Cognitive-Enhancing Effect of Marine Brown Algae-Derived Phenolics through S100B Inhibition and Antioxidant Activity in the Rat Model of Ischemic Stroke
Abstract
:1. Introduction
2. Results
2.1. Characterization of Selected Seaweeds on Phenolic Compounds and Antioxidant Activity
2.2. Gallic Acid Content in S. polycystum Extracts by HPLC Analysis
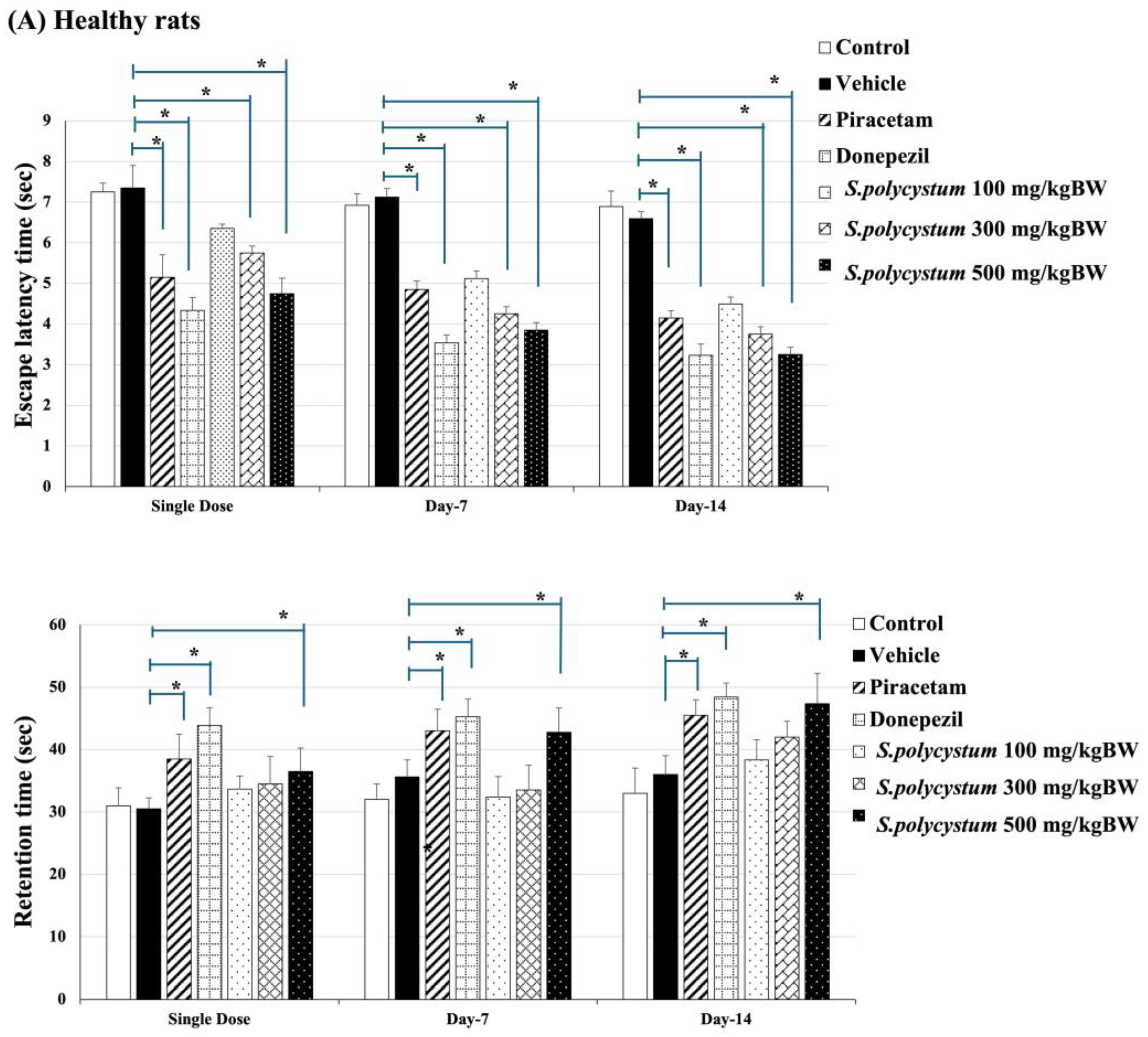
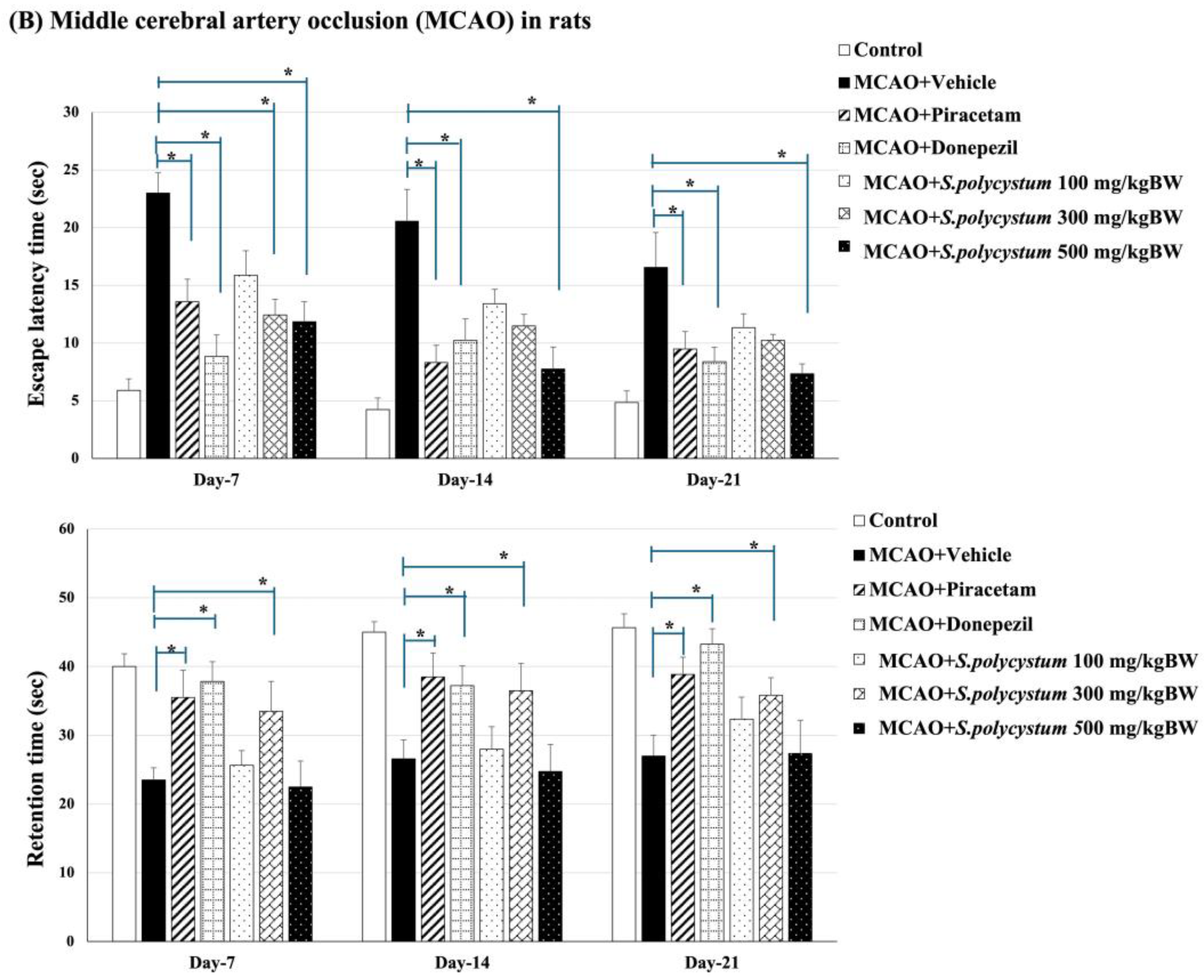
2.3. Effect of S. polycystum on Cognitive Functions by Morris Water Maze
2.4. Effect of S. polycystum on Cognitive Functions by Novel Object Recognition Test (NOR)
2.5. Effect of S. polycystum Extract on the Oxidative Stress Status
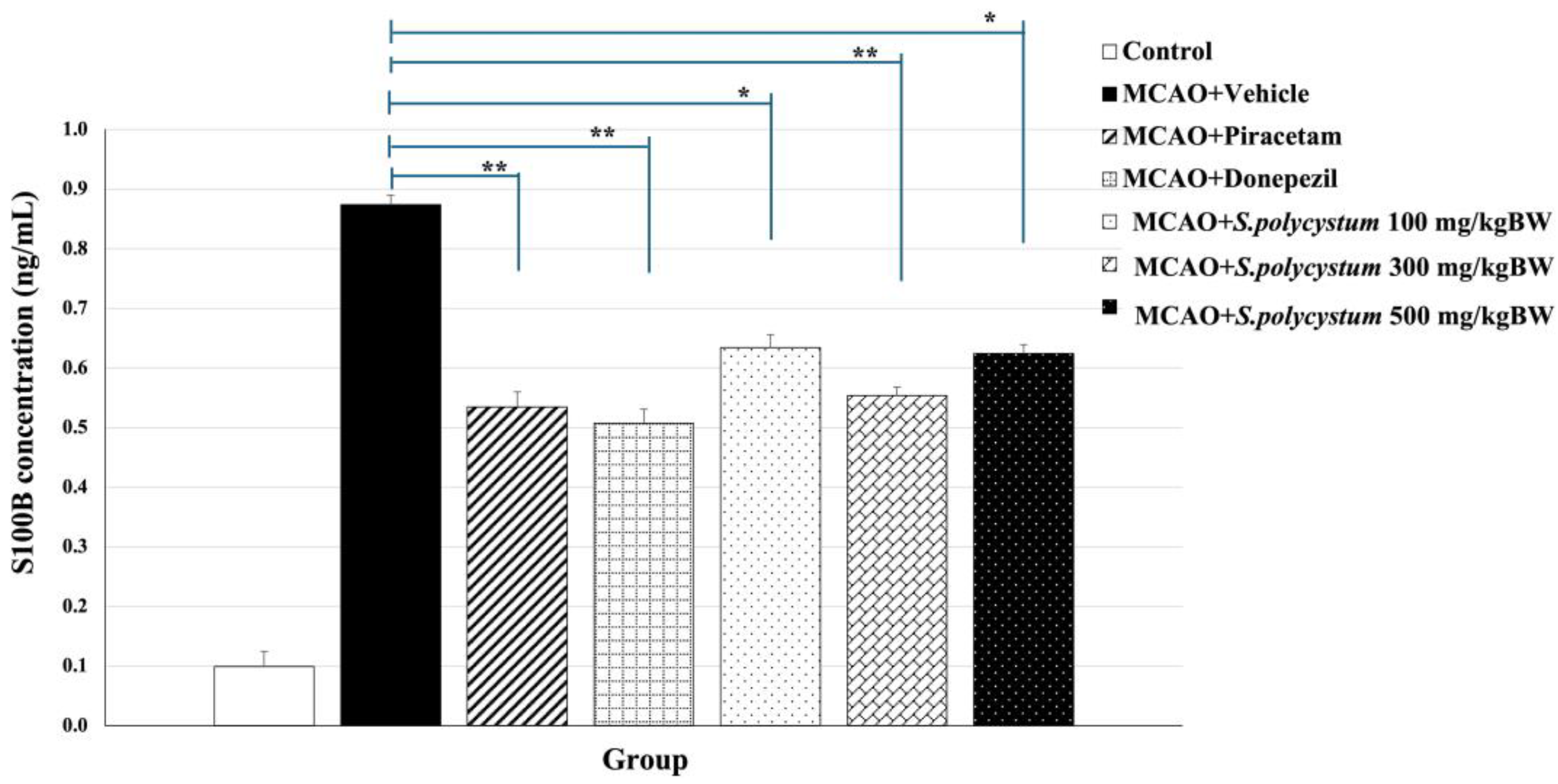
2.6. Effect of S. polycystum Extract on Serum S100B Concentration
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Preparation and Extraction
4.3. Determination of Phenolic Contents
4.4. Determination of Antioxidant Activity Using DPPH Radical Scavenging Activity and Ferric Reducing Antioxidant Power (FRAP) Assay
4.5. Determination of Gallic Acid Content by HPLC Analysis
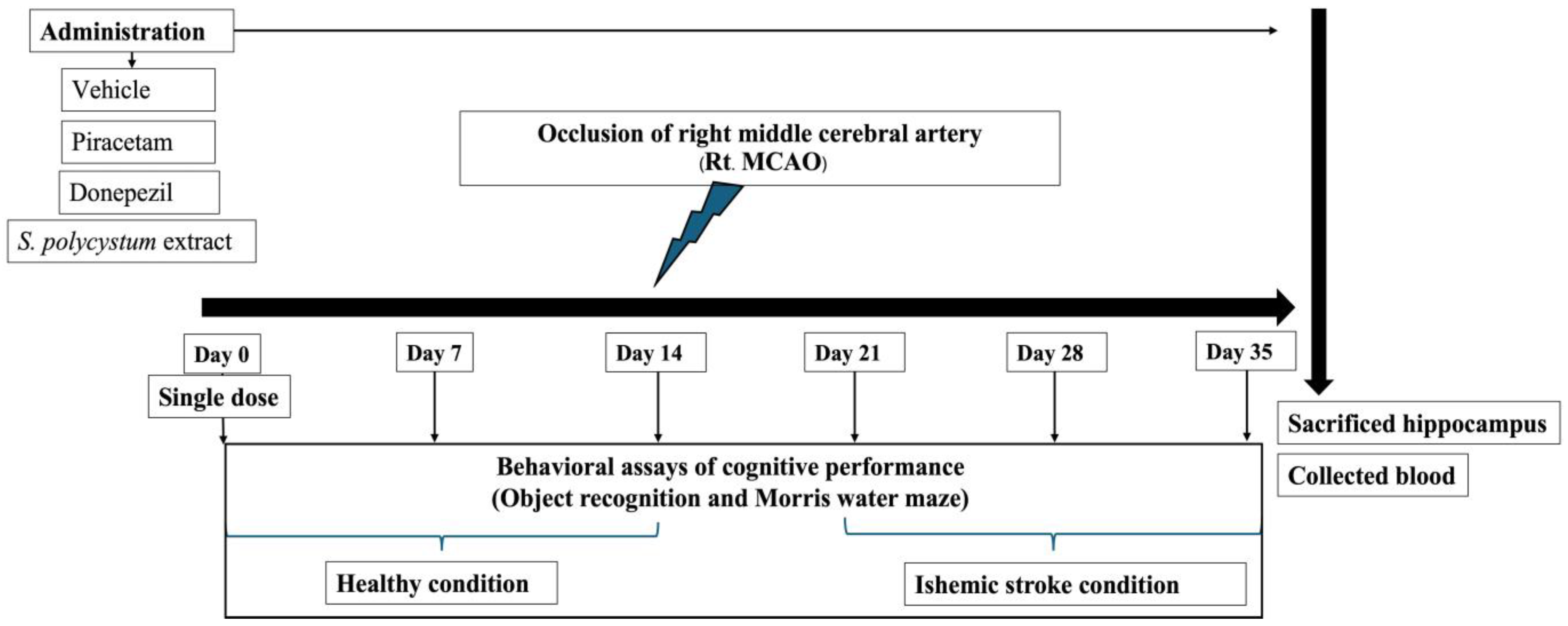
4.6. Experimental Animals
- Group I: Naïve intact; rats in this group received no treatment.
- Group II: Vehicle plus MCAO; rats in this group received a dimethyl sulfoxide (DMSO) solution, which was used as the vehicle treatment, 14 days before and 21 days after exposure to the occlusion of the right middle cerebral artery (Rt. MCAO).
- Group III: Piracetam plus MCAO; rats in this group received piracetam (200 mg/kg BW), 14 days before and 21 days after exposure to Rt. MCAO.
- Group IV: Donepezil plus MCAO; rats received donepezil (1 mg/kg BW), 14 days before and 21 days after exposure to Rt. MCAO.
- Group V–VI: The selected extract plus MCAO; rats received S. polycystum extract (at doses of 100, 300, and 500 mg/kg BW), 14 before and 21 days after exposure to Rt. MCAO.
4.7. Animal Protocol
4.8. Determination the Cognitive-Enhancing Effect
4.8.1. The Morris Water Maze Test
4.8.2. Novel Object Recognition Test (NOR)
4.9. Biochemical Assay
4.9.1. Determination of Oxidative Stress Status
4.9.2. Determination of S100B Serum Level
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, R.O.; Majeed, A.A.; Margiana, R.; Alkadir, O.K.; Almalki, S.G.; Ghildiyal, P.; Samusenkov, V.; Jabber, N.K.; Mustafa, Y.F.; Elawady, A. Therapeutic gene delivery by mesenchymal stem cell for brain ischemia damage: Focus on molecular mechanisms in ischemic stroke. Cell Biochem. Funct. 2024, 42, e3957. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Weijs, R.W.; Shkredova, D.A.; Brekelmans, A.C.; Thijssen, D.H.; Claassen, J.A. Longitudinal changes in cerebral blood flow and their relation with cognitive decline in patients with dementia: Current knowledge and future directions. Alzheimer’s Dement. 2023, 19, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, M.O.; Musa, S.A.; Oladele, S.B.; Iliya, A.I. Amyloid-beta aggregation implicates multiple pathways in Alzheimer’s disease: Understanding the mechanisms. Front. Neurosci. 2023, 17, 1081938. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, L.K.; Adel, S.; Hamza, S.A.; Alsadany, M.A.; Ali, S.M. Investigating the association between blood oxidative stress markers and dementia in Egyptian elderly women. Egypt. J. Neurol. Psychiat. Neurosurg. 2024, 60, 73. [Google Scholar] [CrossRef]
- Mori, T.; Asano, T.; Town, T. Targeting S100B in cerebral ischemia and in Alzheimer′ s disease. Cardiovasc. Psychiatry Neurol. 2010, 2010, 687067. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Mahdkhah, A.; Panahi, F. S100B protein as a post-traumatic biomarker for prediction of brain death in association with patient outcomes. Arch. Trauma. Res. 2013, 2, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Santotoribio, J.D.; Lozano, P.; Cañavate-Solano, C.; Corral-Pérez, J.; O’Ferrall-González, C. Evaluation of the neuroprotective effect of antipsychotics by serum quantification of protein S100B. Farm. Hosp. 2024, S1130. [Google Scholar] [CrossRef]
- Rodrigues, L.; Wartchow, K.M.; Buchfelder, M.; Souza, D.O.; Gonçalves, C.A.; Kleindienst, A. Longterm increased S100B enhances hippocampal progenitor cell proliferation in a transgenic mouse model. Int. J. Mol. Sci. 2022, 23, 9600. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive exploration of marine algae diversity, bioactive compounds, health benefits, regulatory issues, and food and drug applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva-Reis, R.; Chouh, A.; Silva, S.; Braga, S.S.; Silva, A.M.; Cardoso, S.M. Applications of antioxidant secondary metabolites of Sargassum spp. Mar. Drugs 2023, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, S.A.; Renu, K.; Zahra, H.A.; Abdallah, B.M.; Ali, E.M.; Veeraraghavan, V.P.; Sivalingam, K.; Ronsard, L.; Ammar, R.B.; Vidya, D.S.; et al. Polyphenols mediate neuroprotection in cerebral ischemic stroke—An update. Nutrients 2023, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.J.; Soekamto, N.H.; Firdaus, F.; Latip, J. Liquid chromatography-mass spectrometry characterization of bioactive compounds from Sargassum polycystum with the larval and in vitro anticancer activity. Rasayan J. Chem. 2021, 14, 676–683. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Oxidative stress, the blood–brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef] [PubMed]
- Arsianti, A.; Bahtiar, A.; Wangsaputra, V.K.; Azizah, N.N.; Fachri, W.; Nadapdap, L.D.; Fajrin, A.M.; Tanimoto, H.; Kakiuchi, K. Phytochemical composition and evaluation of marine algal Sargassum polycystum for antioxidant activity and in vitro cytotoxicity on hela cells. Pharmacogn. J. 2020, 12, 88–94. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; de la Luz, C.G.M.; Segura, C.A.; Mirjalili, M.H. Natural phenolic compounds with neuroprotective effects. Neurochem. Res. 2024, 49, 306–326. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ibhiedu, J.O.; Egbunu, E.O.; Ndam, A.R.; Ogala, F.; Ologunde, T.; Peterson, J.C.; Boluwatife, A.I.; et al. Stroke and cognitive impairment: Understanding the connection and managing symptoms. Ann. Med. Surg. 2023, 85, 6057–6066. [Google Scholar] [CrossRef]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J. Neurochem. 2010, 115, 814–828. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar]
- Liu, Z.; Liu, Y.; Tu, X.; Shen, H.; Qiu, H.; Chen, H.; He, J. High serum levels of malondialdehyde and 8-OHdG are both associated with early cognitive impairment in patients with acute ischaemic stroke. Sci. Rep. 2017, 7, 9493. [Google Scholar] [CrossRef]
- Vani, J.R.; Mohammadi, M.T.; Foroshani, M.S.; Jafari, M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 2016, 15, 378–390. [Google Scholar]
- Zheng, M.; Wang, X.; Yang, J.; Ma, S.; Wei, Y.; Liu, S. Changes of complement and oxidative stress parameters in patients with acute cerebral infarction or cerebral hemorrhage and the clinical significance. Exp. Ther. Med. 2019, 19, 703–709. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom. J. Intern. Med. 2013, 51, 97–106. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Bloomfield, S.M.; Mcknney, J.; Smith, L.; Brisman, J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care 2007, 6, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Yardan, T.; Erenler, A.K.; Baydin, A.; Aydin, K.; Cokluk, C. Usefulness of S100B protein in neurological disorders. J. Pak. Med. Assoc. 2011, 61, 276–281. [Google Scholar]
- Stammet, P. Blood biomarkers of hypoxic-ischemic brain injury after cardiac arrest. Semin. Neurol. 2017, 37, 75–80. [Google Scholar] [CrossRef]
- Holzschuh, M.; Metz, C.; Brawanski, A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J. Trauma. 1999, 47, 1126–1130. [Google Scholar]
- Langeh, U.; Singh, S. Targeting S100B protein as a surrogate biomarker and its role in various neurological disorders. Curr. Neuropharmacol. 2021, 19, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Zareian, L.; Azarhomayoun, A.; Alimohamadi, M.; Khajavi, M.; Razeghi, J.S. Impact of acute phase epigallocatechin-3-gallate supplementation on consciousness and S100B serum levels in TBI patients: A double blind randomized clinical trial. Iran. J. Neurosurg. 2017, 3, 51–57. [Google Scholar] [CrossRef]
- Qin, B.; Panickar, K.; Anderson, R.A. Cinnamon polyphenols regulate S100β, sirtuins, and neuroactive proteins in rat C6 glioma cells. Nutrition 2014, 30, 210–217. [Google Scholar] [CrossRef]
- Machin, A.; Suprapto, D.A.; Hanifah, A.; Suharjanti, I.; Shodiq, J.; Fatihuddin, M.F.; Kim, B.J.; Firdha, A.A. Potential effect of green tea extract for adjuvant treatment of acute ischemic stroke by S100ß upregulation in non thrombolysis patient. Jurnal Ners 2023, 18, 145–153. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Korani, M.S.; Farbood, Y.; Sarkaki, A.; Moghaddam, H.F.; Mansouri, M.T. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. Eur. J. Pharmacol. 2014, 733, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, L.; Mao, Y. Gallic acid attenuates cerebral ischemia/re-perfusion-induced blood–brain barrier injury by modifying polarization of microglia. J. Immunotoxicol. 2022, 19, 17–26. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.M.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab-Aziz, M.F.; Sazili, A.Q. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Sobuj, M.K.; Shemul, M.S.; Islam, M.S.; Islam, M.A.; Mely, S.S.; Ayon, M.H.; Pranto, S.M.; Alam, M.S.; Bhuiyan, M.S.; Rafiquzzaman, S.M. Qualitative and quantitative phytochemical analysis of brown seaweed Sargassum polycystum collected from Bangladesh with its antioxidant activity determination. Food Chem. Adv. 2024, 4, 100565. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Devel Ther. 2015, 9, 3445–3454. [Google Scholar] [PubMed]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Yan-Hwa, C.; Chao-Lin, C.; Hsia-Fen, H. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.O.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre. 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 126, e55718. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of super oxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Goldblith, S.A.; Proctor, B.E. Photometric determination of catalase activity. J. Biol. Chem. 1950, 187, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Gueroui, M.; Kechrid, Z. Evaluation of some biochemical parameters and brain oxidative stress in experimental rats exposed chronically to silver nitrate and the protective role of vitamin E and selenium. Toxicol. Res. 2016, 32, 301–309. [Google Scholar] [CrossRef] [PubMed]




| Division of Seaweed, Species | Total Phenolic Content (mg GAE/g Dry Extract) | Radical Scavenging Activity of DPPH (µg/mL) | Reducing Power (mg AAE/g Extract) |
|---|---|---|---|
| Chiorophyta, Caulerpa lentillifera | 3.42 ± 0.25 | 32.71 ± 0.43 | 9.56 ± 4.23 |
| Chiorophyta, Chaetomorpha antenina | 16.42 ± 1.21 | 45.26 ± 3.51 | 15.00 ± 0.84 |
| Chiorophyta, Enteromorpha prolofera | 32.72 ± 2.54 | 82 ± 2.47 | 63.00 ± 6.92 |
| Phaeophyta, Sargassum polycystum | 92.25 ± 3.26 | 124.36 ± 5.04 | 63.24 ± 2.79 |
| Phaeophyta, Padina Minor | 54.27 ± 1.59 | 205.32 ± 2.01 | 34.52 ± 3.42 |
| Phaeophyta, Padina australis | 76.29 ± 2.42 | 159.42 ± 1.83 | 35.82 ± 2.54 |
| Rhodophyta, Gelidium pusillum | 15.95 ± 1.38 | 114.25 ± 3.24 | 29.45 ± 3.52 |
| Rhodophyta, Jania Rubens | 22.47 ± 1.05 | 62.42 ± 4.27 | 12.08 ± 2.54 |
| Rhodophyta, Gelidiella acerosa | 32.98 ± 1.32 | 56.42 ± 5.62 | 16.34 ± 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongrum, J.; Yingthongchai, P.; Tateing, S.; Kaewkaen, P. Cognitive-Enhancing Effect of Marine Brown Algae-Derived Phenolics through S100B Inhibition and Antioxidant Activity in the Rat Model of Ischemic Stroke. Mar. Drugs 2024, 22, 451. https://doi.org/10.3390/md22100451
Khongrum J, Yingthongchai P, Tateing S, Kaewkaen P. Cognitive-Enhancing Effect of Marine Brown Algae-Derived Phenolics through S100B Inhibition and Antioxidant Activity in the Rat Model of Ischemic Stroke. Marine Drugs. 2024; 22(10):451. https://doi.org/10.3390/md22100451
Chicago/Turabian StyleKhongrum, Jurairat, Pratoomporn Yingthongchai, Suriya Tateing, and Pratchaya Kaewkaen. 2024. "Cognitive-Enhancing Effect of Marine Brown Algae-Derived Phenolics through S100B Inhibition and Antioxidant Activity in the Rat Model of Ischemic Stroke" Marine Drugs 22, no. 10: 451. https://doi.org/10.3390/md22100451







