Investigating the Anti-Inflammatory Activity of Various Brown Algae Species
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
2.1.1. Fucoidans
2.1.2. Polyphenols
2.2. Proinflammatory Cytokines
2.3. Anti-Inflammatory Cytokines
2.4. In Vivo Anti-Inflammatory Effects
2.5. Antioxidant Effects
2.6. Mechanisms Contributing to Anti-Inflammatory Effects
2.6.1. Protein Denaturation
2.6.2. Inhibition of Nitric Oxide
2.6.3. Anti-Inflammatory Pathways
2.7. Toxicity
2.8. Dermal Effects
2.9. Permeability through Skin
Molecular Size
3. Discussion
4. Future Directions
4.1. Optimization of Extraction Techniques
4.2. Enhancing Skin Penetrability
4.3. Targeted Therapeutic Dosages
4.4. Expanding the Scope of Anti-Inflammatory Research
4.5. Addressing Practical and Logistical Challenges
4.6. Consumer Education and Regulatory Compliance
4.7. Clinical Studies
4.8. Market Viability
4.9. Different Algae Species and Their Chemical Composition
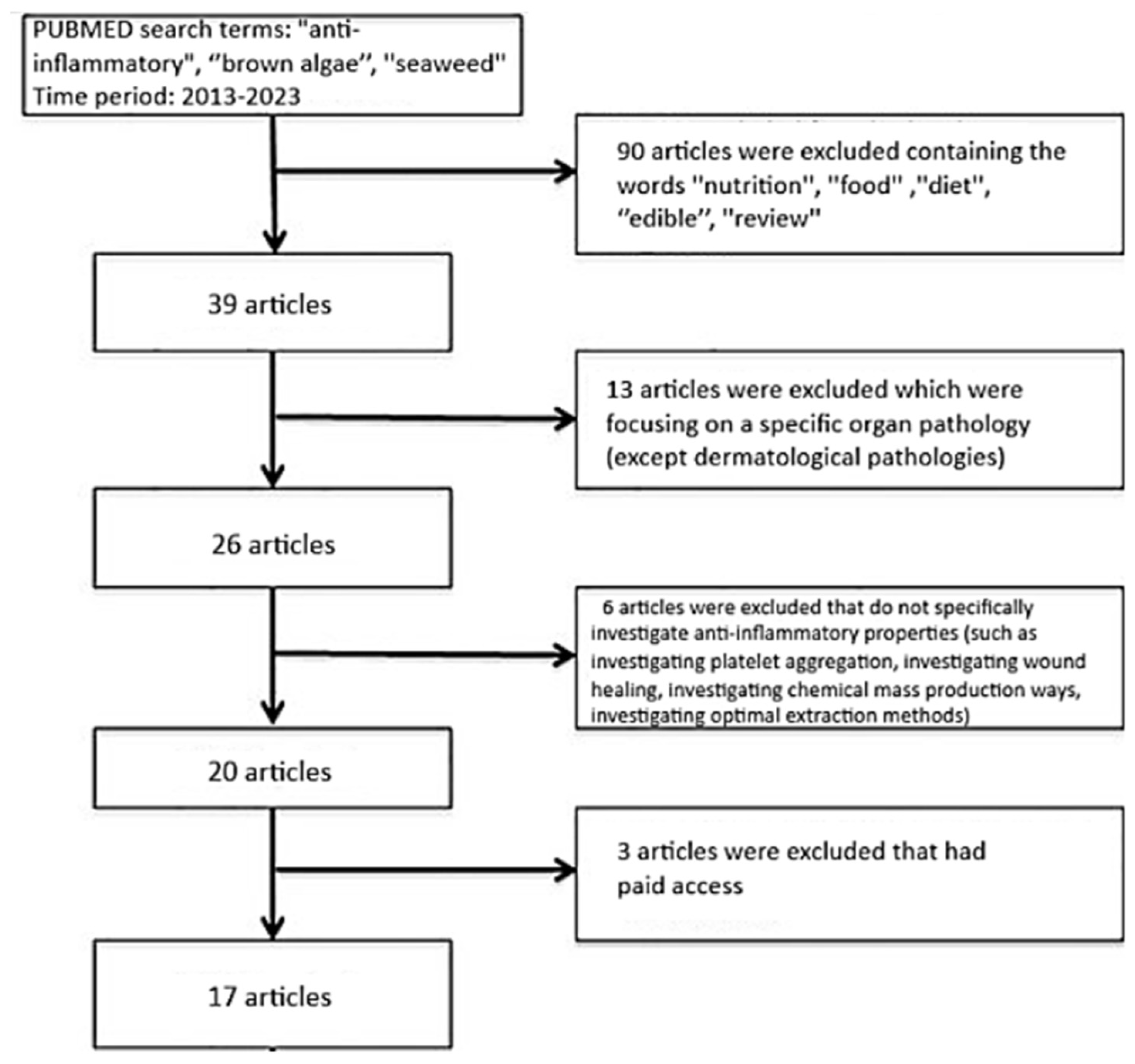
5. Materials and Methods
5.1. Inclusion and Exclusion Criteria
5.2. Critical Appraisal
5.3. Data Extraction
6. Strengths and Limitations of the Literature Study
6.1. Strengths
6.2. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gatmaitan, J.G.; Lee, J.H. Challenges and Future Trends in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 11380. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Saini, K.; Singh, K.K. Challenges of Current Treatment and Exploring the Future Prospects of Nanoformulations for Treatment of Atopic Dermatitis. Pharmacol. Rep. 2023, 75, 1066–1095. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Jee, S.H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef] [PubMed]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Devel. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. 2014, 13, 207–220. [Google Scholar]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Thomas, N.; Kim, S.K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Bai, B.; Song, G.; Zhang, J.; Yu, H.; Huang, S.; Wang, Z.; Lu, G. Topical Drug Delivery Strategies for Enhancing Drug Effectiveness by Skin Barriers, Drug Delivery Systems, and Individualized Dosing. Front. Pharmacol. 2024, 14, 10825035. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, S.-H.; Dong, C.-D.; Huang, C.-Y.; Chang, C.-C.; Chang, J.-S. Effect of Molecular Mass and Sulfate Content of Fucoidan from Sargassum siliquosum on Antioxidant, Anti-Lipogenesis, and Anti-Inflammatory Activity. J. Biosci. Bioeng. 2021, 132, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component. Mar. Drugs 2023, 21, 385. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Pinteus, S.; Gaspar, H.; Goettert, M.I.; Pedrosa, R. Saccorhiza polyschides—A Source of Natural Active Ingredients for Greener Skincare Formulations. Molecules 2022, 27, 6496. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from Seaweed Fucus vesiculosus Inhibits 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jouini, M.; Amor, H.B.H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical Analysis and Evaluation of the Antioxidant, Anti-inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Calado, M.d.L.; Silva, J.; Alves, C.; Susano, P.; Santos, D.; Alves, J.; Martins, A.; Gaspar, H.; Pedrosa, R.; Campos, M.J. Marine Endophytic Fungi Associated with Halopteris scoparia (Linnaeus) Sauvageau as Producers of Bioactive Secondary Metabolites with Potential Dermocosmetic Application. PLoS ONE 2021, 16, e0250954. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Jayawardena, T.; Jeon, Y.-J. Anti-fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Leaves in Macrophages via Inhibiting Inflammatory Signaling Pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [CrossRef]
- Almurshedi, A.S.; El-Masry, T.A.; Selim, H.; El-Sheekh, M.M.; Makhlof, M.E.; Aldosari, B.N.; Alfagih, I.M.; AlQuadeib, B.T.; Almarshidy, S.S.; El-Bouseary, M.M. New Investigation of Anti-inflammatory Activity of Polycladia crinita and Biosynthesized Selenium Nanoparticles: Isolation and Characterization. Microb. Cell Fact. 2023, 22, 21. [Google Scholar] [CrossRef]
- Hadjkacem, F.; Elleuch, J.; Aitouguinane, M.; Chakou, F.Z.; Ursu, A.V.; Dubessay, P.; Bourgougnon, N.; Traikia, M.; Le Cerf, D.; El Alaoui-Talibi, Z.; et al. Primary Structural Features, Physicochemical and Biological Properties of Two Water-Soluble Polysaccharides Extracted from the Brown Tunisian Seaweed Halopteris scoparia. Int. J. Biol. Macromol. 2023, 253, 126757. [Google Scholar] [CrossRef]
- Neelakandan, Y.; Venkatesan, A. Antinociceptive and Anti-inflammatory Effect of Sulfated Polysaccharide Fractions from Sargassum wightii and Halophila ovalis in Male Wistar Rats. Indian J. Pharmacol. 2016, 48, 562. [Google Scholar] [CrossRef]
- Simpi, C.; Nagathan, C.; Karajgi, S.; Kalyane, N. Evaluation of Marine Brown Algae Sargassum ilicifolium Extract for Analgesic and Anti-inflammatory Activity. Pharmacogn. Res. 2013, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A.; Ibrahim, M.; Hasan, M.M.U.; Jilani, T.; Shafique, S.; Rasheed, M. Phycochemical Analyses and Pharmacological Activities of Seven Macroalgae of Arabian Sea (Northern Coast Line). Pak. J. Pharm. Sci. 2021, 34, 963–969. [Google Scholar]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton Rule for the Skin Penetration of Chemical Compounds and Drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The Pharmacokinetics ofFucoidan After Topical Application to Rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.; FitzGerald, R.; Smyth, T. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal Penetration Studies of Potential Phenolic Compounds Ex Vivo and Their Antioxidant Activity In Vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of Squalane-Based Emulsion on Polyphenols Skin Penetration: Ex Vivo Skin Study. Colloids Surf. B Biointerfaces 2022, 218, 112779. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.; Leite, E.L.; Nader, H.B.; Rocha, H.A. Evaluation of Anti-Nociceptive and Anti-Inflammatory Activities of a Heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef]
- Marques, M.S.; Varela, C.; Cabral, M.L.; Cabral, C. Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis. Pharmaceutics 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front. Nutr. 2021, 8, 759953. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Yang, H.W.; Choi, C.S.; Jeon, Y.J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.A. Future Challenges and Opportunities of Marine Algae in Cosmeceuticals. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 25–32. [Google Scholar] [CrossRef]
- Alexander, H.; Paller, A.S.; Traidl-Hoffmann, C.; Beck, L.A.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.D.; Spuls, P.; Su, J.; et al. The Role of Bacterial Skin Infections in Atopic Dermatitis: Expert Statement and Review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2019, 182, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-E.; Zheng, P.; Ye, S.-Z.; Ma, X.; Liu, E.; Pang, Y.-B.; He, Q.-Y.; Zhang, Y.-X.; Li, W.-Q.; Zeng, J.-H.; et al. Microbiome: Role in Inflammatory Skin Diseases. J. Inflamm. Res. 2024, 17, 1057–1082. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the Potential of Using Algae in Cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Alvarado-Sansininea, J.J.; Tavera-Hernández, R.; Jiménez-Estrada, M.; Coronado-Aceves, E.W.; Espitia-Pinzón, C.I.; Díaz-Martínez, S.; Hernández-Anaya, L.; Rangel-Corona, R.; Avila-Ortiz, A.G. Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora. Int. J. Plant Biol. 2022, 14, 63–76. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Aydin, B. Antibacterial Activities of Methanolic Extracts of Different Seaweeds from Iskenderun Bay, Turkey. Int. J. Sec. Metab. 2021, 7, 117–123. [Google Scholar] [CrossRef]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Skin Pharmacol. Physiol. 2024, 37, 174–200. [Google Scholar] [CrossRef]
- Choi, E.H. Effects of Seaweed Laminaria japonica Extracts on Skin Moisturizing Activity In Vivo. J. Cosmet. Sci. 2014, 64, 193–202. [Google Scholar]
- Fonseca, S.; Amaral, M.; Pinto Reis, C.; Custódio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Mar. Drugs 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
| Brown Algae Species Investigated | Chemical Compounds Investigated |
|---|---|
| Fucus vesiculosus | Fucoidan |
| Sargassum wightii | Sulphated polysaccharides |
| Saccharina japonica | Fucoidan |
| Fucus distichus | Fucoidan |
| Fucus serratus | Fucoidan |
| Ascophyllum nodosum | Fucoidan |
| Dictyota menstrualis | Heterofucan |
| Saccorhiza polyschides | Not specified |
| Sargassum siliquosum | Fucoidan |
| Carpomitra costata | Not specified |
| Sargassum vulgare | Phlorotannins |
| Ecklonia maxima | Fucoidan |
| Sargassum ilicifolium | Not specified |
| Polycladia crinita | Polysaccharides, lipids, terpenoids, fucosterol, phlorotannins |
| Cystoseira crinita | Fucoidan |
| Cystoseira compressa | Fucoidan |
| Cystoseira sedoides | Fucoidan |
| Halopteris scoparia (Linnaeus) Sauvageau | Not specified |
| Sargassum swartzii | Not specified |
| Cystoseira indica | Not specified |
| Codium flabellatum | Not specified |
| Cystoseira sedoides (Fucales) | Fucoidan |
| Cladostephus spongeosis | Phlorotannins |
| Padina pavonica | Phlorotannins |
| Padina boergesenii | Chlorophylls, carotenoids, fucoxanthin, phycoerythrin, phycocyanin, lycopene, amino acids, polyphenols, proteins |
| Halopteris scoparia | Not specified |
| Title | Year | Algae Species and Compounds | Study Design | Anti-Inflammatory Assays | Outcome Measures |
|---|---|---|---|---|---|
| In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds | 2022 | Algae species: Saccharina japonica, Fucus vesiculosus, Fucus distichus, Fucus serratus, and Ascophyllum nodosum. Compounds: Fucoidan | In vitro | 1. 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay | This study measures the anti-inflammatory outcomes of the fucoidans through: 1. Their ability to scavenge free radicals 2. Inhibit protein denaturation. |
| Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis | 2013 | Algae species: Dictyota menstrualis Compounds: Heterofucan | In vivo | 1. Assessment of pro-inflammatory cytokines, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) | This study measured the impact of Dictyota menstrualis on: 1. Leukocyte migration into the peritoneal cavity after chemical stimulation 2. Its effect on the expression of pro-inflammatory cytokines |
| Saccorhiza polyschides—A Source of Natural Active Ingredients for Greener Skincare Formulations | 2022 | Algae species: Saccorhiza polyschides Compounds: No specific compound | In vitro | 1. Enzyme-linked immunosorbent assay (ELISA) to determine the levels of pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokine (IL-10) in RAW 264.7 cells 2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay | 1. Measurement of pro-inflammatory and anti-inflammatory cytokines 2. Inhibition of nitric oxide (NO) production 3. Cell viability and cytotoxicity assessment 4. Reactive oxygen species (ROS) production |
| Fucoidan from seaweed Fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis | 2019 | Algae species: Fucus vesiculosus Compounds: Fucoidan | In vitro | 1. Ear Swelling and Erythema Score 2. Histology (hematoxylin and eosin staining) 3. Immunofluorescence staining for scavenger receptor class A (SR-A) and CD4+ T cells in ear tissues 4. Enzyme-linked immunosorbent assay (ELISA) to measure serum levels of immunoglobulin E (IgE) and interleukin-4 (IL-4) 5. Real-time PCR for quantification of mRNA levels of SR-A, pro-inflammatory cytokines (TNF-α, IL-12), anti-inflammatory cytokines (IL-4, IL-10, TGF-β), and GAPDH (housekeeping gene) 6. Spleen Index calculation of spleen weight relative to body weight 7. Cell proliferation assay to evaluate splenocyte proliferation using the Cell Counting Kit-8 (CCK-8) 8. Flow cytometry: analysis of CD4+ T cell subsets, including Th1, Th2, Th17, and regulatory T cells (Treg). | Primary outcome measures included the assessment of: 1. Ear swelling 2. Skin lesions 3. Inflammatory cell infiltration Secondary outcomes included the measurement of: 1. Serum levels of IgE and IL-4 2. Histological analysis of ear tissues 3. Evaluation of spleen index 4. Splenocyte proliferation 5. Characterization of CD4+ T cell subsets |
| Effect of molecular mass and sulphate content of fucoidan from Sargassum siliquosum on antioxidant, anti-lipogenesis, and anti-inflammatory activity | 2021 | Algae species: Sargassum siliquosum Compounds: Fucoidan | In vitro | 1. Assessment of the proinflammatory cytokine TNF-α production in RAW264.7 cells | The primary outcome measures include the antioxidant capacity, anti-lipogenesis activity, and anti-inflammatory activity, which were evaluated based on: 1. Scavenging effect on DPPH radicals (antioxidant) 2. Inhibition of lipid synthesis in HepG2 cells (anti-lipogenesis), 3. Reduction of TNF-α production in RAW264.7 cells (anti-inflammatory). The effects were compared among different fucoidan fractions with varying molecular masses and sulphate contents. |
| Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata | 2021 | Algae species: Carpomitra costata Compounds: No specific compound | In vitro | 1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay 2. Assessment of anti-inflammatory cytokines (TNF-α, IL-6, and IL-10) after mouse macrophage cells (RAW 264.7) exposure to lipopolysaccharide (LPS) as an inflammation mediator | 1. Production of nitric oxide (NO) in mouse macrophage cells 2. The response to lipopolysaccharide (LPS) exposure 3. The levels of inflammatory and anti-inflammatory cytokines (TNF-α, IL-6, and IL-10) The results are expressed as a percentage of the control or in terms of inhibitory effects. |
| Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast | 2022 | Algae species: Sargassum vulgare Compounds: Phlorotannins | In vitro | 1. Inhibition of albumin denaturation | 1. Inhibition of albumin denaturation serves as the outcome measure for the anti-inflammatory activity, and the effectiveness of different fractions 2. Assessing the ability of the samples to scavenge free radicals and prevent oxidation 3. Characterization of phlorotannins within the ethyl acetate fraction, identifying various compounds and derivatives based on LC-ESI-MS/MS analysis |
| Anti-Fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Laves in Macrophages via Inhibiting Inflammatory Signalling Pathways | 2022 | Algae species: Ecklonia maxima Compounds: Fucoidan | In vitro | 1. MTT test for cell viability assessment of particulate matter stimulated RAW 264.7 macrophages 2. Griess assay for the determination of NO production in particulate matter stimulated cells 3. ELISA assay for measuring PGE2 production in particulate matter stimulated RAW 264.7 macrophages 4. Competitive enzyme immunoassay kits for quantifying TNF-α, IL-6, and IL-1β concentrations in the supernatant 5. Western blot analysis for evaluating protein expression levels of iNOS, COX-2, NF-κB, and MAPK in particulate matter stimulated RAW 264.7 cells 6. RT-qPCR for assessing pro-inflammatory cytokine expression levels (iNOS, COX2, IL-1β, IL-6, TNF-α, TLR2, and TLR4) in E. maxima fucoidan-treated RAW 264.7 cells. | The inhibitory effects of E. maxima fucoidan on the: 1. Production of NO 2. Production of PGE2 3. Production of pro-inflammatory cytokines The impact of E. maxima fucoidan on the expression of iNOS, COX-2, NF-κB, and MAPK proteins. |
| Antinociceptive and anti-inflammatory effect of sulphated polysaccharide fractions from Sargassum wightii and Halophila ovalis in male Wistar rats | 2016 | Algae species: Sargassum wightii Compounds: sulphated polysaccharides | In vivo | 1. Carrageenan-induced rat paw oedema 2. Peritonitis model in rats 3. Freund’s adjuvant-induced arthritis | 1. Reduction in paw volume variation over time 2. Decrease in total leukocyte count in peritoneal fluid 3. Percentage of inhibition of arthritis development |
| Evaluation of marine brown algae Sargassum ilicifolium extract for analgesic and anti-inflammatory activity | 2013 | Algae species: Sargassum ilicifolium Compounds: No specific compound | In vivo | 1. Carrageenan-induced rat paw oedema | 1. Reduction in paw swelling over time, expressed as a percentage of inhibition compared to control |
| New investigation of anti-inflammatory activity of Polycladia crinita and biosynthesized selenium nanoparticles: isolation and characterization | 2023 | Algae species: Polycladia crinita Compounds: polysaccharides, lipids, terpenoids, fucosterol, and phlorotannins | In vivo | 1. Carrageenan-induced rat paw oedema | 1. Measurement of paw oedema weight changes 2. Tissue levels of malondialdehyde (MDA) and prostaglandin E2 (PGE2) 3. Histopathological examination of paw tissues 4. Immunohistochemical staining for COX-2 and IL-1β expression in paw tissues |
| Physico-chemical characterization and pharmacological evaluation of sulphated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira | 2015 | Algae species: Cystoseira crinita, Cystoseira compressa, and Cystoseira sedoides Compounds: Fucoidan | In vivo | 1. Carrageenan-induced rat paw oedema 2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay | 1. The reduction in carrageenan-induced paw oedema over time 2. EC50 value, representing the effective concentration at which the antioxidant activity was 50% |
| Marine endophytic fungi associated with Halopteris scoparia (Linnaeus) Sauvageau as producers of bioactive secondary metabolites with potential dermocosmetic application | 2021 | Algae species: Halopteris scoparia (Linnaeus) Sauvageau Compounds: No specific compound | In vitro | 1. MTT assay was used to measure the amount of nitric oxide produced by RAW 264.7 cells incubated in various concentrations of algae extract 2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay | 1. Measuring the production of nitric oxide (NO) in RAW 264.7 cells in response to LPS exposure and evaluate for their ability to suppress NO production |
| Phycochemical analyses and pharmacological activities of seven macroalgae of Arabian Sea (Northern coast line) | 2021 | Algae species: Sargassum wightii, Sargassum swartzii, Cystoseira indica, and Codium flabellatum (*other non brown algae species were also investigated) Compounds: No specific compounds | In vivo | 1. Carrageenan-induced rat paw oedema | 1. Measurement of paw size at specific time points (1st, 2nd, 3rd, 4th, and 5th hours) after the induction of inflammation using the carrageenan suspension |
| Phytochemical Analysis and Evaluation of the Antioxidant, Anti-Inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds | 2018 | Algae species: Cystoseira sedoides (Fucales), Cladostephus spongeosis (Sphacelariales), and Padina pavonica (Dictoyales) Compounds: Phlorotannins | In vivo | 1. Xylene-induced ear oedema in mice 2. Carrageenan-induced paw oedema in rats | 1. Inhibition percentage of ear oedema in the xylene-induced ear oedema model 2. The percentage of inhibition of paw oedema in the carrageenan-induced paw oedema model |
| Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component | 2023 | Algae species: Padina boergesenii Compounds: Chlorophylls, carotenoids, fucoxanthin, phycoerythrin, phycocyanin, lycopene, amino acids, polyphenols, and proteins | In vitro | 1. No specific anti-inflammatory assay was used (*the study identified compounds within algae with known anti-inflammatory effects) | 1. Identification of bioactive compounds with potential cosmetic benefits |
| Primary structural features, physicochemical and biological properties of two water-soluble polysaccharides extracted from the brown Tunisian seaweed Halopteris scoparia | 2023 | Algae species: Halopteris scoparia Compounds: Alginates and fucoidans | In vitro | 1. Denaturation of bovine serum albumin (BSA) induced by heat | 1. The inhibition of albumin denaturation was calculated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoydan, S.; Rustemeyer, T. Investigating the Anti-Inflammatory Activity of Various Brown Algae Species. Mar. Drugs 2024, 22, 457. https://doi.org/10.3390/md22100457
Ersoydan S, Rustemeyer T. Investigating the Anti-Inflammatory Activity of Various Brown Algae Species. Marine Drugs. 2024; 22(10):457. https://doi.org/10.3390/md22100457
Chicago/Turabian StyleErsoydan, Selin, and Thomas Rustemeyer. 2024. "Investigating the Anti-Inflammatory Activity of Various Brown Algae Species" Marine Drugs 22, no. 10: 457. https://doi.org/10.3390/md22100457
APA StyleErsoydan, S., & Rustemeyer, T. (2024). Investigating the Anti-Inflammatory Activity of Various Brown Algae Species. Marine Drugs, 22(10), 457. https://doi.org/10.3390/md22100457





