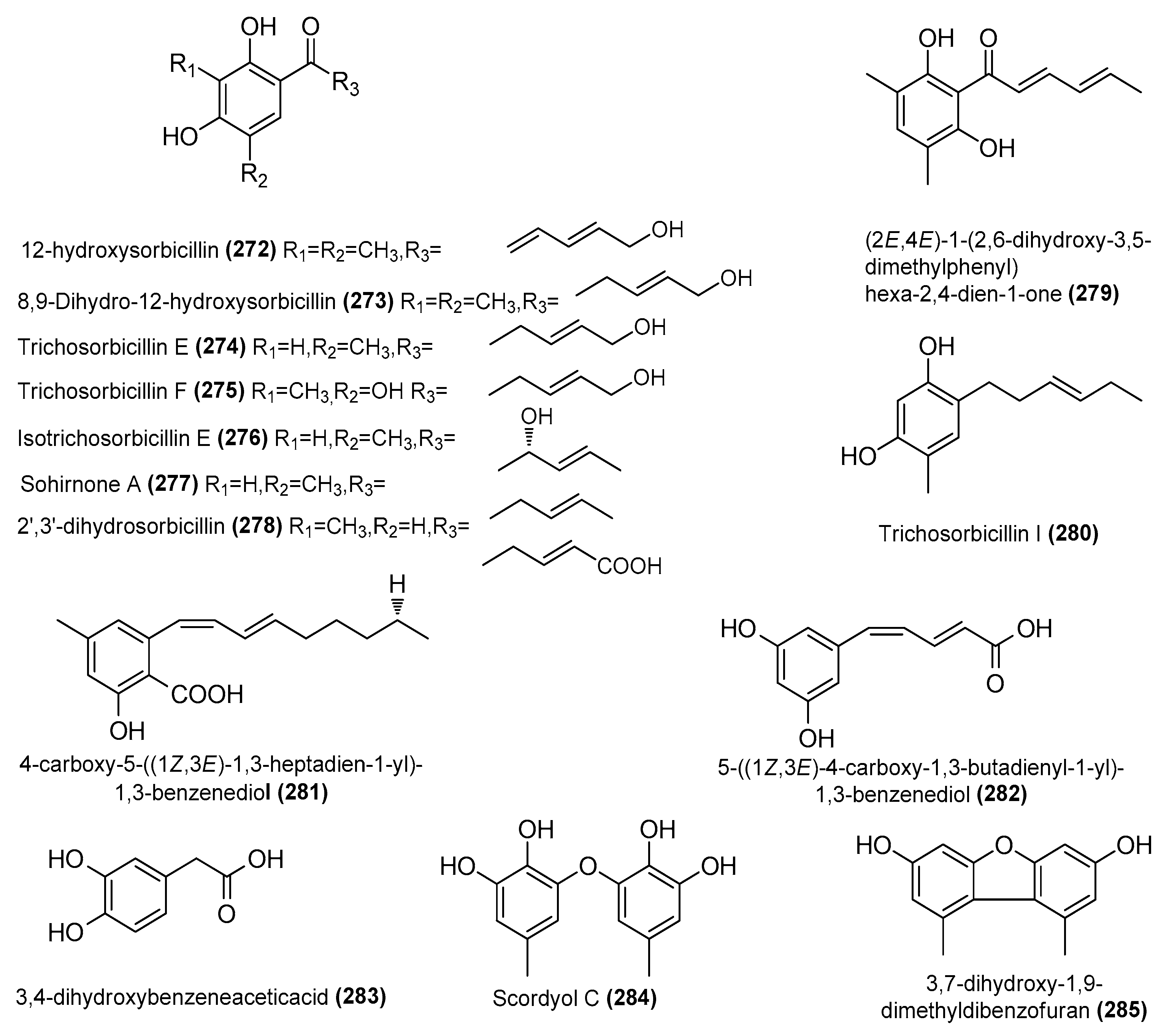
| 12-hydroxysorbicillin (272) | Trichoderma reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.8 μΜ | [63] |
| 8,9-Dihydro-12-hydroxysorbicillin (273) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.9 μΜ | [63] |
| Trichosorbicillin E (274) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.94 μΜ | [63] |
| Trichosorbicillin F (275) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.1 μΜ | [63] |
| Isotrichosorbicillin E (276) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 12 μΜ | [63] |
| Sohirnone A (277) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14 μΜ | [63] |
| 2′,3′-dihydrosorbicillin (278) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 13 μΜ | [63] |
| (2E,4E)-1-(2,6-dihydroxy-3,5-dimethylphenyl) hexa-2,4-dien-1-one (279) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.3 μΜ | [63] |
| Trichosorbicillin I (280) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 13 μΜ | [63] |
| 4-carboxy-5-((1Z,3E)-1,3-heptadien-1-yl)-1,3-benzenediol (281) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| 5-((1Z,3E)-4-carboxy-1,3-butadienyl-1-yl)-1,3-benzenediol (282) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| 3,4-dihydroxybenzeneaceticacid (283) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| Scordyol C (284) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 26.8 ± 1.7μΜ | [72] |
| 3,7-dihydroxy-1,9-dimethyldibenzofuran (285) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.9 ± 0.1 μΜ | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar. Drugs 2024, 22, 433. https://doi.org/10.3390/md22100433
Qiu Y, Chen S, Yu M, Shi J, Liu J, Li X, Chen J, Sun X, Huang G, Zheng C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Marine Drugs. 2024; 22(10):433. https://doi.org/10.3390/md22100433
Chicago/Turabian StyleQiu, Yikang, Shiji Chen, Miao Yu, Jueying Shi, Jiayu Liu, Xiaoyang Li, Jiaxing Chen, Xueping Sun, Guolei Huang, and Caijuan Zheng. 2024. "Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity" Marine Drugs 22, no. 10: 433. https://doi.org/10.3390/md22100433






